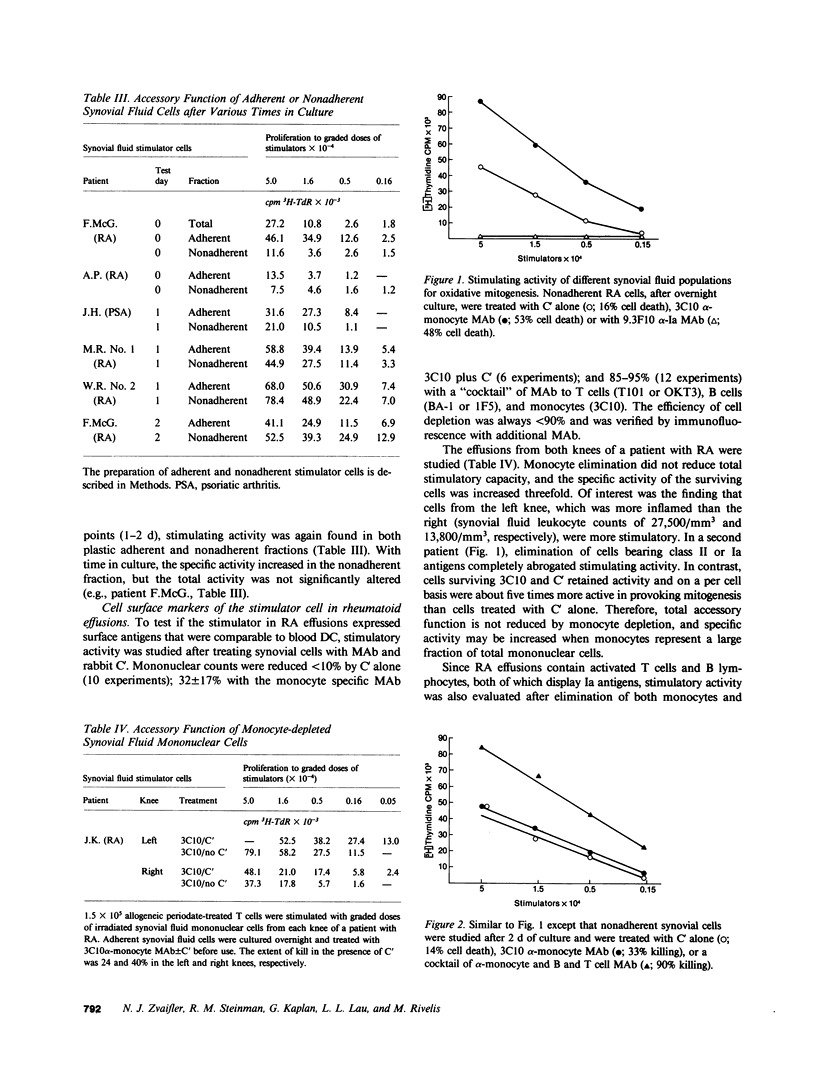
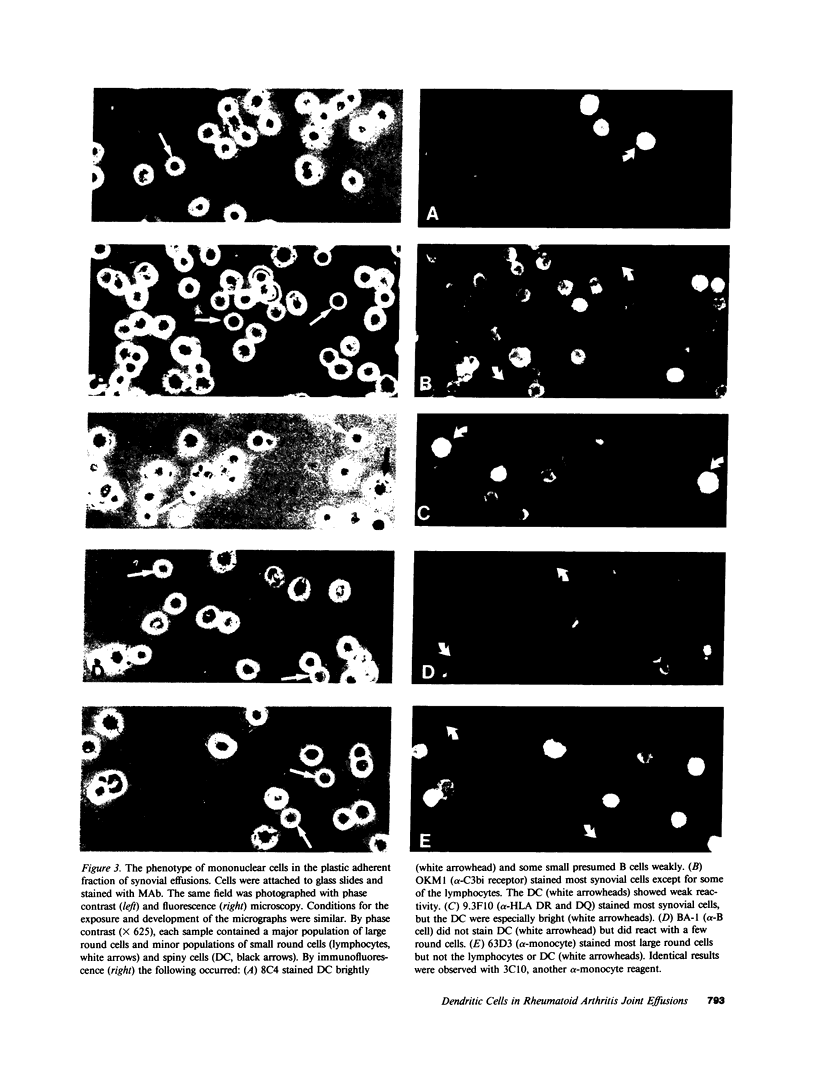
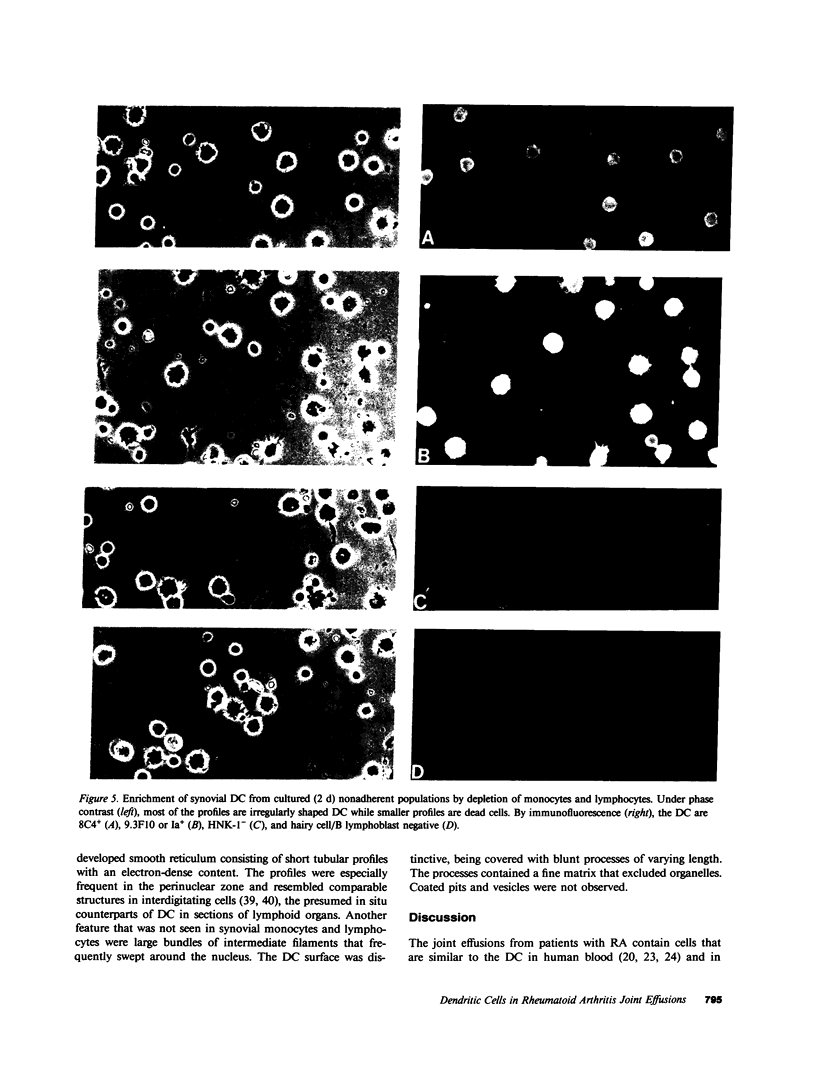
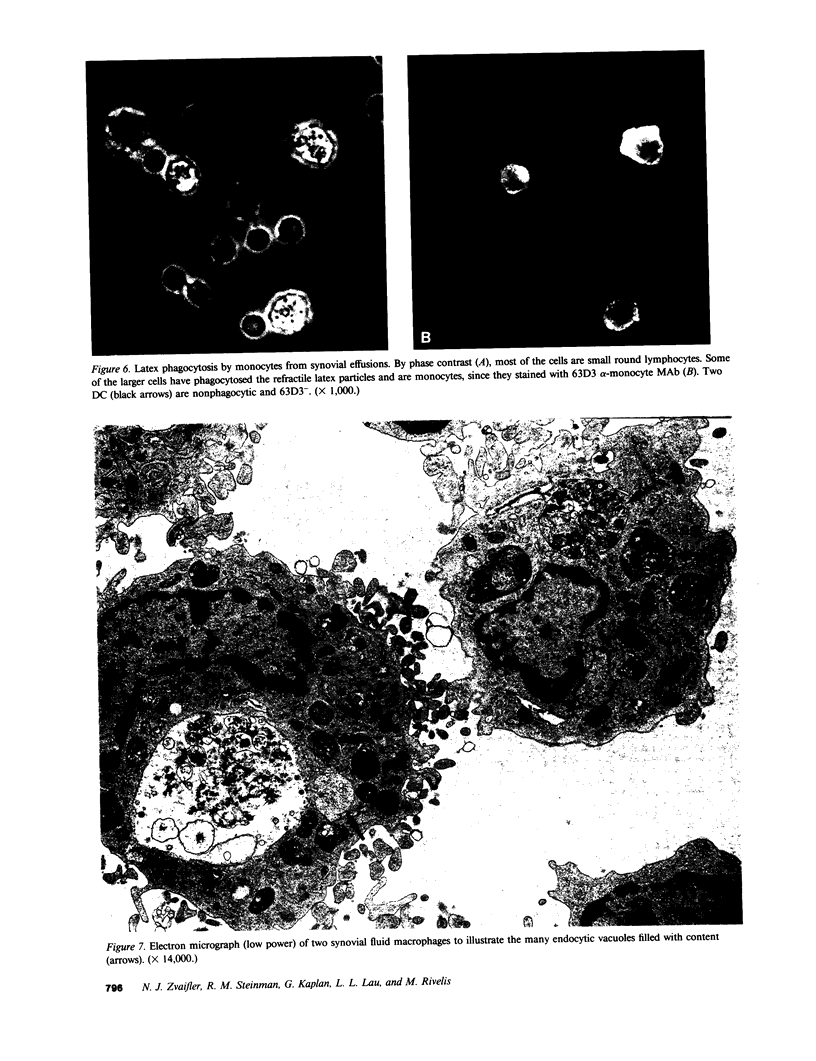
Abstract
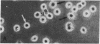
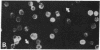
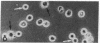
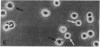
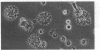
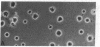
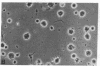
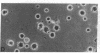
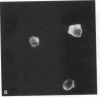
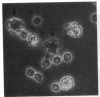
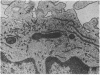
Dendritic cells in the circulation are leukocytes that are rich in Ia antigens and that actively stimulate T cell replication. We have identified dendritic cells in the joint effusions of patients with rheumatoid arthritis. By phase-contrast and immunofluorescence microscopy, synovial mononuclear cells contained 1-5% dendritic profiles that were rich in HLA-DR and DQ, had small amounts of C3bi receptor, and lacked a battery of monocyte and lymphocyte markers. These dendritic cells could be enriched to 60-80% purity by cytolytic depletion of monocytes and lymphocytes with a group of monoclonal antibodies (MAb) and complement. By transmission electron microscopy, the dendritic cell processes were bulbous in shape and lacked organelles. The cytoplasm had few lysosomes or endocytic vacuoles but contained a well-developed smooth reticulum that was comparable to that previously described in the Ia-rich interdigitating cells of lymphoid tissues. The growth of sodium periodate-modified T lymphocytes was used as a rapid quantitative assay of accessory cell function. Synovial mononuclear cells were some ten times more active than normal blood cells. Treatment with alpha-Ia MAb and complement ablated stimulatory function. In contrast, removal of monocytes (MAb, 3C10) or monocytes and B (MAb, BA-1) plus T (MAb, OKT3, or T101) lymphocytes did not significantly alter total activity, and the function per viable cell increased four- to eightfold. We conclude that rheumatoid arthritis synovial fluids contain cells that are comparable in function, phenotype, and structure to blood dendritic cells, although the frequency (1-5%) is 10 times greater in joints. The reason for their accumulation in the articular cavity is not known, but dendritic cells may be important in perpetuating the joint inflammation characteristic of this disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Steinman R. M., Weinstein D. E., Granelli-Piperno A., Palladino M. A. Dendritic cells initiate a two-stage mechanism for T lymphocyte proliferation. J Exp Med. 1983 Apr 1;157(4):1101–1115. doi: 10.1084/jem.157.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D. G., Dayer J. M., Roelke M., Schumacher H. R., Krane S. M. Rheumatoid synovial cell morphologic changes induced by a mononuclear cell factor in culture. Arthritis Rheum. 1983 Jan;26(1):8–14. doi: 10.1002/art.1780260102. [DOI] [PubMed] [Google Scholar]
- Beck P., Burmester G. R., Ledwoch A., Urban C., Kalden J. R. Autologous and allogeneic MLC-reactivity in patients with rheumatoid arthritis. J Clin Lab Immunol. 1981 Jul;6(1):27–33. [PubMed] [Google Scholar]
- Beyer C. F., Bowers W. E. Lymphocyte transformation induced by chemical modification of membrane components. II. Effect of neuraminidase treatment of responder cells on proliferation and cytotoxicity in indirect stimulation. J Immunol. 1978 Nov;121(5):1790–1798. [PubMed] [Google Scholar]
- Burmester G. R., Dimitriu-Bona A., Waters S. J., Winchester R. J. Identification of three major synovial lining cell populations by monoclonal antibodies directed to Ia antigens and antigens associated with monocytes/macrophages and fibroblasts. Scand J Immunol. 1983 Jan;17(1):69–82. doi: 10.1111/j.1365-3083.1983.tb00767.x. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Kalden J. R., Peter H. H., Schedel I., Beck P., Wittenborg A. Immunological and functional characteristics of peripheral blood and synovial fluid lymphocytes from patients with rheumatoid arthritis. Scand J Immunol. 1978;7(5):405–417. doi: 10.1111/j.1365-3083.1978.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Shu G., Ledbetter J. A. Role of the Bp35 cell surface polypeptide in human B-cell activation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1766–1770. doi: 10.1073/pnas.82.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall V., Panayi G. S., Laurent R. Lymphocyte studies in rheumatoid arthritis. III. A comparative study of the responses of peripheral blood and synovial fluid lymphocytes to phytomitogens. Scand J Rheumatol. 1979;8(1):10–16. [PubMed] [Google Scholar]
- Crout J. E., McDuffie F. C., Ritts R. E., Jr Induction of peripheral blood lymphocyte transformation by autologous synovial fluid lymphocytes and synovial fluid. Arthritis Rheum. 1976 May-Jun;19(3):523–531. doi: 10.1002/art.1780190303. [DOI] [PubMed] [Google Scholar]
- Crow M. K., Kunkel H. G. Human dendritic cells: major stimulators of the autologous and allogeneic mixed leucocyte reactions. Clin Exp Immunol. 1982 Aug;49(2):338–346. [PMC free article] [PubMed] [Google Scholar]
- Drexhage H. A., Mullink H., de Groot J., Clarke J., Balfour B. M. A study of cells present in peripheral lymph of pigs with special reference to a type of cell resembling the Langerhans cell. Cell Tissue Res. 1979 Nov;202(3):407–430. doi: 10.1007/BF00220434. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W., Tidman N. Analysis of T cell subsets in the peripheral blood and synovial fluid of patients with rheumatoid arthritis by means of monoclonal antibodies. Ann Rheum Dis. 1983 Aug;42(4):357–361. doi: 10.1136/ard.42.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Galili U., Rosenthal L., Galili N., Klein E. Activated T cells in the synovial fluid of arthritic patients: characterization and comparison with in vitro activated human and murine T cells in cooperation with monocytes in cytotoxicity. J Immunol. 1979 Mar;122(3):878–883. [PubMed] [Google Scholar]
- Galili U., Rosenthal L., Klein E. Activated T cells in the synovial fluid of arthritic patients. II. In vitro activation of the autologous blood lymphocytes. J Immunol. 1981 Aug;127(2):430–432. [PubMed] [Google Scholar]
- Goto M., Zvaifler N. J. Characterization of the natural killer-like lymphocytes in rheumatoid synovial fluid. J Immunol. 1985 Mar;134(3):1483–1486. [PubMed] [Google Scholar]
- Hepburn B., McDuffie F. C., Ritts R. E., Jr Impaired blastogenic response of lymphocytes from synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol. 1976 Jun;3(2):118–123. [PubMed] [Google Scholar]
- Heusermann U., Stutte H. J., Müller-Hermelink H. K. Interdigitating cells in the white pulp of the human spleen. Cell Tissue Res. 1974;153(3):415–417. [PubMed] [Google Scholar]
- Inaba K., Granelli-Piperno A., Steinman R. M. Dendritic cells induce T lymphocytes to release B cell-stimulating factors by an interleukin 2-dependent mechanism. J Exp Med. 1983 Dec 1;158(6):2040–2057. doi: 10.1084/jem.158.6.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Van Voorhis W. C., Muramatsu S. Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and in man. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6041–6045. doi: 10.1073/pnas.80.19.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. H., Balfour B. M., Armstrong J. A., Griffiths S. Functional anatomy of lymph nodes. II. Peripheral lymph-borne mononuclear cells. Anat Rec. 1978 Jan;190(1):5–21. doi: 10.1002/ar.1091900103. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Kabelitz D., Plöen L., Sundström C., Nilsson K., Wigren A., Wigzell H. Immune functions of human synovial cells. Phenotypic and T cell regulatory properties of macrophage-like cells that express HLA-DR. Arthritis Rheum. 1982 May;25(5):488–501. doi: 10.1002/art.1780250502. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Scheynius A., Kabelitz D., Wigzell H. Evidence in support of a self-perpetuating HLA-DR-dependent delayed-type cell reaction in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3632–3636. doi: 10.1073/pnas.79.11.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., Bowers W. E. Accessory and stimulating properties of dendritic cells and macrophages isolated from various rat tissues. J Exp Med. 1982 Jul 1;156(1):1–19. doi: 10.1084/jem.156.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Katchlaski E. Induction of lymphocyte transformation by periodate. FEBS Lett. 1971 Jan 30;12(5):297–300. doi: 10.1016/0014-5793(71)80203-x. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Unkeless J. C., Witmer M. D., Gutchinov B., Cohn Z. A. Studies of the cell surface of mouse dendritic cells and other leukocytes. J Exp Med. 1981 Jul 1;154(1):168–187. doi: 10.1084/jem.154.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Witmer M. D., Gutchinov B. A monoclonal antibody specific for mouse dendritic cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):161–165. doi: 10.1073/pnas.79.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnett D. N., Chiorazzi N., Kunkel H. G. Monoclonal antibodies with specificity for hairy cell leukemia cells. J Clin Invest. 1982 Aug;70(2):254–261. doi: 10.1172/JCI110612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G., Seymour G. The involvement of interdigitating (antigen-presenting) cells in the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. 1983 Feb;51(2):247–254. [PMC free article] [PubMed] [Google Scholar]
- Pugh C. W., MacPherson G. G., Steer H. W. Characterization of nonlymphoid cells derived from rat peripheral lymph. J Exp Med. 1983 Jun 1;157(6):1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. M., Redelman D., Zvaifler N. J., Naides S. Studies of rheumatoid synovial fluid lymphocytes. I. Evidence for activated natural killer- (NK) like cells. J Immunol. 1982 Apr;128(4):1758–1763. [PubMed] [Google Scholar]
- Silver R. M., Redelman D., Zvaifler N. J. Studies of rheumatoid synovial fluid lymphocytes. II. A comparison of their behavior with blood mononuclear cells in the autologous mixed lymphocyte reaction and response to TCGF. Clin Immunol Immunopathol. 1983 Apr;27(1):15–27. doi: 10.1016/0090-1229(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Silverman H. A., Johnson J. S., Vaughan J. H., McGlamory J. C. Altered lymphocyte reactivity in rheumatoid arthritis. Arthritis Rheum. 1976 May-Jun;19(3):509–515. doi: 10.1002/art.1780190301. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Gutchinov B., Witmer M. D., Nussenzweig M. C. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton J. A., Peter J. B. The responses of peripheral blood and synovial fluid lymphocytes of patients with rheumatoid arthritis to in vitro stimulation with mitogens. Clin Immunol Immunopathol. 1978 Jun;10(2):233–241. doi: 10.1016/0090-1229(78)90031-4. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Valinsky J., Hoffman E., Luban J., Hair L. S., Steinman R. M. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983 Jul 1;158(1):174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R. J., Burmester G. R. Demonstration of Ia antigens on certain dendritic cells and on a novel elongate cell found in human synovial tissue. Scand J Immunol. 1981 Oct;14(4):439–444. doi: 10.1111/j.1365-3083.1981.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Brinckerhoff C. E., Mainardi C. L., Vater C. A., Evanson J. M., Harris E. D., Jr Collagenase production by rheumatoid synovial cells: morphological and immunohistochemical studies of the dendritic cell. Ann Rheum Dis. 1979 Jun;38(3):262–270. doi: 10.1136/ard.38.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. L., Adamson T. C., 3rd, Vaughan J. H., Fox R. I. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1984 Jan;27(1):32–39. doi: 10.1002/art.1780270106. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. Pathogenesis of the joint disease of rheumatoid arthritis. Am J Med. 1983 Dec 30;75(6A):3–8. doi: 10.1016/0002-9343(83)90469-2. [DOI] [PubMed] [Google Scholar]
- de Vere Tyndall A., Knight S. C., Edwards A. J., Clarke J. B. Veiled (dendritic) cells in synovial fluid. Lancet. 1983 Feb 26;1(8322):472–473. doi: 10.1016/s0140-6736(83)91468-x. [DOI] [PubMed] [Google Scholar]