Abstract
Cystic hypersecretory pattern is a rare and poorly recognised variant of invasive ductal carcinoma of the breast. Cystic hypersecretory lesions of the breast have a spectrum of morphological features ranging from clearly benign cystic hypersecretory hyperplasia (CHH), CHH with atypia, cystic hypersecretory carcinoma (CHC) to invasive CHC. Until now, no case of invasive CHC has been reported in India, to the best of our knowledge. We report a case of a 57-year-old female with a history of a lump in the inferomedial quadrant of the right breast for three years, gradually increasing in size. A mammography showed a well-defined, lobulated radio-opacity. A modified radical mastectomy was done. Gross examination showed multiple cystic spaces filled with thick gelatinous material and solid areas. On histopathology, cystic hypersecretory variant of invasive ductal breast carcinoma with focal papillary pattern was diagnosed. Cystic hypersecretory ductal carcinoma behaves in a low-grade fashion for many years but has a potential for invasiveness and metastasis, so regular follow-up of such cases is crucial.
Keywords: breast, carcinoma, mastectomy
Introduction
Cystic hypersecretory carcinoma (CHC) and cystic hypersecretory hyperplasia (CHH) was first described in 1984 [1]. CHC is an uncommon distinctive variant of ductal breast carcinoma in situ that arises in the background of CHH and is characterised grossly by the presence of dilated ducts and cysts containing glistening, gelatinous material, and microscopically areas of micropapillary carcinoma in the epithelium lining the cyst [2]. CHC is thought to behave in an indolent manner but has the potential to give rise to invasive carcinoma [3]. An invasive component has been reported approximately in 20% of CHC cases, and it tends to be poorly differentiated ductal carcinoma with solid growth pattern and no secretory activity [2]. Positive reactions for periodic acid schiff (PAS), carcinoembryonic antigen (CEA), alpha-lactalbumin have been observed in the cyst contents, which are consistently negative for thyroglobulin. CHC are usually estrogen receptor (ER) and HER-2/neu positive [4]. There have only been a few cases of invasive CHC reported in the literature. We describe an additional new case of invasive CHC in a 57-year-old female, and the relevant literature is reviewed [Table 1].
Table 1. Review of cases of CHC in the literature.
| Source, y | Age, y | Size/Location | Lymph Node Status | Type of Disease Present | ER/PR | Modality of Diagnosis and Final Therapy |
|---|---|---|---|---|---|---|
| Rosen and Scott [1], 1984 | 62 | NA/L | N0 | In situ | NA | MRM |
| 41 | NA/R | N0 | In situ | NA | MRM | |
| 39 | 2.5 cm first/L | N0 | In situ | NA | 2 x Bx/MRM | |
| 48 | 10 cm/R | NA | In situ | NA | SM | |
| 78 | 8 cm/R | NA | In situ | NA | Bx | |
| ‘old’ | 8 cm/R | NA | In situ | NA | SM | |
| 52 | ‘large’/L | † | Invasive | Pos/NA | Bx | |
| 47 | 2.2 cm/R | N1 | Invasive | NA | MRM | |
| 62 | 1 cm/L | N0 | Invasive | NA | MRM | |
| 34/55 | NA/L | NA | In situ | NA | Bx | |
| Guerry et al [5], 1988 | NA | NA | N1 | Invasive | NA | MRM |
| NA | NA | † | Invasive | NA | MRM | |
| 17 additional cases | N0 | In situ | NA | MRM | ||
| Colandrea et al [6], 1988 | 62/67 | 2 cm/R | N0Mx | In situ | Neg/neg | Cyto/2 x Bx/MRM |
| Adams and Lacey [7], 1990 | 70 | 4.5 cm/L | N0 | Microinvasive | Neg/pos | Bx/MRM/RT/Tam |
| Kim et al [8], 1997 | 37 | 8.8 cm/R | N0 | Invasive | NA | Cyto/MRM |
| Herrmann et al [9] 1999 | 49 | 6 cm/L | N0 | Invasive | Pos/pos | Bx/MRM |
| Shah AK [10], 2000 (two cases) | NA | NA | NA | In situ | NA | NA |
| Park JM [11], 2002 (two cases) | NA | NA | NA | NA | NA | NA |
| Lee JS [12], 2004 | 45 | 4.7 cm/L | N0M0 | Invasive | Neg/neg | Bx/MRM |
| Shin SJ [13], 2004 | ||||||
| Nine cases | 42** | NA | N0 | In situ | NA | Bx |
| One case | 42** | NA | N (micro) | Invasive | NA | Bx (MRM) |
| Park C [14], 2004 | 49/L | NA | NA | In situ | NA | Bx |
| Resetkova E [15], 2005 | NA | NA | NA | NA | NA | NA |
| Skalova A 2005 [16] (five cases) | 66.8** | 7–8 cm | NA | Three in situ Two invasive | One Case Pos/Pos | NA |
| Sahoo S [17], 2008 | 48/L | NA | NA | In situ | NA | Core needle Bx/SM |
| Chen DB [18], 2010 | NA | NA | NA | Microinvasive | NA | NA |
| Song SW [19], 2011 | 43 | NA | NA | Invasive | NA | Bx |
| D’Alfonso TM [20], 2014, Mean (ten case) | 62.8** | 0.9 cm* | NA | Nine in situ | 7 pos/2 pos | NA |
| NA | One microinvasive | Pos/pos | NA | |||
| Bi R [21], 2014 (three cases) | 49.3** | NA | NA | One in situ Two invasive | 1 pos/1 pos | MRM |
| Present case | 57 | 7 cm | N0 | invasive | Neg/neg | MRMs |
ER/PR indicates estrogen receptor/progesterone receptor; NA: not available; L: left breast; R: right breast; pos: positive; neg: negative; MRM: modified radical mastectomy; Bx: biopsy; SM: simple mastectomy; Cyto: cytology; RT: radiation; and Tam: tamoxifen; N (micro): lymph node micrometastasis.
Mean age
Indicates cases with distal metastatic disease.
Case report
A 57-year-old female presented with a palpable mass in the inferomedial quadrant of the right breast. The lump was gradually progressive in size for the last three years. Physical examination revealed a painless, mobile, ill-defined, hard, 8 x 7 cm lump with no retraction of nipple, no nipple discharge, and no axillary lymphadenopathy. The patient had no history of benign breast disease previously or family history of breast cancer. A mammography showed a well-defined, lobulated radio-opacity in the inferomedial quadrant of right breast (Figure 1a).
Figure 1. (a). Mammography, mediolateral oblique view showing well defined, lobulated radio-opacity in the right inferomedial quadrant. (b). The cut surface of the mass showing cysts filled with gelatinous secretions.
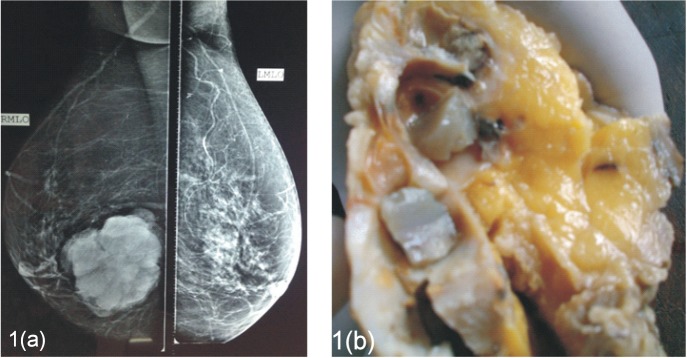
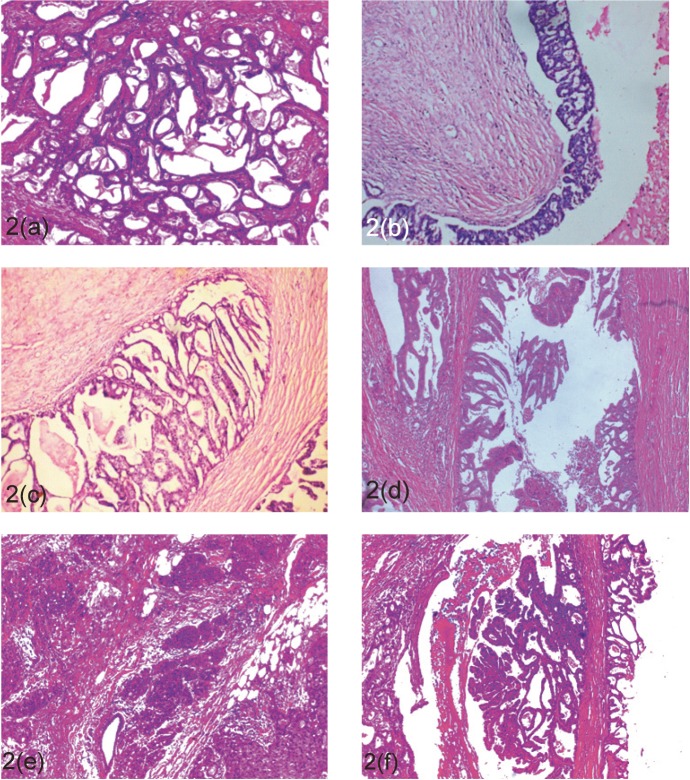
The patient underwent a modified radical mastectomy. Grossly, the cut surface revealed a 7 x 7 cm tumour which showed multiple cystic spaces filled with thick gelatinous material and grey-white solid areas (Figue 1b). Microscopically, the tumour showed varied histological pattern with predominantly multiple variable- sized cystic spaces filled with PAS positive dense eosinophilic material resembling thyroid colloid (Figure 2a). These homogenous secretions were retracted from the surrounding epithelium producing a scalloped margin. The cyst lining epithelium exhibited a variable pattern that in most areas was flat to cuboidal (Figure 2a) and in other areas showed a proliferative change in the form of pseudostratification to knobby epithelial tufts (Figure 2b) to complex branching fronds that extend across duct lumen forming a Roman arch bridging pattern (Figure 2c). Areas of intraductal carcinoma (micropapillary type) (Figure 2d) accompanied by an invasive component comprising of solid pattern of moderately to poorly differentiated ductal carcinoma cells (Figure 2e) was seen. Some areas of tumour tissues also showed papillary pattern with fibrovascular core (Figure 2f). Immunohistochemistry (IHC) shows, the cystic contents were negative for CEA and thyroglobulin. Tumour was ER, PR, HER-2/neu negative.
Figure 2. Microscopic findings (H & E). The lesion is composed of multiple cyst and ducts containing eosinophilic secretions (a; 40x). Some of the cysts are lined by flattened epithelium (a; 40x) while others show epithelial proliferations in form of pseudostratification, knobby tufts (b; 100x), Roman arch (c; 100x), to micro-papillary carcinoma in situ (d; 630X), and invasive pattern in form of solid sheets (e; 100x), and papillary pattern of ductal carcinoma (f; 100x).
A diagnosis of invasive cystic hypersecretory carcinoma was made with focal papillary pattern. None of the axillary lymph nodes were positive for tumour metastasis.
Discussion
Rosen and Scott defined cystic hypersecretory carcinoma as a subtype of intraductal carcinoma of the breast and described this entity in a series of 10 cases [1]. Since then few authors have put this entity forward generally in the form of case reports and that further invasive CHC is much rarer (Table 1). Cystic hypersecretory lesions of the breast have a spectrum of morphological features ranging from clearly benign (CHH), a combination of benign and atypical epithelium (CHH with atypia), to cases that combine benign, atypical, and frankly malignant epithelium [5]. The characteristic findings of invasive CHC are formation of dilated ducts filled with eosinophilic colloid-like material in their lumens and lined by pseudostratified to micropapillary epithelium along with foci of invasion. Extravasation of cyst material into the stroma does not indicate invasion [1, 5, 11]. Invasion is heralded by solid nests of malignant cells and is usually poorly differentiated with no secretory characteristic. Among the reported cases of cystic hypersecretory breast lesion, most cases are of in situ CHC, with only a few cases of invasive CHC [Table 1]. So this makes our case extremely rare in the scenario that it is invasive CHC and also harbours foci of CHH and CHC in situ within the lesion and shows an additional morphology of papillary pattern. If no cytological atypia is present and the epithelium is flat or cuboidal, the lesion is characterised as CHH. Foci of the similar picture is seen in our case along with invasive areas and in accordance with long history of gradually progressing breast lump; it appears that the CHH has progressed to CHC in situ to invasive CHC showing the spectrum of evolution of this tumour within the same lesion.
The differential diagnosis of invasive CHC includes juvenile secretory carcinoma, mucinous carcinoma, malignant mucocele-like tumour, and metastatic thyroid carcinoma. Juvenile secretory carcinoma contains vacuolated cytoplasm and more bubbly secretions which are not typical features of CHC [22]. Mucinous carcinoma and malignant mucocele-like tumour also show cystically dilated ducts. However secretions of these lesions are rather pale and basophilic and do not show linear cracking artefacts, and the mucinous content are formed from extravasation of mucin within the stroma. Metastatic follicular thyroid cancer may mimic CHC, so a histological differentiation requires IHC stain for thyroglobulin [12, 13, 15]. CHC usually behaves in a non-aggressive manner but few reported invasive CHC cases, including our case, emphasise the need for follow-up and genetic studies for future risk assessment.
Conclusion
CHC in situ of the breast is a rare distinctive variant of ductal carcinoma that behaves in a low-grade fashion for many years but, nevertheless, has a potential for invasive growth and development of distant metastasis. Under-diagnosis of CHC as a benign lesion that is CHH is a recognised phenomenon and should be avoided by extensive sampling of the lesion and looking for invasion. It is a persistent condition with the potential to evolve into carcinoma. Longer follow-up and study of additional cases will be necessary to determine if this lesion has distinctive clinical characteristics.
References
- 1.Rosen PP, Scott M. Cystic hypersecretory duct carcinoma of the breast. Am J Surg Pathol. 1984;8(1):31–41. doi: 10.1097/00000478-198401000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Erdinç Kamer, et al. Invasive cystic hypersecretory ductal carcinoma of the breast. Meme Sağlığı Dergisi. 2008. Cilt 4 Sayı 2.
- 3.Skálová A, et al. Cystic hypersecretory carcinoma: rare and potentially aggressive variant of intraductal carcinoma of the breast. [Report of five cases] CeskaGynekol. 2005;70(1):73–8. [PubMed] [Google Scholar]
- 4.Paul Peter Rosen. Rosen’s breast pathology chapter cystic hypersecretory carcinoma and cystic hypersecretory hyperplasia. 2009:581–589. [Google Scholar]
- 5.Guerry P, Erlandson RA, Rosen PP. Cystic hypersecretory hyperplasia and cystic hypersecretory duct carcinoma of the breast Cancer. 1988;61(8):1611–1620. doi: 10.1002/1097-0142(19880415)61:8<1611::aid-cncr2820610819>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 6.Colandrea JM, et al. Cystic hypersecretory duct carcinoma of the breast. Arch Pathol Lab Med. 1988;112(5):560–3. [PubMed] [Google Scholar]
- 7.Adams GD, Lacey S. Cystic hypersecretory breast carcinoma: an unusual breast cancer. Nebr Med J. 1990;75(5):104–108. [PubMed] [Google Scholar]
- 8.Kim MK, Ghee-Young K, Gyung-Yub G. Fine needle aspiration cytology of cystic hypersecretory carcinoma of the breast. Acta Cytol. 1997;41(3):892–896. doi: 10.1159/000332724. [DOI] [PubMed] [Google Scholar]
- 9.Herrmann ME, McClatchey KD, Siziopikou KP. Invasive cystic hypersecretory ductal carcinoma of breast: a case report and review of the literature. Arch Pathol Lab Med. 1999;123(11):1108–10. doi: 10.1043/1543-2165-123.20.1108. [DOI] [PubMed] [Google Scholar]
- 10.Shah AK, et al. Cystic hypersecretory duct carcinoma of the breast. Breast J. 2000;6(4):269–272. doi: 10.1046/j.1524-4741.2000.98036.x. [DOI] [PubMed] [Google Scholar]
- 11.Park JM, Seo MR. Cystic hypersecretory duct carcinoma of the breast: report of two cases. Clin Radiol. 2002;57(4):312–5. doi: 10.1053/crad.2001.0825. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Lee YJ. Invasive cystic hypersecretory carcinoma of the breast: a case report. J Korean Med Sci. 2004;19(1):149–51. doi: 10.3346/jkms.2004.19.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin SJ, Rosen PP. Carcinoma arising from preexisting pregnancy-like and cystic hypersecretory hyperplasia lesions of the breast: a clinicopathologic study of 9 patients. Am J Surg Pathol. 2004;28(6):789–93. doi: 10.1097/01.pas.0000126060.20455.27. [DOI] [PubMed] [Google Scholar]
- 14.Park C, et al. Sonographic findings in a patient with cystic hypersecretory duct carcinoma of the breast. J Clin Ultrasound. 2004;32(1):29–32. doi: 10.1002/jcu.10223. [DOI] [PubMed] [Google Scholar]
- 15.Resetkova E, et al. Pathologic quiz case: a large, ill-defined cystic breast mass. Invasive cystic hypersecretory duct carcinoma. Arch Pathol Lab Med. 2005;129(3):e79–80. doi: 10.5858/2005-129-e79-PQCALI. [DOI] [PubMed] [Google Scholar]
- 16.Skalova A, et al. Cystic hypersecretory carcinoma: rare and poorly recognized variant of intraductal carcinoma of the breast. Report of five cases. Histopathology. 2005;46(1):43–9. doi: 10.1111/j.1365-2559.2005.02055.x. [DOI] [PubMed] [Google Scholar]
- 17.Sahoo S, et al. Cystic hypersecretory carcinoma of the breast with paget disease of the nipple: a diagnostic challenge. Int J Surg Pathol. 2008;16(2):208–12. doi: 10.1177/1066896907306843. [DOI] [PubMed] [Google Scholar]
- 18.Chen DB, Kan X. Cystic hypersecretory carcinoma with microinvasive carcinoma and cystic hypersecretory hyperplasia of breast: report of a case. Zhonghua Bing Li Xue Za Zhi. 2010;39(1):54–5. Chinese. [PubMed] [Google Scholar]
- 19.Song SW, Whang IY, Chang ED. Cystic hypersecretory ductal carcinoma of the breast: a rare cause of cystic breast mass. Jpn J Radiol. 2011;29(9):660–2. doi: 10.1007/s11604-011-0601-y. [DOI] [PubMed] [Google Scholar]
- 20.D’Alfonso TM, et al. Cystic hypersecretory (in situ) carcinoma of the breast: a clinicopathologic and immunohistochemical characterization of 10 cases with clinical follow-up. Am J Surg Pathol. 2014;38(1):45–53. doi: 10.1097/PAS.0b013e31829fc47b. [DOI] [PubMed] [Google Scholar]
- 21.Bi R, et al. Clinicopathologic features of cystic hypersecretory lesion of the breast. Zhonghua Bing Li Xue Za Zhi. 2014;43(1):25–9. Chinese. [PubMed] [Google Scholar]
- 22.Hamele-Bena D, Cranor ML, Rosen PP. Mammary mucocele-like lesions, benign and malignant. Am J Surg Pathol. 1996;20(9):1081–1085. doi: 10.1097/00000478-199609000-00005. [DOI] [PubMed] [Google Scholar]