Abstract
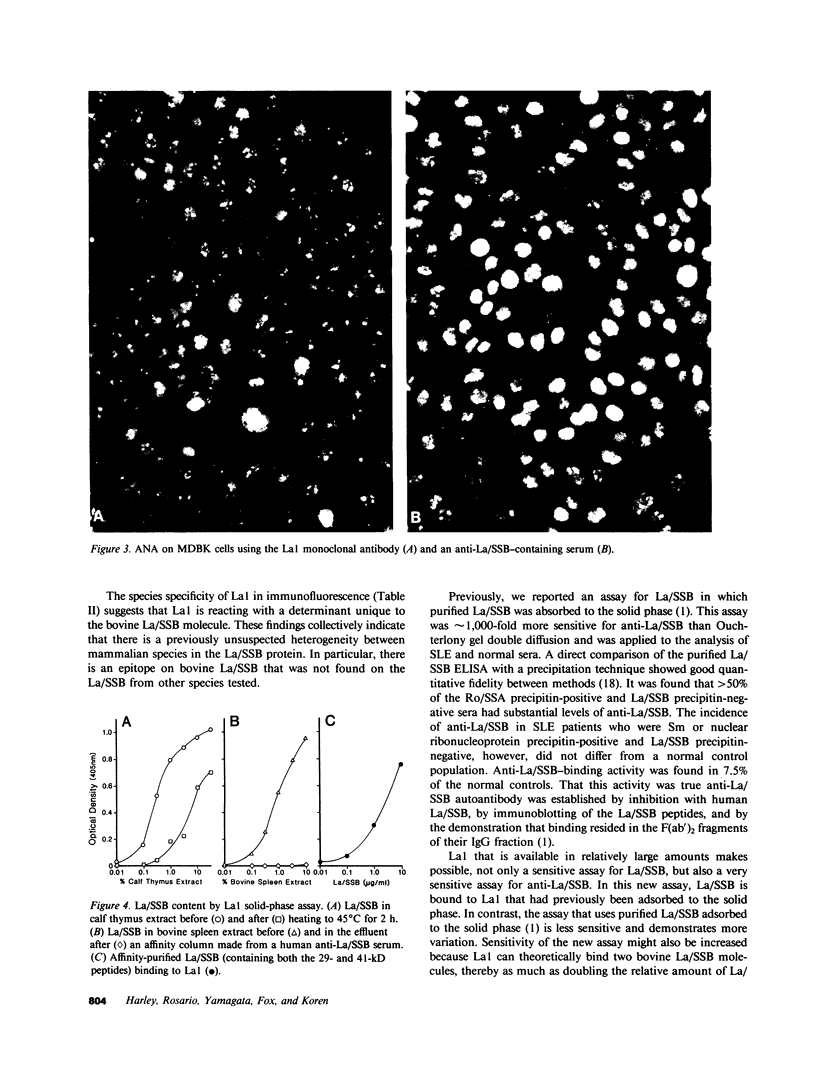
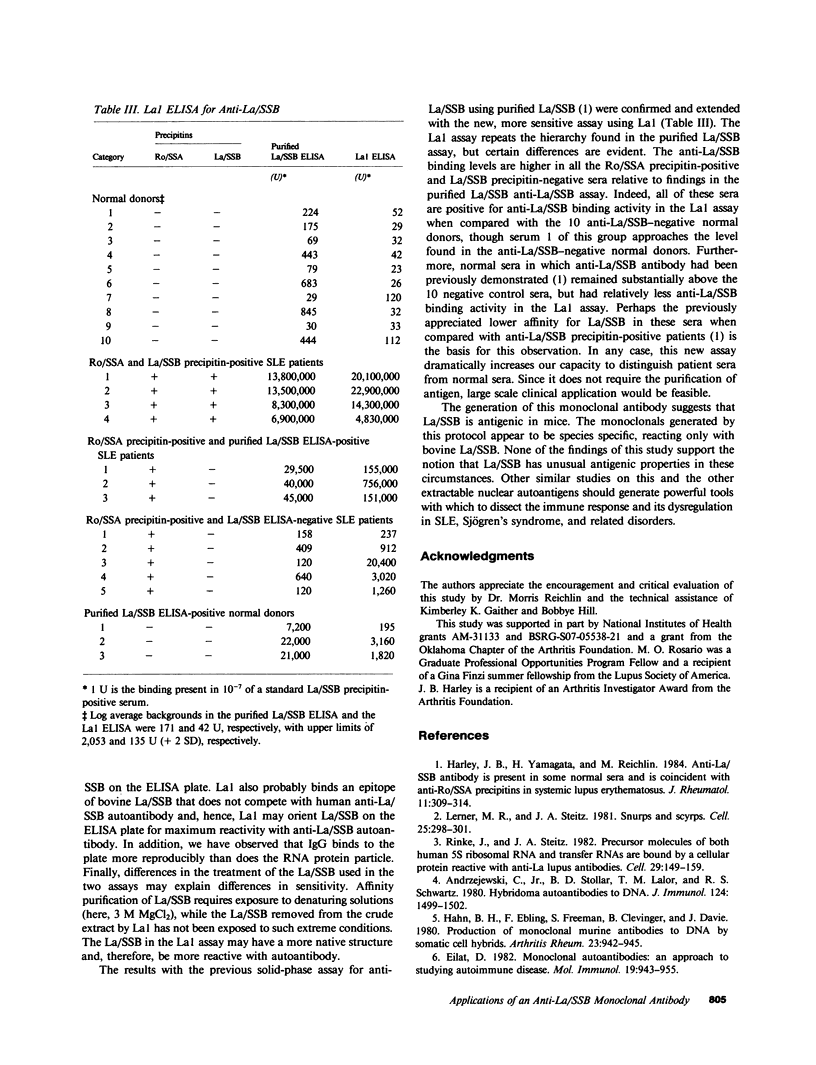
La/SSB is a small nuclear RNA protein against which precipitating autoantibodies are made in many patients with systemic lupus erythematosus or Sjögren's syndrome. The recent purification of La/SSB has made structural and immunologic studies possible. Consequently, a mouse hybridoma antibody (La1) was raised, after immunization and fusion, that reacted with bovine La/SSB. Results of inhibition tests with tissue extracts and fluorescent antinuclear antibody tests demonstrated that La1 reacted with bovine extracts and cells, but not with those from human, mouse, or rabbit sources. La1 reacted in Western blot and in an adapted anti-La/SSB enzyme-linked immunosorbent assay with only the 41-kD bovine La/SSB peptide and not with the smaller 29-kD bovine La/SSB peptide. RNA gels showed that La1 bound the La/SSB particle that contained the predominant La/SSB RNA species near 90 nucleotides as well as the minor RNA species, both of which were bound by the human autoimmune anti-La/SSB serum. A solid-phase assay for human autoimmune anti-La/SSB antibody using La1 was more sensitive for the detection of human anti-La/SSB than was a comparable assay using purified La/SSB, and showed that anti-La/SSB is present in nearly all Ro/SSA precipitin-positive sera. Thus, this study demonstrates that monoclonal antibody can be raised against La/SSB; that the protein moiety of bovine La/SSB differs from human, mouse, and rabbit at an epitope on the 41-kD La/SSB peptide; that the RNA bound to the La1-reactive particle was as heterogeneous as that binding the anti-La/SSB autoimmune serum; and that anti-Ro/SSA and anti-La/SSB are closely associated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrzejewski C., Jr, Stollar B. D., Lalor T. M., Schwartz R. S. Hybridoma autoantibodies to DNA. J Immunol. 1980 Mar;124(3):1499–1502. [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- DeHeer D. H., Pages J. M., Bussard A. E. Specificity of antierythrocyte autoantibodies secreted by a NZB-derived hybridoma and NZB peritoneal cells. Cell Immunol. 1980 Jan;49(1):135–141. doi: 10.1016/0008-8749(80)90063-5. [DOI] [PubMed] [Google Scholar]
- Eilat D. Monoclonal autoantibodies: an approach to studying autoimmune disease. Mol Immunol. 1982 Jul;19(7):943–955. doi: 10.1016/0161-5890(82)90360-1. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Ebling F., Freeman S., Clevinger B., Davie J. Production of monoclonal murine antibodies to DNA by somatic cell hybrids. Arthritis Rheum. 1980 Aug;23(8):942–945. doi: 10.1002/art.1780230811. [DOI] [PubMed] [Google Scholar]
- Harada F., Kato N. Nucleotide sequences of 4.5S RNAs associated with poly(A)-containing RNAs of mouse and hamster cells. Nucleic Acids Res. 1980 Mar 25;8(6):1273–1285. doi: 10.1093/nar/8.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley J. B., Yamagata H., Reichlin M. Anti-La/SSB antibody is present in some normal sera and is coincident with anti-Ro/SSA precipitins in systemic lupus erythematosus. J Rheumatol. 1984 Jun;11(3):309–314. [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Snurps and scyrps. Cell. 1981 Aug;25(2):298–300. doi: 10.1016/0092-8674(81)90047-7. [DOI] [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Ro-Choi T. S., Redy R., Henning D., Takano T., Taylor C. W., Busch H. Nucleotide sequence of 4.5 S ribonucleic acid of Novikoff hepatoma cell nuclei. J Biol Chem. 1972 May 25;247(10):3205–3222. [PubMed] [Google Scholar]
- Venables P. J., Smith P. R., Maini R. N. Purification and characterization of the Sjögren's syndrome A and B antigens. Clin Exp Immunol. 1983 Dec;54(3):731–738. [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Harley J. B., Reichlin M. Molecular properties of the Ro/SSA antigen and enzyme-linked immunosorbent assay for quantitation of antibody. J Clin Invest. 1984 Aug;74(2):625–633. doi: 10.1172/JCI111460. [DOI] [PMC free article] [PubMed] [Google Scholar]







