Abstract
Background
PTPRD, encoding protein tyrosine phosphatases receptor type D, is located at chromosome 9p23–24.1, a loci frequently lost in many types of tumors. Recently, PTPRD has been proposed to function as a tumor suppressor gene. The current study aimed to investigate PTPRD expression and its prognostic significance in primary gastric adenocarcinoma.
Methods and Results
Quantitative real time reverse transcription PCR (qRT-PCR) and western blotting were used to examine PTPRD expression in paired gastric tumourous and paracancerous tissues. Compared with the matched normal gastric mucosa tissues, both the mRNA (P = 0.0138) and protein (P = 0.0093) expression of PTPRD in fresh surgical specimens were significantly reduced. Clinicopathological and prognostic roles of PTPRD in gastric adenocarcinoma were investigated using immunohistochemistry with 513 paraffin-embedded gastric adenocarcinoma tissue blocks. Statistical analysis revealed that reduced PTPRD expression was significantly associated with T stage (P = 0.004), TNM stage (P<0.001) and tumor size (P = 0.003). Furthermore, Kaplan-Meier survival analysis revealed that low expression of PTPRD significantly correlated with poor survival of gastric cancer patients (P<0.001). Cox regression analysis confirmed PTPRD expression as independent predictor of the overall survival of gastric cancer patients. The MTT assay determined the effects of PTPRD on cell proliferation of MGC803 and GES1 cell lines. Restoring PTPRD expression in MGC803 cells significantly inhibited their growth rate. Silencing PTPRD expression by siRNA treatment in GES1 significantly enhanced cell proliferation compared with mock siRNA treatment. Methylation analysis of PTPRD promoter CpG island in 3 primary GC samples showed one case with partial methylation.
Conclusions
These results indicated that PTPRD is a candidate tumour suppressor in gastric cancer. Thus, PTPRD may play an important role in gastric tumorigenesis and serve as a valuable prognostic marker of gastric adenocarcinoma.
Introduction
Globally, gastric cancer (GC) is currently the fourth most common malignancy and the second leading cause of cancer mortality [1]. More new cases of GC are diagnosed in China each year than in any other country [2]. The incidence of GC has declined over time, due to improving living standards, improvements in early diagnosis, advanced surgical techniques and combined therapy (surgery, chemotherapy and radiotherapy) [3]. However, distant metastasis and local recurrence cannot be avoided easily in most cases, and the prognosis of GC patients remains far from satisfactory [2], [3]. Tumorigenesis of GC has been considered a multifactorial and multistep process that involves the activation of oncogenes and the inactivation of tumor suppressor genes at different stages [4]. Further understanding of these alterations and the molecular mechanisms involved in gastric carcinogenesis will be critical for improved diagnosis, therapy and prognosis of GC.
Protein tyrosine phosphatases (PTPs) are signaling molecules that regulate a variety of cellular processes, including cell growth, differentiation, cell cycle and oncogenic transformation. The constitutive activation of PTPs signaling pathways is a biochemical hallmark of cancer [5]. This is mostly occurs via activation of tyrosine kinase receptors, such as amplification of HER2/Neu and mutations of the epidermal growth factor receptor [5]. The protein encoded by the PTPRD gene (protein tyrosine phosphatase, receptor type, D) is one of 38 known human receptor-type PTPs, a group of proteins that are increasingly thought to be important in human neoplasia and cancer progression [6], [7].
The PTPRD gene is located at chromosome 9p23–24.1, a locus frequently lost in neuroblastoma, gliomas, lung cancer and other malignancies [8]–[11]. Weir et al. detected homozygous deletions and missense mutations of PTPRD in adenocarcinoma of the colon and lung [12], [13]. David et al. identified frequent deletion and mutation of PTPRD in glioblastoma multiforme and malignant melanoma, and showed that these mutations were inactivating [5]. A recent study showed reduced PTPRD expression in the majority (>80%) of cell lines and surgical specimens of lung cancer, indicating that PTPRD is a candidate tumor suppressor [14]. These researches suggested that PTPRD might be one of a select group of tumor suppressor genes that are inactivated in a wide range of common human tumor types.
However, the role of PTPRD in human gastric adenocarcinoma has not yet been investigated. In the present study, we detected PTPRD expression level in gastric adenocarcinoma using quantitative real-time reverse transcription PCR (qRT-PCR), western blotting and immunohistochemistry. Meanwhile, prognostic and clinicopathological features of PTPRD were investigated in 513 gastric adenocarcinoma tissue samples. Furthermore, we evaluated the functional role of PTPRD in the proliferation of the GC cell line MGC803 and gastric epithelial mucosa cell line GES1. We further designed methylation-specific PCR assays to assess the methylation status of PTPRD promoter CpG island in primary GC tissues. Taken together, our research suggested that PTPRD was a candidate tumor suppressor in GC. Low expression of PTPRD was a reliable indicator of disease progression and poor prognosis of GC.
Materials and Methods
Ethics Statement
The research was approved by the Ethics Committee of Shandong Academy of Medical Sciences. Written informed consent was obtained from each patient involved in the study.
Cell line and culture conditions
The GC cell line MGC803 and gastric epithelial mucosa cell line GES1 were obtained from the Committee of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). The cells were cultured in RPMI 1640 media containing 10% heat-inactive fetal bovine serum (FBS). The cells were incubated at 37°C in a humidified 5% CO2 atmosphere.
Human tissue samples
A total of 42 paired fresh GC specimens and matched adjacent noncancerous tissue samples were collected from GC patients undergoing radical gastrectomy at Sun Yat-sen University Cancer Center between 2010 and 2012, and the diagnosis was confirmed by pathological examination. None of the patients had been treated before sugery. After surgical resection, fresh tissues were immediately immerged in RNA later (Ambion, Inc., USA) to avoid RNA degradation, and then stored at 4°C overnight to allow thorough penetration of RNA later into the tissue. Next, all the samples were frozen at −80°C until RNA and protein extraction was performed. Another 3 fresh GC specimens, used for methylation analysis of PTPRD, were collected from GC patients undergoing radical gastrectomy at Sun Yat-sen University Cancer Center in September 2014. The tumor-Node-Metastasis (TNM) staging was recorded based on the 7th edition of the International Union Against Cancer (UICC).
Gastric cancer patients and follow-up
Five hundred and thirteen paraffin-embedded primary gastric carcinoma samples were obtained from the postoperative patients in Sun Yat-sen University Cancer Center between January 2003 and December 2006. All patients in our study belonged to the same ethnic group. The patients were selected according to the criteria: (1) diagnosis of gastric adenocarcinoma with histopathological identification; (2) limited or extended surgical history that included gastrectomy plus lymphadenectomy; (3) no chemotherapy and radiotherapy before surgery; (4) availability of complete follow-up data; (5) no history of other synchronous malignancies or familial malignancy; (6) no recurrent gastric cancer or remnant gastric cancer; (7) no death in the perioperative period. The surgery was performed by experienced surgeons followed procedures of Japanese Gastric Cancer Association (JGCA) guidelines.
Postoperative follow-up included clinical and laboratory examinations every 3 months for the first 2 years, every 6 months during the third to fifth years, annually for an additional 5 years or until patient death, whichever occurred first. The overall survival was defined as the time from the operation to the death or last follow-up. The characteristics of these patients are listed in table 1.
Table 1. Correlation between PTPRD expression and clinicopathological parameters of 513 gastric adenocarcinoma cases.
| Clinicopathological parameters | n a | PTPRD expression | χ2 | P value | |
| High | Low | ||||
| All | 513 | 252 | 261 | ||
| Age (years) | |||||
| <55 | 230 | 117 | 113 | 2.713 | 0.257 |
| ≧55 | 281 | 133 | 148 | ||
| Gender | 0.037 | 0.853 | |||
| Male | 338 | 165 | 173 | ||
| Female | 175 | 87 | 88 | ||
| Tumor size | 9.241 | 0.003* | |||
| <3 cm | 84 | 54 | 30 | ||
| ≧3 cm | 429 | 198 | 231 | ||
| Tumor infiltration | 15.182 | 0.004* | |||
| T1 | 67 | 42 | 25 | ||
| T2 | 53 | 34 | 19 | ||
| T3 | 175 | 86 | 89 | ||
| T4a | 179 | 73 | 106 | ||
| T4b | 39 | 17 | 22 | ||
| Local lymph node metastasis | 4.237 | 0.238 | |||
| N0 | 189 | 103 | 86 | ||
| N1 | 119 | 58 | 61 | ||
| N2 | 82 | 38 | 44 | ||
| N3 | 123 | 53 | 70 | ||
| Distant metastasis | 2.426 | 0.128 | |||
| M0 | 466 | 234 | 232 | ||
| M1 | 47 | 18 | 29 | ||
| TNM staging | 20.785 | <0.001* | |||
| 1 | 95 | 64 | 31 | ||
| 2 | 192 | 98 | 94 | ||
| 3 | 177 | 71 | 106 | ||
| 4 | 49 | 19 | 30 | ||
aNumbers of cases in each group. * Statistically significant (P<0.05).
Extraction of total RNA and qRT-PCR
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, California, USA) according to the protocol of manufacturer's. RNAse-free DNase I was used to remove DNA contamination. Total RNA concentration was assessed by measuring absorbance at 260 nm using a NANO DROP spectrophotometer (ND-1000, Thermo Scientific, USA). Reverse transcription (RT) to synthesize the first-strand of cDNA was performed in a 25 µl reaction volume containing 2 µg total RNA, 0.5 µg Oligo (dt), 200 U M-MLV reverse transcriptase (Promega, USA), 25 U RNase inhibitor and 2.5 mM dNTP. The reaction system was incubated at 70°C for 5 minutes, 42°C for1 hour and resulting cDNA was stored at −20°C. The cDNA was then subjected to real-time quantitative PCR for evaluation of the relative mRNA levels of PTPRD and GAPDH (glyceraldehyde-3-phosphate dehydrogenase, as an internal control) with the following primers: PTPRD forward: 5′-TTATCAGTGCCAATCTTC-3′, and reverse: 5′-TCTGTTGTCTGTATCCAT-3′; GAPDH forward: 5′-CTCCTCCTGTTCGACAGTCAGC-3′, and reverse: 5′-CCCAATACGACCAAATCCGTT-3′. Gene-specific amplification was performed using an ABI 7900HT real-time PCR system (Life Technologies, Carlsbad, California, USA) with a 15 µl PCR reaction system containing 0.5 µl cDNA, 7.5 µl of 2 x SYBR Green master mix (Invitrogen, Carlsbad, California, USA), and 200 nM of the appropriate oligo nucleotide primers. The reaction procedure was performed as follows: preheated at 95°C for 10 min, and then 45 cycles of amplified at 95°C for 30 secands 60°C for 1 min. The resolution curve was measured at 95°C for 15 sec, 60°C for 15 sec and 95°C for 15 sec. The Ct (threshold cycle) value of each sample was calculated from the threshold cycles with the instrument's software (SDS 2.3). Relative expression levels of PTPRD were normalized to the geometric mean of the internal control gene GAPDH. Data were analyzed using the comparative threshold cycle (2−ΔCT) method.
Western blotting analysis
The frozen gastric cancer tissue samples, including tumor and non-tumor tissues, as well as cell lines, were lysed in RIPA lysis buffer at 4°C for 15 min. The lysates were cleared by centrifugation (12,000 rpm) at 4°C for 30 min to collect total protein. About 50 µg protein samples were then separated by electrophoresis in a 12% SDS (sodium dodecyl sulfate) polyacrylamide gel and the transferred onto a polyvinylidene fluoride membrane. After blocking the non-specific binding sites for 60 min with 5% non-fat milk, the membranes were incubated with a rabbit polyclonal antibody against PTPRD (LifeSpan BioScineces, USA, at 1∶1000 dilution) at 4°C overnight. The membranes were then washed with TBST (tris-buffered saline with tween-20) three times at room temperature for 15 min. After washing, the target protein was probed with the horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (SantaCruz, USA, at 1∶2000 dilution) at 37°C for 1 hour. After three washes, the membranes were developed by an enhanced chemiluminescence system (Cell Signaling Technology, Danvers, Massachusetts, USA). The band intensity was measured by densitometry using the Quantity One software (Bio-Rad Laboratories, Inc. Hercules, CA, USA). The protein levels were normalized with respect to GAPDH protein level which was detected using mouse anti-human GAPDH monoclonal antibody (Shanghai Kangchen, China, at 1∶5000 dilution).
Immunohistochemistry analysis
After deparaffinization with dimethylbenzene, the gastric cancer tissue sections were rehydrated through 100%, 95%, 90%, 80% and 70% ethanol. After three washes in PBS (phosphate-buffered saline), the tissue sections were boiled in antigen retrieval buffer containing 0.01 M sodium citrate-hydrochloric acid (pH = 6.0) for 15 min using a microwave oven. After rinsing with PBS, the sections were incubated with primary antibody and then rinsed in 3% peroxidase quenching solution (Invitrogen) to block endogenous peroxidase. The sections were then incubated with a rabbit polyclonal antibody against PTPRD (LifeSpan BioScineces, USA, at 1∶100 dilution) at 4°C overnight. After washing with PBS, the sections were incubated with a biotinylated secondary antibody (Zhongshan Golden Bridge Biotech, Beijing, China) at room temperature for 30 min. The visualization signal of the slides was treated with 3, 3′-diaminobenzidine (DAB) solution, and all of the slides were counterstained with hematoxylin for 15 min. As negative controls, adjacent sections were processed as described above except incubating at 4°C overnight in blocking solution without the primary antibody.
Semi-quantitative evaluation
The PTPRD protein expression level was assessed by immunostaining score, which was calculated as the sum of the percent of positively stained tumor cells and the staining intensity. Briefly, the percentage of positive staining was scored as 0 (0–9%, negative), 1 (10%–25%, sporadic), 2 (26%–50%, focal) or 3 (51%–100%, diffuse), and the intensity as 0 (no staining), 1 (weak staining, visible at high magnification), 2 (moderate staining, visible at low magnification) and 3 (dark staining, strikingly positive at low magnification). The total immunostaining score was calculated with the value of percent positivity score × staining intensity score, which ranged from 0 to 9. The expression level of PTPRD was defined as following: “−” (negative, score 0), “+” (weakly positive, score 1–3), “++” (positive, score 4–6), and “+++” (strongly positive, score7–9). Based on the PTPRD expression levels, we divided the gastric cancer patients into two groups: low PTPRD expression group (PTPRD “−” or PTPRD “+”) and high PTPRD expression group (PTPRD “++”or PTPRD “+++”).
Expression plasmid and transient transfections
A eukaryotic expression plasmid pCMV6-XL4 containing the full-length of human PTPRD cDNA was obtained from the ORIGENE Company (Beijing, China). Empty vector was used as negative control. MGC803 cells (2×105) were cultured in 6-well plates until they reached 85–90% confluence, and then transient transfections were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. At 48 hours after transfection, gene expression was examined by qRT-PCR and western blotting analysis. After that, cell proliferation assay was performed.
RNA oligonucleotides and cell transfection
For knockdown of PTPRD expression, the siRNAs were synthesized by GenePharma Company (Shanghai, China). The siRNA sequences were as follows: siRNA1-PTPRD, forward: 5′-GAGCCACACAGAAGUUAAUUU-3′, reverse: 5′-AUUAACUUCUGUGUGGCUCUU-3′, and siRNA2-PTPRD, forward: 5′-GUGGGUCUGUUAUCAUUAUTT-3′, reverse: 5′-AUAAUGAUAACAGACCCACTT-3′. The negative control (NC), forward: 5′-UUCUCCGAACGUGUCACGUTT-3′, reverse: 5′-ACGUGACACGUUCGGAGAATT-3′. 400 pmol siRNA-PTPRD or NC was transfected into 2×105 GES1 cells using Lipofectamine RNAi MAX reagent (Invitrogen, USA) for 48 hours according to the manufacturer's protocol. After detecting by qRT-PCR and western blotting, cell proliferation assay was performed.
Cell proliferation assay
Cell growth rate was detected by MTS assay. Cells were seeded in a 96-well plate at a density of 3×102 cells per well. The cell growth rate was detected using cell proliferation MTS kit according to the manufacturer's instruction (Promega, USA). Each experiment was performed in triplicate.
Methylation analysis
The genomic DNA of 3 fresh GC tissues was isolated with the DNeasy Tissue Kit (Qiagen Inc., Valencia, CA). Bisulfite modification of genomic DNA was carried out using the CpGenome DNA Modification Kit (Chemicon). Bisulfite modified DNA was amplified by PCR using two sets of PTPRD specific primer pairs that recognize either the methylated or unmethylated CpG island and then analyzed by electrophoresis. Selection of primers used for methylation-specific PCR was accomplished using MSPPrimer [15]. Primer sequences are as follows: M-PTPRD (methylated), forward: 5′-GGGGTTCGTTTAGGTCGC-3′, reverse: 5′-CGCCCGCTAAAAAAAAAAACGACG-3′, and U-PTPRD (unmethylated), forward: 5′-TGGTGGGGTTTGTTTAGGTTGTG-3′, reverse: 5′-ATACTCCAAACACCCACTAAAAAAAAAAACAACA-3′.
Statistical analysis
Differences in PTPRD mRNA and protein expression between paired tumor and the adjacent non-tumor tissue samples were evaluated with the paired Student's t-test. The χ2test was used to analyze the relationships between PTPRD expression and various clinicopathological parameters. Survival curves were calculated using the Kaplan–Meier method and compared by the log-rank test. Univariate and multivariate analyses were performed to detect PTPRD expression and the clinicopathological variables by using Cox proportional hazards regression model. The two-tailed unpaired Student's t-test was used to assess differences in cell growth rate. All the statistical analyses were performed with the software of SPSS (Statistical Package for the Social Sciences, version 17.0, Chicago, IL, USA), and a two-sided P value less than 0.05 was considered to be statistically significant.
Results
qRT-PCR analysis of PTPRD mRNA expression
The mRNA level of PTPRD was examined by qRT-PCR assays in 42 paired gastric cancerous and matched adjacent normal mucosa tissues. As shown in figure 1, the PTPRD expression level was significantly lower in 32 (76.19%) tumor-bearing tissues compared with the adjacent non-tumor tissues (P = 0.0138, Figure 1).
Figure 1. The mRNA expression of PTPRD in human primary gastric adenocarcinoma surgical specimens was evaluated by qRT-PCR.
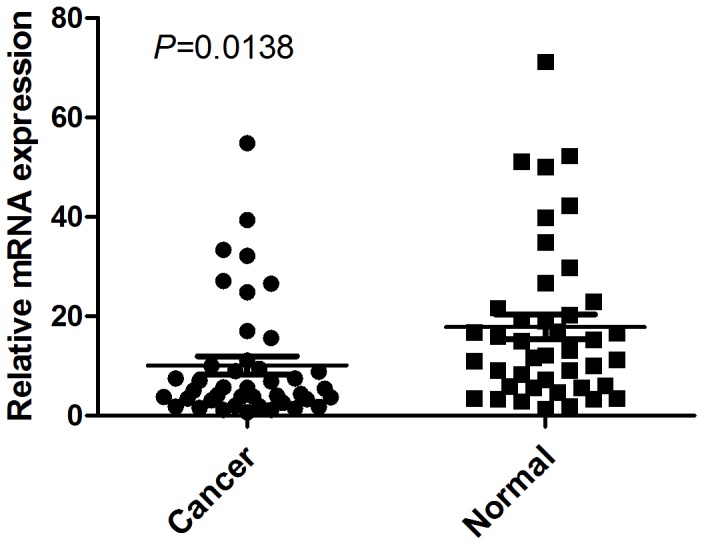
The relative mRNA expression of PTPRD was significantly decreased in GC tissues compared with the matched adjacent noncancerous tissues (n = 42, P = 0.0138). Horizontal lines represent the mean.
Western blotting analysis of PTPRD protein expression
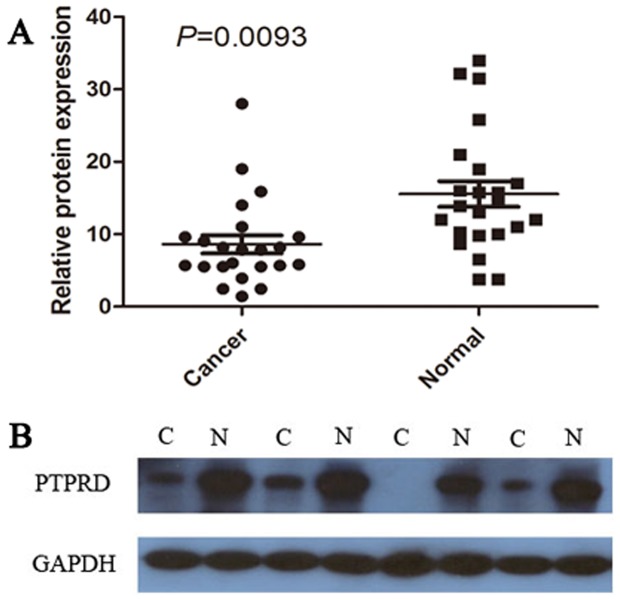
To further investigate if the expression of PTPRD is reduced at the protein level in gastric adenocarcinoma, western blotting was performed on 23 paired gastric cancerous and noncancerous specimens. The results showed a PTPRD band at the expected size of 250 kDa, and the amount of PTPRD protein present was further measured by densitometry (normalized to GAPDH expression as a loading control). Fifteen of 23 (65.22%) tumors showed decreased PTPRD expression. The statistical evaluation showed significantly decreased expression of PTPRD in gastric tumor tissues compared with matched adjacent noncancerous tissues (P = 0.0093, Figure 2A). Representative examples of western blotting results were shown in Figure 2B.
Figure 2. Decreased protein expression of PTPRD in gastric adenocarcinoma was assessed by western blotting.
(A) Relative PTPRD protein expression levels in GC tissues and the matched normal paracancerous tissues (PTPRD/GAPDH, n = 23, P = 0.0093). Horizontal lines represent the mean. (B) Representative results of PTPRD protein expression in 4 paired GC tissues and the matched adjacent noncancerous tissues (C, GC tissues; N, matched noncancerous gastric mucosa).
Immunohistochemical analysis and clinicopathological characteristics
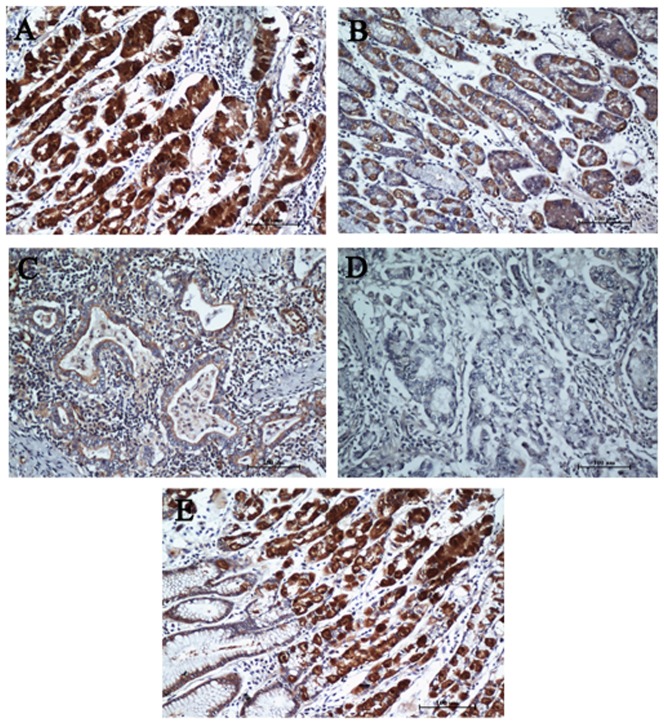
To further investigate the clinicopathological and prognostic roles of PTPRD expression, 513 paraffin-embedded gastric adenocarcinoma tissue blocks were used for immunohistochemical analysis. Results indicated that 261 of 513 (50.87%) cases showed reduced PTPRD expression in cancerous tissues (Figure 3C&D), whereas 252 (49.12%) cases showed strong immunostaining (Figure 3B). Normal gastric mucosa showed the strongest PTPRD positive staining (Figure 3A). Immunostaining of a gastric cancer sample from the same patient showed a sharp contrast of PTPRD staining intensity (Figure 3E). In addition, low expression of PTPRD was significantly correlated with tumor size (P = 0.003), depth of tumor infiltration (T stage, P = 0.004), and TNM stage (P<0.001), but not with age, gender, local lymph node metastasis (N stage), or distant metastasis (M stage) (Table 1). Representative photomicrographs are shown in Figure 3.
Figure 3. PTPRD protein expression in gastric adenocarcinoma surgical specimens evaluated by immunohistochemistry.
(A) Strong PTPRD staining was observed in noncancerous gastric mucosa. (B) Strong PTPRD staining in well-differentiated gastric cancer. (C) Weak PTPRD staining in moderately differentiated GC. (D) Negative PTPRD staining in poorly differentiated GC. (E) Immunostaining of GC and adjacent nontumorous tissues showing a sharp contrast of PTPRD staining intensity.
Expression of PTPRD and clinical outcome
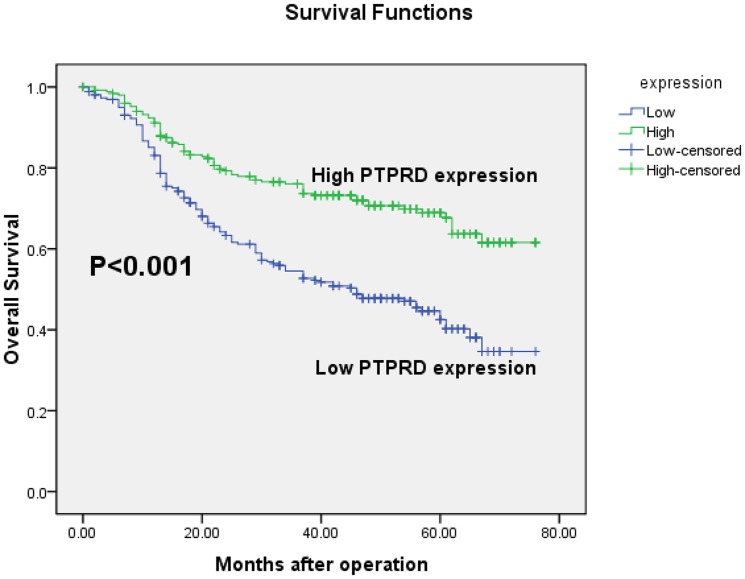
The prognostic value of PTPRD for gastric adenocarcinoma patients' overall survival was evaluated between patients with high and low PTPRD protein levels. The 5-year overall survival rates in patients with high and low PTPRD expression were 67.7% and 42.5%, respectively. Kaplan-Meier curve assessment showed that the overall survival of patients with low PTPRD expression was significantly worse than that of PTPRD-high patients (P<0.001, log-rank test, Figure 4).
Figure 4. Kaplan-Meier survival curves of gastric adenocarcinoma patients (n = 513) after gastrectomy.
The survival rate of patients in PTPRD-high group was significantly higher than that of the patients in the PTPRD-low group (log-rank test, P<0.001).
To identify the potentially significant prognostic variables in all the patients with gastric adenocarcinoma, univariate Cox regression analysis of each variable was performed in relation to the survival time. Data showed that tumor infiltration, local lymph node metastasis, distant metastasis, TNM stage, tumor size and PTPRD expression were significantly associated with overall survival (Table 2). Furthermore, multivariate Cox regression analysis confirmed local lymph node metastasis (P = 0.011), TNM stage (P = 0.038) and PTPRD expression (P = 0.002) as independent predictors of overall survival of GC patients (Table 2). The relative risk of death in patients with high-PTPRD tumors was significantly lower than that of patients with low-PTPRD tumors (HR = 0.629).
Table 2. Univariate and multivariate analyses of overall survival of gastric adenocarcinoma patients.
| Variables | n a | Univariate analyses | Multivariate analyses | ||||
| HR | (95% CI) | P value | HR | (95% CI) | P value | ||
| Age (years) | 0.332 | ||||||
| <55 | 230 | 1.000 | |||||
| ≧55 | 281 | 1.139 | 0.875–1.483 | ||||
| Gender | 0.719 | ||||||
| Female | 175 | 1.000 | |||||
| Male | 338 | 0.951 | 0.723–1.251 | ||||
| Tumor size | <0.001* | 0.690 | |||||
| <3 cm | 84 | 1.000 | 1.000 | ||||
| ≧3 cm | 429 | 4.257 | 2.321–7.810 | 1.144 | 0.591–2.213 | ||
| Tumor infiltration | <0.001* | 0.167 | |||||
| T1 | 67 | 1.000 | 1.000 | ||||
| T2 | 53 | 7.847E3 | 0.000–8.853E29 | 1.075E4 | 0.000–4.118E37 | ||
| T3 | 175 | 3.322E4 | 0.000–3.734E30 | 1.416E4 | 0.000–5.437E37 | ||
| T4a | 179 | 3.914E4 | 0.000–4.400E30 | 1.299E4 | 0.000–4.987E37 | ||
| T4b | 39 | 7.998E4 | 0.000–8.997E30 | 2.229E4 | 0.000–8.566E37 | ||
| Local lymph node metastasis | <0.001* | 0.011* | |||||
| N0 | 189 | 1.000 | 1.000 | ||||
| N1 | 119 | 2.477 | 1.608–3.817 | 1.644 | 1.020–2.651 | ||
| N2 | 82 | 4.022 | 2.605–6.209 | 1.915 | 1.118–3.281 | ||
| N3 | 123 | 5.931 | 3.996–8.803 | 2.416 | 1.435–4.068 | ||
| Distant metastasis | <0.001* | 0.600 | |||||
| M0 | 466 | 1.000 | 1.000 | ||||
| M1 | 47 | 6.330 | 4.479–8.946 | 0.673 | 0.153–2.957 | ||
| TNM staging | <0.001* | 0.038* | |||||
| 1 | 95 | 1.000 | 1.000 | ||||
| 2 | 192 | 10.615 | 3.329–33.849 | 2.414 | 0.552–10.551 | ||
| 3 | 177 | 26.111 | 8.293–82.210 | 3.211 | 0.673–15.306 | ||
| 4 | 49 | 91.390 | 28.270–295.440 | 18.176 | 2.174–151.950 | ||
| PTPRD | <0.001* | 0.002* | |||||
| Low | 261 | 1.000 | 1.000 | ||||
| High | 252 | 0.474 | 0.357–0.630 | 0.629 | 0.469–0.845 | ||
HR, hazard ratio; CI, confidence interval; a Numbers of cases in each group; * Statistically significant (P<0.05).
The role of PTPRD in cell proliferation in MGC803 and GES1 cell lines
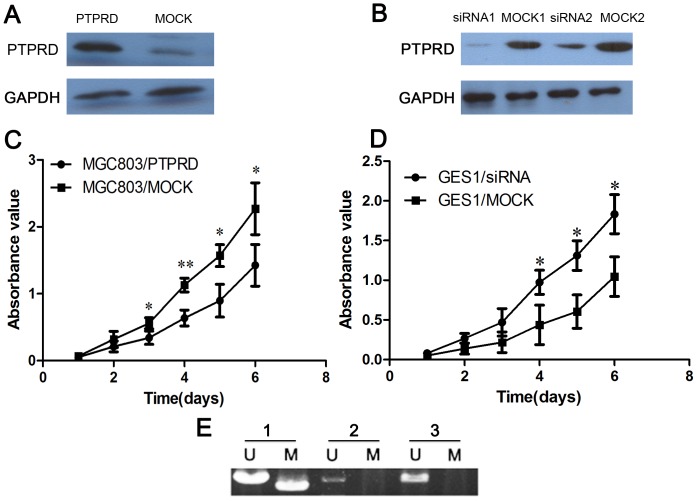
To further evaluate the effects of PTPRD on cell proliferation, a PTPRD expression vector and control vector were transfected separately into MGC803 cells. PTPRD expression in transfected cells were detected by western blotting (Figure 5A). Cell growth assay revealed that the cell growth rate in PTPRD-transfected GC cells was significantly lower than that of control vector-transfected GC cells (Figure 5C). Meanwhile, we silenced PTPRD expression in the gastric epithelial mucosa cell line GES1 using PTPRD-specific siRNA oligonucleotides. The PTPRD expression in transfected GES1 cells were detected by western blotting (Figure 5B). Silencing the expression of PTPRD in GES1 significantly enhanced cell proliferation compared with mock siRNA treatment (Figure 5D).
Figure 5. The growth suppressor role of PTPRD in cell proliferation and DNA methylation analysis of PTPRD.
(A) Western blotting analysis of PTPRD overexpression in MGC803 cells. (B) Western blotting analysis of decreased PTPRD expression in GES1 cells. (C) Cell proliferation assay showing the suppressive effect of restoring PTPRD expression on the proliferation of MGC803 cell line. (D) Results showing significantly enhanced proliferation rate of PTPRD-silenced GES1 cells compared with mock siRNA treatment GES1 cells. (E) Methylation analysis of PTPRD promoter CpG island in primary GC tissues. Among 3 GC samples, one case showed partial methylation and 2 cases were unmethylated. *, P<0.05 versus the mock control; **, P<0.01 versus the mock control.
Abnormal DNA methylation of PTPRD in GC
We further designed methylation-specific PCR assays to assess the methylation status of PTPRD promoter CpG island in primary GC tissues. Results showed that, among 3 GC samples, one case showed partial methylation and 2 cases were unmethylated (Figure 5E).
Discussion
Receptor protein tyrosine phosphatase delta (PTPRD) is a member of the highly conserved family of receptor protein tyrosine phosphatases (PTPs) [15]. The PTPs are a superfamily of enzymes that function in a coordinated manner with protein tyrosine kinases to control signalling pathways that underlie a broad spectrum of fundamental physiological processes [7]. These enzymes are divided into the classical group, phosphotyrosine (pTyr)-specific phosphatases and the dual specificity phosphatases [7], [16], [17]. There are 107 PTPs encoded in the human genome, of which 38 belong to the group of classical PTPs, which show specificity for phosphotyrosine [6]. PTPs are signaling molecules that regulate a variety of cellular processes, including cell growth, differentiation, mitotic cycle and oncogenic transformation [6], [7]. Recently, several classical PTPs have been identified as potential tumor suppressors, including receptor PTPs such as DEP1 (densityenhanced phosphatase-1, encoded by PTPRJ) [18], PTPκ (encoded by PTPRK) [19] and PTPρ (encoded by PTPRT) [20]. This group of genes is increasingly thought to be important in cancer development and progression.
The PTPRD gene is located at chromosome 9p23–24.1, an area of human genome that is frequently lost in many kinds of tumors [8]–[11]. Urushibara et al. described a selective reduction in PTPRD expression in hepatomas and first proposed PTPRD as a tumor suppressor [21]. Subsequent studies reported homozygous deletions of PTPRD in a broad spectrum of human tumor types, such as lung adenocarcinoma [9], [12], [22], [23], pancreatic carcinoma [24], melanoma [25] and glioblastoma [5], etc. Kohno et al. observed reduced PTPRD expression in the majority (>80%) of cell lines and surgical specimens of lung cancer [14]. Veeriah et al. found that PTPRD was mutated in 6% of glioblastoma multiformes, 13% of head and neck squamous cell carcinomas, and in 9% of lung cancers [15]. Their study revealed that loss of expression of PTPRD predicts for poor prognosis in glioma patients [15]. These studies have established that PTPRD has a growth suppressive role in many types of human cancer [26]. However, thus far, the expression, clinical significance and biological functions of PTPRD in gastric adenocarcinoma have not been explored.
In our present study, we detected the mRNA and protein levels of PTPRD in GC patients by western blotting and qRT-PCR, respectively. PTPRD was expressed at both lower mRNA and protein levels in GC tissues compared with corresponding non-cancerous tissues. Moreover, immunohistochemistry showed decreased PTPRD expression in 261 out of 513 samples of gastric cancer patients. These results indicated that PTPRD might be a candidate tumour suppressor in GC. Our observation is in agreement with a series studies revealing that PTPRD expression is frequently lost or reduced in a number of human cancer tissues and cell lines, including lung cancer and glioblastoma multiforme [14], [15].
The correlation of PTPRD and clinical outcome was analyzed by immunohistochemical staining of specimens in large series of gastric cancer patients (n = 513). Reduced expression of PTPRD was significantly correlated with tumor size (P = 0.003), depth of tumor infiltration (T stage, P = 0.004), and TNM stage (P<0.001). The overall survival of patients with low PTPRD expression was significantly worse than that of PTPRD-high patients (P<0.001). These findings were similar to the previous studies in lung cancer and glioblastoma by Veeriah et al. [15]. Taken together, these results demonstrated that PTPRD might serve as a tumor suppressor in a broad spectrum of human tumor types.
Univariate and multivariate analysis demonstrated that PTPRD was an independent risk factor in the prognosis of GC patients. Thus, PTPRD may serve as a valuable prognostic biomarker for GC patients after surgery and as a potential target for gene therapy in the treatment of GC.
PTPRD encodes a transmembrane protein with a cytoplasmic tyrosine phosphatase domain [15]. Recently, a study by Veeriah et al. revealed that loss of PTPRD resulted in altered growth of astrocytes [15]. PTPRD directly dephosphorylates the oncoprotein STAT3 and regulates the STAT3 pathway. Mutations in PTPRD abrogate the ability to regulate STAT3 [15]. Their results suggest that PTPRD may act as a tumor suppressor by regulating cell growth, and the loss of this gene plays an important role in progression, rather than the initiation of malignant gliomas [15]. In the current study, we found that the loss of PTPRD expression was significantly correlated with a higher T stage of gastric cancer, implying that absence of PTPRD expression may promote tumor growth and invasion. Moreover, we detected lower PTPRD immunoreactivity in poorly differentiated gastric cancer tissues than in well-differentiated ones, suggesting that decreased PTPRD expression might play a role in tumor de-differentiation. Furthermore, we investigated the functional role of PTPRD in MGC803 and GES1 cell lines. Restoring PTPRD expression in GC cells significantly inhibited cell proliferation. Whereas, silencing PTPRD expression in gastric epithelial cells significantly enhanced the cell growth rate. These results indicated that PTPRD might play an import role in regulating gastric cancer cell growth. Recently, Veeriah's research showed that human astrocytes lacking PTPRD exhibited increased growth [15]. Our study, together with that of Veeriah et al., suggested that PTPRD might serve as a candidate tumour suppressor in a wide range of common human tumor types. However, the functional role and molecular underpinnings of PTPRD in GC have not been fully explored, requiring further investigation in future research.
DNA hypermethylation in promoter CpG island has been shown to be a predominant mechanism by which tumor suppressors are inactivated in cancers [27]. In the present study, methylation analysis of PTPRD promoter CpG island in 3 primary GC samples showed one case with partial methylation. Low PTPRD expression in most GC tissues was probably due to DNA methylation of promoter CpG island. Recently, two studies showed DNA hypermethylation of PTPRD in glioblastoma and breast cancer cell lines [15], [28]. These researches indicated that the methylation of CpG in the PTPRD promoter was might be involved in the inactivation of PTPRD in many types of human cancers.
In conclusion, we demonstrated reduced PTPRD expression in gastric adenocarcinoma and its correlation with a more malignant phenotype and poorer prognosis in a large number of clinical samples. In addition, the data generated in the current study represent a valuable report correlating the presence of PTPRD with clinicopathological characteristics and the overall survival of gastric cancer patients. Further studies are needed to fully evaluate the molecular mechanism of low expression of PTPRD in gastric oncogenesis. We confirmed that PTPRD might serve as a candidate tumor suppressor gene and prognostic biomarker in gastric adenocarcinoma.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by the Shandong Science&Development Program (No. 2012GSF12115) and National Natural Science Foundation of China (81273776). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2. Wang YC WL, Liu JT (2012) Comparison of Cancer Incidence between China and the USA. Cancer Biol Med 9:128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, et al. (2009) Recent patterns in gastric cancer: a global overview. Int J Cancer 125:666–673. [DOI] [PubMed] [Google Scholar]
- 4. Nobili S, Bruno L, Landini I, Napoli C, Bechi P, et al. (2011) Genomic and genetic alterations influence the progression of gastric cancer. World J Gastroenterol 17:290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solomon DA, Kim JS, Cronin JC, Sibenaller Z, Ryken T, et al. (2008) Mutational inactivation of PTPRD in glioblastoma multiforme and malignant melanoma. Cancer Res 68:10300–10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ostman A, Hellberg C, Bohmer FD (2006) Protein-tyrosine phosphatases and cancer. Nat Rev Cancer 6:307–320. [DOI] [PubMed] [Google Scholar]
- 7. Tonks NK (2006) Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol 7:833–846. [DOI] [PubMed] [Google Scholar]
- 8. Stallings RL, Nair P, Maris JM, Catchpoole D, McDermott M, et al. (2006) High-resolution analysis of chromosomal breakpoints and genomic instability identifies PTPRD as a candidate tumor suppressor gene in neuroblastoma. Cancer Res 66:3673–3680. [DOI] [PubMed] [Google Scholar]
- 9. Sato M, Takahashi K, Nagayama K, Arai Y, Ito N, et al. (2005) Identification of chromosome arm 9p as the most frequent target of homozygous deletions in lung cancer. Genes Chromosomes Cancer 44:405–414. [DOI] [PubMed] [Google Scholar]
- 10. Purdie KJ, Harwood CA, Gulati A, Chaplin T, Lambert SR, et al. (2009) Single nucleotide polymorphism array analysis defines a specific genetic fingerprint for well-differentiated cutaneous SCCs. J Invest Dermatol 129:1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Purdie KJ, Lambert SR, Teh MT, Chaplin T, Molloy G, et al. (2007) Allelic imbalances and microdeletions affecting the PTPRD gene in cutaneous squamous cell carcinomas detected using single nucleotide polymorphism microarray analysis. Genes Chromosomes Cancer 46:661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weir BA, Woo MS, Getz G, Perner S, Ding L, et al. (2007) Characterizing the cancer genome in lung adenocarcinoma. Nature 450:893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, et al. (2006) The consensus coding sequences of human breast and colorectal cancers. Science 314:268–274. [DOI] [PubMed] [Google Scholar]
- 14. Kohno T, Otsuka A, Girard L, Sato M, Iwakawa R, et al. (2010) A catalog of genes homozygously deleted in human lung cancer and the candidacy of PTPRD as a tumor suppressor gene. Genes Chromosomes Cancer 49:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veeriah S, Brennan C, Meng S, Singh B, Fagin JA, et al. (2009) The tyrosine phosphatase PTPRD is a tumor suppressor that is frequently inactivated and mutated in glioblastoma and other human cancers. Proc Natl Acad Sci USA 106:9435–9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, et al. (2004) Protein tyrosine phosphatases in the human genome. Cell 117:699–711. [DOI] [PubMed] [Google Scholar]
- 17. Andersen JN, Jansen PG, Echwald SM, Mortensen OH, Fukada T, et al. (2004) A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J 18:8–30. [DOI] [PubMed] [Google Scholar]
- 18. Ruivenkamp CA, van Wezel T, Zanon C, Stassen AP, Vlcek C, et al. (2002) Ptprj is a candidate for the mouse colon-cancer susceptibility locus Scc1 and is frequently deleted in human cancers. Nat Genet 31:295–300. [DOI] [PubMed] [Google Scholar]
- 19. Nakamura M, Kishi M, Sakaki T, Hashimoto H, Nakase H, et al. (2003) Novel tumor suppressor loci on 6q22–23 in primary central nervous system lymphomas. Cancer Res 63:737–741. [PubMed] [Google Scholar]
- 20. Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, et al. (2004) Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science 304:1164–1166. [DOI] [PubMed] [Google Scholar]
- 21. Urushibara N, Karasaki H, Nakamura K, Mizuno Y, Ogawa K, et al. (1998) The selective reduction in PTPdelta expression in hepatomas. Int J Oncol 12:603–607. [PubMed] [Google Scholar]
- 22. Zhao X, Weir BA, LaFramboise T, Lin M, BeroukhimR, et al (2005) Homozygous deletions and chromosome amplifications in human lung carcinomas revealed by single nucleotide polymorphism array analysis. Cancer Res 65:5561–5570. [DOI] [PubMed] [Google Scholar]
- 23. Nagayama K, Kohno T, Sato M, Arai Y, Minna JD, et al. (2007) Homozygous deletion scanning of the lung cancer genome at a 100-kb resolution. Genes Chromosomes Cancer 46:1000–1010. [DOI] [PubMed] [Google Scholar]
- 24. Calhoun ES, Hucl T, Gallmeier E, West KM, Arking DE, et al. (2006) Identifying allelic loss and homozygous deletions in pancreatic cancer without matched normals using high-density single-nucleotide polymorphism arrays. Cancer Res 66:7920–7928. [DOI] [PubMed] [Google Scholar]
- 25. Stark M, Hayward N (2007) Genome-wide loss of heterozygosity and copy number analysis in melanoma using high-density single-nucleotide polymorphism arrays. Cancer Res 67:2632–2642. [DOI] [PubMed] [Google Scholar]
- 26. Solomon DA, Kim JS, Yang XR, Tucker MA, Goldstein AM, et al. (2009) Lack of inherited mutations of PTPRD in familial melanoma and melanoma-astrocytoma syndrome. Pigment Cell Melanoma Res 22:489–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herman JG, Baylin SB (2003) Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 349:2042–2054. [DOI] [PubMed] [Google Scholar]
- 28. Chan TA, Glockner S, Yi JM, Chen W, Van Neste L, et al. (2008) Convergence of mutation and epigenetic alterations identifies common genes in cancer that predict for poor prognosis. PLoS Med 5(5):e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.