Abstract
Objective
The Wnt/β-catenin signaling pathway has been implicated in human heart valve disease and is required for early heart valve formation in mouse and zebrafish. However, the specific functions of Wnt/β-catenin signaling activity in heart valve maturation and maintenance in adults have not been previously determined.
Approach and Results
Here, we show that Wnt/β-catenin signaling inhibits Sox9 nuclear localization and proteoglycan expression in cultured chicken embryo aortic valves (AoVs). Loss of β-catenin in vivo in mice, using Periostin(Postn)Cre-mediated tissue-restricted loss of β-catenin (Ctnnb1) in valvular interstitial cells (VICs), leads to the formation of aberrant chondrogenic nodules and induction of chondrogenic gene expression in adult AoVs. These nodular cells strongly express nuclear Sox9, and Sox9 downstream chondrogenic extracellular matrix (ECM) genes, including Aggrecan, Col2a1, and Col10a1. Excessive chondrogenic proteoglycan accumulation and disruption of stratified ECM maintenance in the AoV leaflets are characteristics of myxomatous valve disease. Both in vitro and in vivo data demonstrate that loss of Wnt/β-catenin signaling leads to increased nuclear expression of Sox9 concomitant with induced expression of chondrogenic ECM proteins.
Conclusions
β-catenin limits Sox9 nuclear localization and inhibits chondrogenic differentiation during valve development and in adult AoV homeostasis.
Keywords: myxomatous valve disease, Wnt/β-catenin signaling, valvular interstitial cells, chondrogenesis, aortic valve, proteoglycan
Introduction
Adult heart valves are stratified into extracellular matrix (ECM) compartments, defined as collagen-rich fibrosa, proteoglycan-rich spongiosa, and elastin-rich ventricularis/atrialis layers.1 In diseased heart valves, disruption of ECM layers and deposition of abnormal matrix leads to valve dysfunction. Myxomatous valve disease is characterized by excessive proteoglycan accumulation and degradation of collagen and elastin fibers, leading to valve prolapse and insufficiency.1–4 The proteoglycan-rich spongiosa is similar to the ECM of cartilage, and regulatory pathways that control chondrogenesis also are active in valve development.1, 5, 6 Although dysregulation and reactivation of early developmental programs has been described in heart valve disease,7–11 it remains unclear whether the expansion of proteoglycan-rich spongiosa in myxomatous valve disease is an active chondrogenic process.
During the initiation of heart valve development, Wnt/β-catenin signaling is required for early endocardial cushion formation.12, 13 At later stages, Wnt/β-catenin promotes the expression of fibrosa-related and osteogenic-like ECM genes in cultured chicken embryo valve interstitial cells (VICs).14 Wnt/β-catenin signaling is active in normal heart valves in mice at 1 month of age and also is increased in human calcific aortic valve disease.14, 15 Together, these data suggest that Wnt/β-catenin signaling has multiple roles in valve development and disease. However, the specific regulatory requirements for Wnt/β-catenin signaling in valvular ECM stratification and maintenance have not been previously reported.1, 6
Sox9, a SRY transcription factor required for cartilage lineage development,16–18 is crucial for normal valve formation and promotes expression of cartilage-associated genes.19, 20 In addition, Sox9 is required to prevent calcification in adult aortic valves (AoVs).21, 22 In diseased human and mouse heart valves,10, 11, 23, 24 expression of Sox9 and cartilage-related genes is induced, suggesting that induction of a chondrogenic gene program contributes to heart valve disease. In endochondral bone development, Wnt/β-catenin signaling inhibits Sox9-driven chondrogenesis and promotes osteogenic differentiation.25 However, whether reduction of Wnt/β-catenin signaling directly promotes Sox9-mediated proteoglycan expansion in normal heart valves or in myxomatous valve disease remains untested.
Here, we used embryonic chicken aortic valve organ cultures to investigate the role of Wnt/β-catenin signaling in layer-specific ECM expression during valve stratification. In vivo, we used VIC-specific loss of β-catenin driven by PostnCre26 in mice to determine requirements for β-catenin in valve ECM maturation and adult homeostasis. We show that Wnt/β-catenin limits Sox9 nuclear localization and inhibits chondrogenic differentiation of aortic VIC during heart valve development and maintenance in adults. Moreover, loss of β-catenin in mice leads to the formation of hypertrophic nodules with excessive proteoglycan accumulation in adult AoV leaflets, and similarly increased nuclear localization of Sox9 and proteoglycan expression are observed in human myxomatous valve disease.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Wnt/β-catenin signaling inhibits chondrogenic gene expression in cultured chicken aortic valves
Previously, we reported that Wnt3a treatment induces early osteogenic gene induction in cultured dissociated chicken embryonic day (E)14 aortic VICs.14 However, these dissociated cells do not retain the structural architecture of stratified heart valve leaflets, and therefore ECM compartmentalization cannot be examined using this method. In order to analyze diversified ECM production and regulation in a more physiological context, a novel aortic valve organ culture (aVOC) was devised. Whole aortic valves including adjacent aortic root were isolated from E14 chicken embryos and cultured in vitro as aVOCs. Although valve morphology is abnormal in cultured aVOCs, ECM compartmentalization is maintained, as shown by Movat’s Pentachrome staining of proteoglycans, collagen fibers and elastin, as well as immunostaining for Elastin (ventricularis), Aggrecan and Collagen 2 (Col2) (spongiosa), and also Col3 (fibrosa) (Fig. SIA–D). Thus, this system can be used for examination of regulatory mechanisms that control ECM compartmentalization in developing valves.
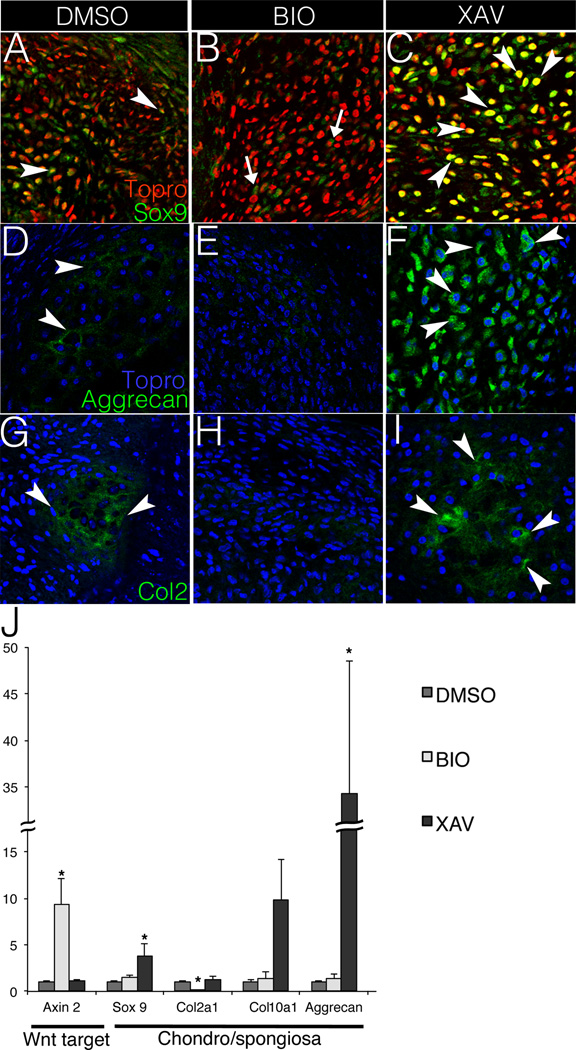
The role of Wnt/β-catenin signaling in heart valve ECM composition and compartmentalization was examined using the E14 chicken aVOC cultures. Cultures were treated with BIO to increase Wnt/β-catenin signaling,27 or XAV-939 to decrease Wnt/β-catenin signaling.28 When treated with BIO (Wnt activation), the proteoglycan-expressing area, as indicated by Alcian Blue staining, decreases compared to vehicle-treated controls (DMSO) (Fig. SII). Although the area of proteoglycan expression was not increased in XAV-939-treated cultures, increased intensity of Alcian Blue staining was noted (Fig. SIIC). In contrast, XAV-939 treatment, but not BIO, decreases the fibrillar collagen-expressing area in cultured aVOC as indicated by Saffron staining (Fig. SII). Thus Wnt/β-catenin signaling inhibits proteoglycan expression (spongiosa) and is required for collagen expression (fibrosa) in cultured aVOCs. Immunostaining for the chondrogenic transcription factor Sox9 demonstrates that Sox9 protein expression is decreased in cultured aVOCs upon BIO treatment, whereas XAV-939 addition promotes Sox9 expression and nuclear localization (Fig. 1A–C). Expression of cartilage matrix proteins Aggrecan and Col2 also are repressed by BIO (Fig. 1E, H) and increased with XAV-939 (Fig. 1F, I). Quantification of gene expression by qRT-PCR confirms Wnt activation with BIO treatment, as indicated by increased Axin2 gene expression. Likewise BIO treatment dampens Col2a1 expression but has no apparent effect on Sox9, Col10, or Aggrecan gene expression levels. By contrast, Wnt inhibition with XAV-939 induces chondrogenic markers Sox9 and Aggrecan, but not Col2a1 (Fig. 1J). The differing effects of altered Wnt signaling on chondrogenic protein versus mRNA expression support complex downstream transcriptional and post-transcriptional regulatory mechanisms. However, together, these data support a role for Wnt/β-catenin in ECM compartmentalization by limiting nuclear localization of Sox9 and expression of chondrogenic matrix proteins.
Figure 1. Wnt/β-catenin signaling inhibits chondrogenic gene expression in chicken embryo aortic valve organ cultures (aVOC).
A–C. Sox9 expression (green) is shown by immunofluorescent staining in sectioned aVOCs after treatment with DMSO, BIO (Wnt activation) or XAV-939 (Wnt inhibition). Arrowheads indicate cells with positive nuclear Sox9 staining (orange) in A and C, whereas arrows in B indicate cells with cytosolic Sox9 (green). D–F. Aggrecan expression (green) is detected by immunofluorescent staining in treated aVOCs. Arrowheads indicate positive Aggrecan staining in both D and F. G–I. Col2 expression (green, arrowheads) is decreased in BIO-treated aVOCs (H), compared to either DMSO controls (G) or XAV-treated aVOCs (I). Arrowheads indicate positive Col2 staining in both G and I. Nuclei are counterstained with Topro 3, and pseudo-colored in red (A–C), or blue (D–I). Representative images are shown from four aVOCs analyzed for each condition. J. Expression of the Wnt/β-catenin pathway target gene Axin2 and chondrogenic genes Sox9, Col2a1, Col10 and Aggrecan was evaluated by qRT-PCR in cultured aVOC treated with DMSO, BIO or XAV (n=6). Normalized relative gene expression levels were calculated and compared to DMSO controls that were set to 1.0. Statistical significance was determined using paired Student’s t-tests. * indicates p<0.05. Error bars represent SEM.
Loss of β-catenin in aortic VICs results in formation of proteoglycan rich nodules in adult mice
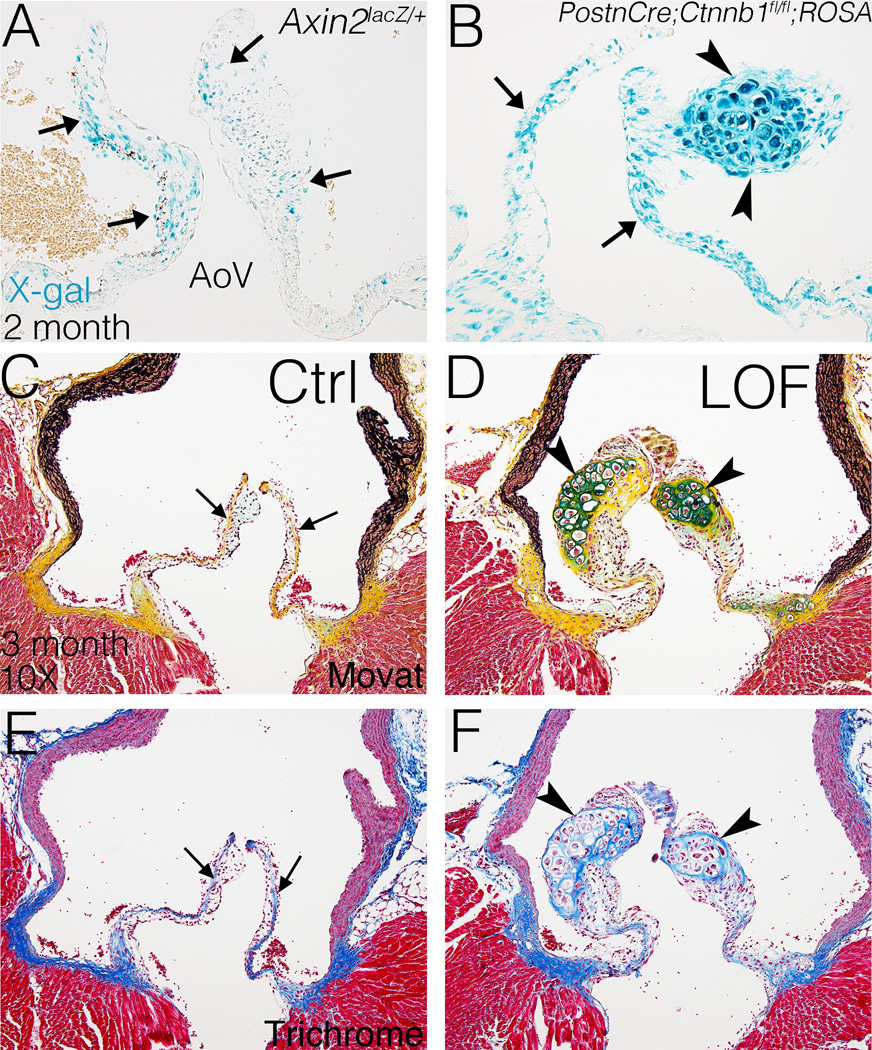
The role of Wnt/β-catenin signaling in adult valves in vivo was examined in mice with loss of β-catenin in VICs. Wnt/β-catenin signaling is active in semilunar valves before birth as previously reported,14 and also at 2 weeks and in adult aortic valves as indicated by an Axin2lacZ/+ reporter line29 (Fig. 2A, SIII). Interestingly, Wnt/β-catenin signaling is active preferentially at the distal tip and the hinge of the AoV at 2 weeks after birth and also in adult mice. PostnCre26 was used for conditional recombination of floxed exons 2–6 in the β-catenin allele30 (Ctnnb1fl/fl), and is active in AoV interstitial cells but not endothelium, as indicated by positive X-gal staining in adult PostnCre;ROSA26 mice (Fig. SIVB).31
Figure 2. Wnt/β-catenin signaling is active in adult AoVs and loss of β-catenin leads to formation of proteoglycan-rich hypertrophic cartilage-like nodules in the AoVs of PostnCre;Ctnnb1fl/fl mice.
A. Wnt/β-catenin signaling, as indicated by Axin2lacZ reporter activity, is active in the distal tip of a normal aortic valve (AoV) at 2 months-of-age. Arrows indicate cells with β-gal activity (blue) expressed from the Axin2lacZ locus in X-gal stained sections. B. PostnCre is active throughout the leaflets (arrows) of AoV, including the nodule (arrowheads), in PostnCre;Ctnnb1fl/fl;ROSA26 mice at 3 months of age. C, D. AoVs are stained by Movat’s Pentachrome to visualize the ECM distribution and morphology at 3 months. Arrows in C indicate normal valve stratification, whereas arrowheads in D indicate proteoglycan-rich (blue) nodules. E, F. AoVs were stained by Masson’s Trichrome for detection of collagen (blue) at 3 months of age (n=4). Arrows in E indicate normal collagen deposition, whereas arrowheads in F indicate collage expression surrounding the nodules.
The efficacy of PostnCre-mediated loss of β-catenin protein was determined in semilunar valves of PostnCre;Ctnnb1fl/fl mice at post-natal day (P)0 and 2 weeks of age. β-catenin protein expression is reduced in the AoV interstitium, but not endothelium, of PostnCre;Ctnnb1fl/fl mice at P0 and is not detectable at 2 weeks, compared to Cre-negative controls (Fig. SVA–D). Furthermore, Axin2 mRNA, a downstream target of Wnt/β-catenin signaling, is significantly decreased by ~70% in PostnCre;Ctnnb1fl/fl animals, compared to Cre-negative controls, in isolated AoV leaflets at 3 months of age (Fig. SVE). Therefore, β-catenin protein and canonical Wnt signaling are reduced in the AoV interstitium of PostnCre;Ctnnb1fl/fl mice. Although β-catenin protein is reduced, the PostnCre;Ctnnb1fl/fl mice have apparently normal semilunar valve morphology at P0 (Fig. SVIA–D) and initial compartmentalization of collagen and proteoglycan as indicated by Pentachrome staining (Fig. SVIE–F). No differences in cell density or morphometry were observed at early postnatal stages (Fig SVIG, H). In addition, PostnCre;Ctnnb1fl/fl mice survive through gestation and do not exhibit obvious morbidity or mortality.
The requirement for β-catenin in valve homeostasis and ECM maintenance was determined in adult PostnCre;Ctnnb1fl/fl mice. Histological analysis of PostnCre;Ctnnb1fl/fl animals demonstrates that proteoglycan-rich nodules containing hypertrophic cells are present in the AoV at 2 months, with 100% penetrance at 3 months (Fig. 2B). Cre-mediated recombination occurs throughout the valve leaflets, as apparent in PostnCre;Ctnnb1fl/fl;ROSA26lacZ reporter mice at 3 months (Fig. 2B). However, the nodules are restricted to the distal tip of the AoV leaflet where Wnt/β-catenin signaling is active, as indicated by Axin2lacZ expression (Fig 2A). The formation of proteoglycan-rich nodules is specific to the semilunar valves with a lower frequency of nodule formation in the pulmonary valve and no apparent abnormalities noted in the mitral or tricuspid valves in PostnCre;Ctnnb1fl/fl animals up to 6 months of age (Fig. SVII).
To visualize the morphology and stratification of the AoV, Movat’s Pentachrome and Masson’s Trichrome staining were performed. In controls, the AoV is stratified with defined layers of proteoglycans and collagen (Fig. 2C, E). However, all 3-month-old PostnCre;Ctnnb1fl/fl mice exhibit large nodules almost exclusively in the distal tip of the AoV (Fig. 2D, F). These nodules in PostnCre;Ctnnb1fl/fl AoVs are densely stained by Alcian Blue, indicating strong proteoglycan deposition (Fig. 2D). By contrast, fibrillar collagen deposition is apparently reduced in the nodules (Fig. 2F), but accumulation is obvious surrounding the nodules, as indicated by Masson’s Trichrome staining. Strikingly, the nodular cells also exhibit dramatic morphological changes including the presence of hypertrophic cells (Fig. 2B, D, F). However, cell death was not apparent in the nodules of PostnCre;Ctnnb1fl/fl animals, as indicated by cleaved caspase-3 immunoreactivity (Fig. SVIII). In addition, the PostnCre;Ctnnb1fl/fl nodules do not calcify at 1 year of age (data not shown). The increased proteoglycan deposition and hypertrophic cellular morphology of the nodular cells in PostnCre;Ctnnb1fl/fl AoVs at 3 months are similar to hypertrophic chondrocytes.18 Thus β-catenin is required for normal ECM maintenance, and loss of β-catenin leads to the formation of hypertrophic cartilage-like nodules in the adult AoV.
Aortic valve nodules express chondrogenic genes as a result of loss of β-catenin
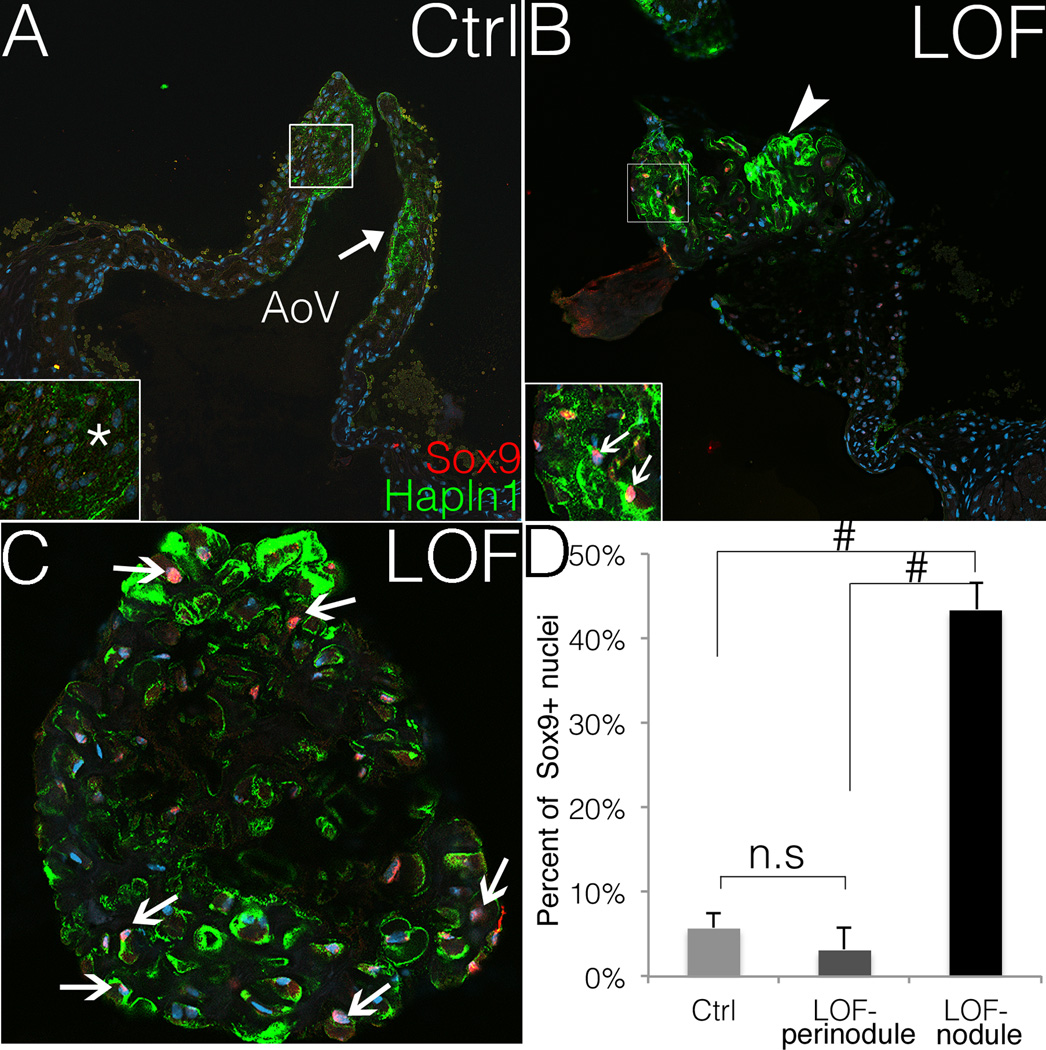
Spongiosa and fibrosa ECM compartmentalization, as well as induction of chondrogenic regulatory mechanisms, were examined in PostnCre;Ctnnb1fl/fl AoV nodules. Sox9 is required for chondrogenesis,18 and for the expression of Hyaluronan and Proteoglycan Link protein 1 (Hapln1) in developing heart valves.32, 33 Hapln1 is normally restricted to the ventricularis side of Cre-negative control AoVs (Fig. 3A, C), but its expression is expanded throughout the proteoglycan-rich nodules in PostnCre;Ctnnb1fl/fl AoVs, as detected by immunofluorescent staining (Fig. 3B, C). Likewise, the transcription factor Sox9 is strongly expressed in the nuclei of nodular cells at the distal tip of AoV in PostnCre;Ctnnb1fl/fl animals (Fig. 3B, C), and the percentage of Sox9 positive nuclei is higher in the nodules compared to either peri-nodular areas or entire AoV leaflets in Cre-negative controls (Fig. 3D). Thus, loss of β-catenin leads to increased nuclear Sox9 expression in proteoglycan-rich nodular cells in the adult AoV. Similarly, nuclear localization of Sox9 and increased Hapln1 expression also are apparent in human myxomatous mitral valve disease (Fig. SIX).
Figure 3. The nodular cells PostnCre;Ctnnb1fl/fl AoVs express nuclear Sox9.
A, B. Sox9 expression (red) is shown in PostnCre;Ctnnb1fl/fl (LOF) nodular cells but is not detected (asterisk) in Cre-negative control (Ctrl) AoVs. The arrow in A indicates normal expression of Hapln1 (green) in controls. The arrowhead in B indicates the Hapln1-positive (green) LOF nodule, and strong nuclear Sox9 expression (red) in LOF nodular cells (arrows, inset in B). Nuclei are counterstained in blue by Topro3. Staining shown is representative of n=4 specimens analyzed, and a minimum of 2 different slides per sectioned sample was stained. C. Nuclear Sox9 (red) is shown at higher magnification within a Hapln1-positive (green) nodule in a PostnCre;Ctnnb1fl/fl AoV. Arrows indicate nuclear Sox9 positive nodular cells. D. The percent of Sox9 positive nuclei is significantly higher in the LOF nodules than in control AoVs or perinodular regions (Hapln-negative) in LOF AoVs (LOF n=6, control n=8; ANOVA). # indicates p<0.01. n.s indicates not significant (p=0.401). Error bars represent SEM.
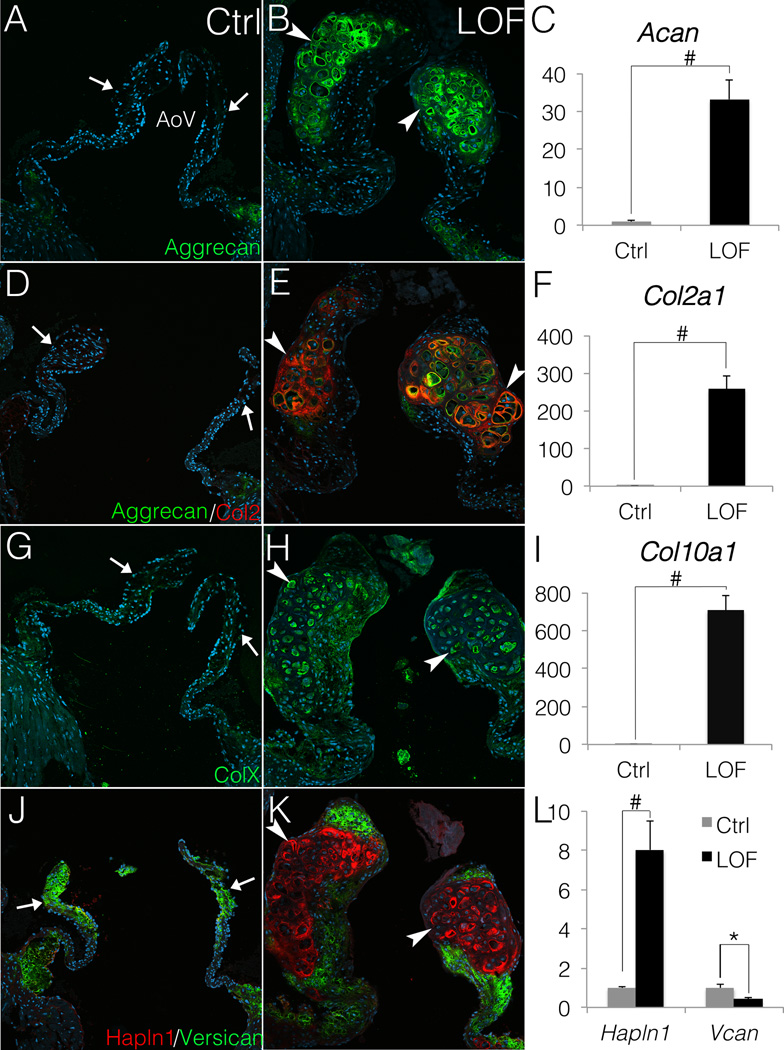
The expression of Sox9 target genes Aggrecan (Acan), Col2, and ColX, which are expressed in cartilage, was examined in AoV lacking β-catenin. Both Aggrecan and Col2 are strongly expressed in the nodules of PostnCre;Ctnnb1fl/fl AoVs (Fig. 4B, E), in contrast to no detectable expression in the distal tip of AoV in Cre-negative controls (Fig. 4A, D). Consistent with the morphology similar to hypertrophic cartilage (Fig. 2F, H), the nodular cells in PostnCre;Ctnnb1fl/fl AoVs also express ColX (Fig. 4H), which is a hypertrophic chondrocyte marker18 and is not normally expressed in heart valves (Fig. 4G). Likewise, qRT-PCR analysis of gene expression demonstrates that Acan is increased by 33-fold (Fig. 4C), Col2a1 by ~260-fold (Fig. 4F) and Col10a1 by ~700-fold (Fig. 4I) in PostnCre;Ctnnb1fl/fl AoVs compared to controls. Thus loss of β-catenin leads to induction of a chondrogenic gene program apparent in increased nuclear localization of Sox9 and induced expression of downstream target genes Acan, Col2a1, Hapln1, and Col10a1.
Figure 4. PostnCre;Ctnnb1fl/fl nodular cells express chondrocyte markers Aggrecan, Col2 and ColX.
A–I, Chondrogenic ECM genes are induced and expressed at high levels in PostnCre;Ctnnb1fl/fl (LOF) nodules compared to Cre-negative controls (Ctrl). Aggrecan (A, B, D & E; green), Col2 (D, E; red) and ColX (G, H; green) expression is shown in control AoVs and LOF nodules. Arrows indicate the lack of endogenous chondrogenic ECM expression in control AoV (A, D, and G), whereas arrowheads indicate induced expression in LOF nodules (B, E, and H). By qRT-PCR (C, F and I), Aggrecan (Acan), Col2a1 and Col10a1 are significantly upregulated in LOF AoV, compared to Cre-negative controls (n=3). J, K. The relative distribution of Versican (green) and Halpn1 (red) expression is shown in LOF and control AoVs. Arrows indicate normal Versican expression and limited expression of Hapln1 in control AoVs (J). Arrowheads indicate markedly reduced Versican expression (green) and increased Hapln1 expression (red) in LOF nodules (K). L. By qRT-PCR, Hapln1 is significantly increased in LOF AoV relative to controls, whereas Versican (Vcan) expression is decreased in LOF AoV compared to controls (n=3). Student’s t-tests were performed to determine the statistical significance of gene expression differences between LOF and control AoVs. # indicates p<0.01, and * indicates p<0.05. All error bars represent SEM.
The proteoglycan composition and timing of induction were further examined in PostnCre;Ctnnb1fl/fl AoVs. Versican (Vcan) is normally the predominant proteoglycan in the spongiosa layer of AoVs (Fig. 4J), but is dramatically reduced in the nodules of PostnCre;Ctnnb1fl/fl AoVs, demonstrating a switch in the predominant proteoglycan to Aggrecan, with loss of β-catenin (Fig. 4K). Consistent with the transition to a chondrogenic ECM, the expression of Hapln1 expands markedly to encompass the entire nodule in PostnCre;Ctnnb1fl/fl AoVs (Fig. 4K). qRT-PCR analysis also demonstrates that Hapln1 is increased by 8-fold and Vcan is decreased by 60% (Fig. 4L). The initiation of nodule formation and proteoglycan alterations were examined at early post-natal stages. At P0 and 2 weeks, Aggrecan, not normally expressed in mouse aortic valves, is detected in PostnCre;Ctnnb1fl/fl AoVs (Fig. SXB, D). Other chondrogenic ECM markers including Col2 and ColX are not expressed in PostnCre;Ctnnb1fl/fl AoV at this early stage, indicating that formation of the hypertrophic nodules develops over time. Thus, loss of β-catenin leads to induction of proteoglycans and collagens characteristic of cartilage, beginning with Aggrecan. Together, these data suggest that loss of β-catenin drives VICs in PostnCre;Ctnnb1fl/fl AoVs to undergo progressive chondrogenic differentiation and maturation after birth.
The fibrosa ECM is dysregulated in adult AoV lacking β-catenin
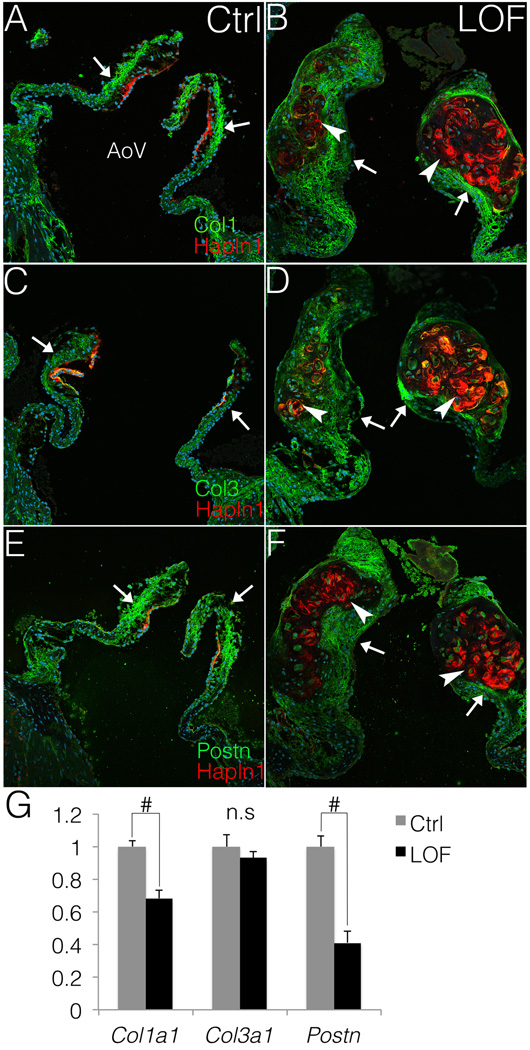
The initial formation and compartmentalization of the fibrosa layer relative to the spongiosa was examined in PostnCre;Ctnnb1fl/fl AoVs at P0 and 2 weeks. The initial organization of collagen and proteoglycan-rich regions of semilunar valves is apparently normal, as indicated by Movat’s pentachrome staining (Fig. SVIE, F). Localized expression of Col1 and Hapln1 also is apparently normal at 2 weeks in both PostnCre;Ctnnb1fl/fl animals and Cre-negative controls (Fig. SXG, H). In adult mice, Col1 expression normally is restricted to the fibrosa layer (Fig. 5A). However, this well-defined layer is lost in PostnCre;Ctnnb1fl/fl AoVs, in which Col1 is absent from the nodules but is apparent in the peri-nodular areas (Fig. 5B). Likewise, Col3 is predominantly expressed in the fibrosa layer in Cre-negative control AoVs (Fig. 5C). However, in PostnCre;Ctnnb1fl/fl AoVs, Col3 expression is expanded throughout the thickened leaflets but is only minimally expressed in the proteoglycan-rich nodules (Fig. 5D). Consistent with the expression of Col1 and Col3, endogenous Postn is not detected within the nodules of PostnCre;Ctnnb1fl/fl animals, but is expressed in the thickened distal tip in the collagen-rich region (Fig. 5E, F). In addition, Col1a1 and Postn transcripts are significantly decreased in PostnCre;Ctnnb1fl/fl AoVs, compared to Cre-negative controls (Fig. 5G), at 3 months. Thus, the proteoglycan rich nodules lose the expression of fibrillar collagens and matricellular Postn, while the fibrosa layer is disrupted in adult PostnCre;Ctnnb1fl/fl AoVs. Together, these data demonstrate that loss of β-catenin signaling disrupts normal ECM maintenance and compartmentalization in the adult AoV.
Figure 5. Fibrosa ECM markers Col1, Col3 and Postn are minimally expressed in the nodules but are present in the perinodular regions.
A, B. Col1 expression is shown in PostnCre;Ctnnb1fl/fl (LOF) and Cre-negative control (Ctrl) AoVs. Arrows indicate normal Col1 expression (green) in the valve leaflets, and Hapln1 (red) restricted to the commissure in control AoVs (A). Arrowheads indicate that the Hapln1-positive (red) LOF nodules are Col1 negative, whereas arrows indicate Col1 expression in perinodular regions in LOF AoVs (B). C–F. Similarly, the protein expression (green) of Col3 (C,D) and Postn (E,F) also is decreased in the Hapln1-positive nodules (red) in LOF AoVs (arrowheads, D, F), compared to controls (arrows, C, E). G. By qRT-PCR, Col1a1 and Postn are significantly (n=3; # indicate p<0.01) decreased in LOF AoVs compared to Cre-negative controls as determine by Student’s t-est. However, no significant (n.s.) difference (p=0.438) is detected in Col3a1 expression between controls and LOF mutants. Error bars represent SEM.
Discussion
β-catenin is required for endothelial-to-mesenchymal transition at the early endocardial cushion stage in heart valve development,13 and upregulation of Wnt/β-catenin signaling has been implicated in heart valve disease.15 Here, we show that β-catenin signaling is required for normal ECM maintenance and heart valve homeostasis in cultured embryonic chicken aortic valves and adult mice. In vitro and in vivo analyses demonstrate increased nuclear localization of Sox9 and induction of downstream chondrogenic gene expression when Wnt/β-catenin signaling is reduced. Thus, Wnt/β-catenin signaling limits nuclear localization of Sox9 in aortic VICs and is required for maintenance of ECM compartmentalization. These regulatory interactions are intrinsic to VICs, since PostnCre is not active in endothelial cells and β-catenin expression is maintained on the surface of PostnCre;Ctnnb1fl/fl AoVs. Together, these studies demonstrate a critical role for Wnt/β-catenin signaling in heart valve ECM homeostasis with implications for myxomatous valve disease mechanisms (see model, Fig SXI).
Embryonic aVOC cultures treated with the Wnt/β-catenin activator BIO have decreased proteoglycan deposition, whereas treatment with the inhibitor XAV leads to increased chondrogenic matrix protein expression and nuclear localization of Sox9. Likewise, treatment of isolated embryonic aortic VICs with Wnt3a induces fibrosa-like gene expression consistent with a role in heart valve ECM compartmentalization.14 However, the initial stratification of PostnCre;Ctnnb1fl/fl AoVs is normal at 2 weeks of age, indicating that Wnt/β-catenin signaling is not required for initiation of valve stratification, but rather for maintenance of normal ECM compartmentalization. Further evidence for active Wnt/β-catenin signaling in adult valve homeostasis is the expression of a Axin2lacZ/+ reporter in the distal regions of adult AoV and reduction of Axin2 transcripts in PostnCre;Ctnnb1fl/fl AoVs. Interestingly, the formation of hypertrophic cartilage-like nodules in PostnCre;Ctnnb1fl/fl mice also occurs at the distal tips of the AoVs. The lack of an initial effect on valve stratification and the localization of the chondrogenic nodules to the distal tips of the aortic valve leaflets in PostnCre;Ctnnb1fl/fl mice is in contrast to the results observed in cultured embryonic aVOCs and suggests that Wnt/β-catenin signaling is modulated by physiological factors in vivo. It has been reported that mechanical tension and compression stimulate the expression of Wnt10b, and co-receptor Lrp5 in cultured osteoblast cells.34 The intracellular mechanosensing RhoA/ROCK cascade also affects Sox9 expression and activity.35, 36 Thus mechanical stimulation may have a role in the interaction between Wnt/β-catenin signaling and Sox9-mediated chondrogenic differentiation in the aortic VICs.
Loss of β-catenin in VICs leads to increased expression and nuclear localization of Sox9, as well as increased expression of Col2a1, Acan, and Col10a1, in cultured aVOC and adult AoVs. This same regulatory hierarchy is active in hypertrophic cartilage,37–39 and loss of β-catenin in mesenchymal progenitors leads to increased expression of Sox9 and ectopic cartilage formation in the developing skull.40, 41 Here, we demonstrate that loss of Wnt/β-catenin signaling leads to increased nuclear localization of Sox9 and chondrogenic gene induction in valve progenitors and adult valves. Although Sox9 and β-catenin proteins have been demonstrated to interact in cartilage39, 42 and gonad development43, the mechanism by which loss of β-catenin leads to increased nuclear localization of Sox9 is not known. In developing valves, Sox9 is required for expression of cartilage-related ECM genes, including Hapln1 and Col2a1.20 Haploinsufficiency of Sox9 in heart valves is sufficient to promote ectopic calcification in adult mice,21 and Sox9 suppresses Osteopontin expression preventing matrix mineralization in postnatal mouse heart valve explants.22 Increased Wnt/β-catenin signaling has also been implicated in human calcific aortic valve disease, potentially by promoting active osteogenesis in the fibrosa layer.15, 44 Here, we show that loss of β-catenin leads to abnormal chondrogenic differentiation of aortic VICs with excessive accumulation of proteoglycans in mice. Likewise, nuclear Sox9 and Hapln1 protein expression are increased in human myxomatous valve disease. Together, these data provide further evidence for shared regulatory mechanisms in skeletal lineages and in heart valve development and disease.1, 5, 6
Excessive proteoglycan accumulation is one of the characteristics of myxomatous valve disease.1–4 Although its etiology appears complex, mutations in the cytoskeletal protein Filamin A (FLNA) and a variety of ECM protein mutations have been linked to human myxomatous valve disease.2, 3, 45–47 Additional causes include congenital malformation, increased serotonin signaling, or infective endocarditis.48 In each case, the myxomatous valve exhibits increased proteoglycan and decreased collagen composition leading to insufficiency and regurgitation. However, the molecular mechanism(s) of disease pathogenesis and progression are still poorly understood.3, 4 Expression of Aggrecan, Col2 and Sox9 is induced in human myxomatous valve disease, suggesting that chondrogenic differentiation of VICs is integral to disease pathogenesis.24 Similarly, in mice lacking endogenous Postn, inappropriate differentiation of mesenchymal cushion cells and abnormal Aggrecan expression were detected in Postn−/− valves.49 Here, we demonstrate that VICs directly undergo chondrogenic differentiation with excessive proteoglycan accumulation as a result of loss of β-catenin. The chondrogenic nodules observed in mouse aortic valves are not a characteristic feature of human valve pathology. However predominant nuclear Sox9 localization in regions of increased Hapln1 protein expression was observed in both PostnCre;Ctnnb1fl/fl AoV and human myxomatous mitral valve disease, supporting a role for this regulatory interaction in human valve pathogenesis. It remains to be determined if Wnt/β-catenin signaling is repressed by the multiple causes of myxomatous valve disease or contributes to pathogenesis that ultimately leads to valve insufficiency. If this is the case, maintaining or increasing Wnt/β-catenin signaling could be exploited therapeutically as an alternative to surgical repair, which is the current standard of care for severe myxomatous valve disease.3
Supplementary Material
Significance.
The role of Wnt/β-catenin signaling in heart valve stratification and subsequent extracellular matrix (ECM) maintenance is not known. In this study, we found that Wnt/β-catenin signaling is required for heart valve homeostasis, since loss of β-catenin leads to increased chondrogenic differentiation of aortic valve interstitial cells (VICs) in adult mice. In the absence of β-catenin, VICs are susceptible to ectopic chondrogenic differentiation, leading to the formation of nodules containing proteoglycan-rich hypertrophic cartilage-like cells, with nuclear localization of the chondrogenic transcription factor Sox9 and loss of fibrillar collagen. This chondrogenic phenotype is similar to human myxomatous heart valve disease characterized by increased nuclear Sox9 localization and excessive proteoglycan accumulation.
Acknowledgments
We thank Dr A. Colige from Laboratory of Connective Tissues Biology (GIGA, Sart-Tilman, Belgium) and Dr M. Radermecker from Department of Cardiovascular and Thoracic Surgery and Human Anatomy (CHU-Sart-Tilman, Belgium) for sharing human mitral valve samples.
Sources of funding:
This work is supported by the National Institutes of Health Grant R01HL094319 (to K.E.Y.), American Heart Association Predoctoral Fellowship 13PRE16410009 (to M.F.) and R01HL60714 (to S.J.C.).
Abbreviations
- AoV
Aortic valve
- aVOC
aortic valve organ culture
- BIO
6-Bromoindirubin-3'-oxime
- ECM
extracellular matrix
- VIC
valvular interstitial cells
Footnotes
Disclosures: None
References
- 1.Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayek E, Gring CN, Griffin BP. Mitral valve prolapse. The Lancet. 2005;365:507–518. doi: 10.1016/S0140-6736(05)17869-6. [DOI] [PubMed] [Google Scholar]
- 3.Guy TS, Hill AC. Mitral valve prolapse. Annu Rev Med. 2012;63:277–292. doi: 10.1146/annurev-med-022811-091602. [DOI] [PubMed] [Google Scholar]
- 4.Hulin A, Deroanne C, Lambert C, Defraigne J-O, Nusgens B, Radermecker M, Colige A. Emerging pathogenic mechanisms in human myxomatous mitral valve: Lessons from past and novel data. Cardiovasc Pathol. 2013;22:245–250. doi: 10.1016/j.carpath.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Lincoln J, Lange AW, Yutzey KE. Hearts and bones: Shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006;294:292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Combs MD, Yutzey KE. Heart valve development: Regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J-H, Wylie-Sears J, Bischoff J. Opposing actions of notch1 and vegf in post-natal cardiac valve endothelial cells. Biochem Biophys Res Commun. 2008;374:512–516. doi: 10.1016/j.bbrc.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty S, Wirrig EE, Hinton RB, Merrill WH, Spicer DB, Yutzey KE. Twist1 promotes heart valve cell proliferation and extracellular matrix gene expression during development in vivo and is expressed in human diseased aortic valves. Dev Biol. 2010;347:167–179. doi: 10.1016/j.ydbio.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li C, Xu S, Gotlieb AI. The response to valve injury. A paradigm to understand the pathogenesis of heart valve disease. Cardiovasc Pathol. 2011;20:183–190. doi: 10.1016/j.carpath.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Wirrig EE, Hinton RB, Yutzey KE. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J Mol Cell Cardiol. 2011;50:561–569. doi: 10.1016/j.yjmcc.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheek JD, Wirrig EE, Alfieri CM, James JF, Yutzey KE. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. J Mol Cell Cardiol. 2012;52:689–700. doi: 10.1016/j.yjmcc.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Zivkovic D, Plasterk RH, Clevers H. The wnt/β-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- 13.Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. Β-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfieri CM, Cheek J, Chakraborty S, Yutzey KE. Wnt signaling in heart valve development and osteogenic gene induction. Dev Biol. 2010;338:127–135. doi: 10.1016/j.ydbio.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawakami Y, Rodriguez-León J, Belmonte JCI. The role of tgfβs and sox9 during limb chondrogenesis. Curr Opin Cell Biol. 2006;18:723–729. doi: 10.1016/j.ceb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Akiyama H, Lefebvre V. Unraveling the transcriptional regulatory machinery in chondrogenesis. J Bone Miner Metab. 2011;29:390–395. doi: 10.1007/s00774-011-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung Tsang K, Wa Tsang S, Chan D, Cheah KSE. The chondrocytic journey in endochondral bone growth and skeletal dysplasia. Birth Defects Res C. 2014;102:52–73. doi: 10.1002/bdrc.21060. [DOI] [PubMed] [Google Scholar]
- 19.Lincoln J, Alfieri CM, Yutzey KE. Bmp and fgf regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol. 2006;292:290–302. doi: 10.1016/j.ydbio.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 20.Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev Biol. 2007;305:120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peacock JD, Levay AK, Gillaspie DB, Tao G, Lincoln J. Reduced sox9 function promotes heart valve calcification phenotypes in vivo. Circ Res. 2010;106:712–719. doi: 10.1161/CIRCRESAHA.109.213702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peacock JD, Huk DJ, Ediriweera HN, Lincoln J. Sox9 transcriptionally represses spp1 to prevent matrix mineralization in maturing heart valves and chondrocytes. PLoS ONE. 2011;6:e26769. doi: 10.1371/journal.pone.0026769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sainger R, Grau JB, Branchetti E, Poggio P, Seefried WF, Field BC, Acker MA, Gorman RC, Gorman JH, Hargrove CW. Human myxomatous mitral valve prolapse: Role of bone morphogenetic protein 4 in valvular interstitial cell activation. J Cell Physiol. 2012;227:2595–2604. doi: 10.1002/jcp.22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacerda CMR, MacLea HB, Duncan CG, Kisiday JD, Orton EC. Human myxomatous mitral valves exhibit focal expression of cartilage-related proteins. J Hypertens Cardiol. 2013;1:1–10. [Google Scholar]
- 25.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical wnt signals in development and disease: Conditional loss- and gain-of-function mutations of β-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, Kruzynska-Frejtag A, Koushik SV, Lilly B, Burch JBE, Firulli AB, Conway SJ. Identification and characterization of a novel schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer. Dev Biol. 2007;307:340–355. doi: 10.1016/j.ydbio.2007.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of wnt signaling by a pharmacological gsk-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 28.Huang S-MA, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 29.Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the β-catenin gene by wnt1-cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 31.Soriano P. Generalized lacz expression with the rosa26 cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 32.Kou I, Ikegawa S. Sox9-dependent and-independent transcriptional regulation of human cartilage link protein. J Biol Chem. 2004;279:50942–50948. doi: 10.1074/jbc.M406786200. [DOI] [PubMed] [Google Scholar]
- 33.Lockhart MM, Wirrig EE, Phelps AL, Ghatnekar AV, Barth JL, Norris RA, Wessels A. Mef2c regulates transcription of the extracellular matrix protein cartilage link protein 1 in the developing murine heart. PLoS ONE. 2013;8:e57073. doi: 10.1371/journal.pone.0057073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong Z, Zeng X-L, Ni J-H, Huang X-F. Comparison of the biological response of osteoblasts after tension and compression. Eur J Orthodont. 2013;35:59–65. doi: 10.1093/ejo/cjr016. [DOI] [PubMed] [Google Scholar]
- 35.Woods A, Wang G, Beier F. Rhoa/rock signaling regulates sox9 expression and actin organization during chondrogenesis. J Biol Chem. 2005;280:11626–11634. doi: 10.1074/jbc.M409158200. [DOI] [PubMed] [Google Scholar]
- 36.Kumar D, Lassar AB. The transcriptional activity of sox9 in chondrocytes is regulated by rhoa signaling and actin polymerization. Mol Cell Biol. 2009;29:4262–4273. doi: 10.1128/MCB.01779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikegami D, Akiyama H, Suzuki A, Nakamura T, Nakano T, Yoshikawa H, Tsumaki N. Sox9 sustains chondrocyte survival and hypertrophy in part through pik3ca-akt pathways. Development. 2011;138:1507–1519. doi: 10.1242/dev.057802. [DOI] [PubMed] [Google Scholar]
- 38.Leung VYL, Gao B, Leung KKH, Melhado IG, Wynn SL, Au TYK, Dung NWF, Lau JYB, Mak ACY, Chan D, Cheah KSE. Sox9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet. 2011;7:e1002356. doi: 10.1371/journal.pgen.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dy P, Wang W, Bhattaram P, Wang Q, Wang L, Ballock RT, Lefebvre V. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell. 2012;22:597–609. doi: 10.1016/j.devcel.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartman C. Canonical wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between sox9 and β-catenin control chondrocyte differentiation. Genes Dev. 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of β-catenin in xy gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: Not simply a degenerative process: A review and agenda for research from the national heart and lung and blood institute aortic stenosis working group executive summary: Calcific aortic valve disease – 2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kyndt F, Gueffet J-P, Probst V, et al. Mutations in the gene encoding filamin a as a cause for familial cardiac valvular dystrophy. Circulation. 2007;115:40–49. doi: 10.1161/CIRCULATIONAHA.106.622621. [DOI] [PubMed] [Google Scholar]
- 46.Nesta F, Leyne M, Yosefy C, Simpson C, Dai D, Marshall JE, Hung J, Slaugenhaupt SA, Levine RA. New locus for autosomal dominant mitral valve prolapse on chromosome 13: Clinical insights from genetic studies. Circulation. 2005;112:2022–2030. doi: 10.1161/CIRCULATIONAHA.104.516930. [DOI] [PubMed] [Google Scholar]
- 47.Padang R, Bagnall RD, Semsarian C. Genetic basis of familial valvular heart disease. Circ Cardiovasc Genet. 2012;5:569–580. doi: 10.1161/CIRCGENETICS.112.962894. [DOI] [PubMed] [Google Scholar]
- 48.Orton EC, Lacerda CMR, MacLea HB. Signaling pathways in mitral valve degeneration. J Vet Cardiol. 2012;14:7–17. doi: 10.1016/j.jvc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.