Abstract
Objective
The impact of leukotriene production by the 5-Lipoxygenase (5-LO) pathway in the pathophysiology of Abdominal Aortic Aneurysms (AAA) has been debated. Moreover, a clear mechanism through which 5-LO influences AAA remains unclear.
Approach and Results
Aneurysm formation was attenuated in 5-LO−/− mice, and in lethally irradiated WT mice reconstituted with 5-LO−/− bone marrow in an elastase perfusion model. Pharmacologic inhibition of 5-LO attenuated aneurysm formation in both aortic elastase perfused WT and angiotensin II treated LDLr−/− mice, with resultant preservation of elastin and fewer 5-LO and MMP9 producing cells. Separately, analysis of WT mice 7 days after elastase perfusion showed that 5-LO inhibition was associated with reduced polymorphonuclear leukocyte infiltration to the aortic wall. Importantly, 5-LO inhibition initiated 3 days after elastase perfusion in WT mice arrested progression of small AAA. Human AAA and control aorta corroborated these elastin and 5-LO expression patterns.
Conclusions
Inhibition of 5-LO by pharmacologic or genetic approaches attenuates aneurysm formation and prevents fragmentation of the medial layer in two unique AAA models. Administration of 5-LO inhibitor in small AAA slows progression of AAA. Targeted interruption of the 5-LO pathway is a potential treatment strategy in AAA.
Keywords: Aneurysm, Aorta, Inflammation, Immune system, Leukotriene
INTRODUCTION
Abdominal aortic aneurysms (AAA) remain an important cause of cardiovascular mortality, and is the 15th leading cause of death from any cause in men over age 55 1, 2. AAA is a multi-factorial disease associated with aging, smoking and hypertension, where chronic inflammation in the aortic wall, and protease-mediated degradation of structural matrix proteins contribute to aortic dilatation and rupture 3, 4. Surgery is the only established treatment as there are currently no medical therapies to delay the onset or prevent AAA. Pharmacological approaches that slow aneurysm growth by 50% could delay surgery by more than 5 years and will also reduce risk of potentially fatal rupture 5. Thus, understanding the pathogenesis of AAA is critical to developing novel therapies.
Leukotrienes (LT) mediate inflammatory responses in various cardiovascular diseases 6–9 such as atherosclerosis and aortic valve disease 10. 5-Lipoxygenase (5-LO) is the key enzyme in LT biosynthesis 11, catalyzing the initial steps in the conversion of arachidonic acid (AA) to the unstable leukotriene precursor, LTA412, 13. The efficient utilization of endogenous AA by 5-LO requires a helper protein, 5-lipoxygenase activating protein (FLAP).
To date, studies investigating the role of 5-LO in AAA have been equivocal. Zhao et al initially reported a functional role of the 5-LO pathway in aneurysm formation 14 and found that 5-LO/ApoE−/− mice fed a cholate-rich diet had a reduced incidence of aortic aneurysms. Consistent with this, BLT1/ApoE−/− mice that lack the high affinity LTB4 receptor to 5-LO exhibited attenuated aneurysm formation in an Angiotensin II (Ang II) infusion model 15. A similar effect was reproduced using a BLT antagonist in the same model, and was associated with attenuation in aortic wall infiltration by macrophages 16. Expressions of ALOX5 and ALOX5AP, the genes encoding 5-LO and FLAP respectively, have been reported to be higher in human AAA compared to control aorta 10. Furthermore, LTB4 production by polymorphonuclear leukocytes (PMNL) appears to be elevated in patients undergoing aneurysmectomy 10, 17, 18. However in epidemiologic studies, the seven known single nucleotide polymorphisms (SNPs) of ALOX5AP were not associated with human AAA 19, suggesting the lack of a strong genetic association between the 5-LO pathway and AAA. Moreover aneurysm formation and aortic wall inflammation was not attenuated in Ang II infused hyperlipidemic 5-LO/ApoE−/− mice or in ApoE−/− mice treated with a FLAP inhibitor 20. Collectively, these data suggest an unclear role of this pathway in the pathogenesis of aortic aneurysm formation.
As a result of the critical role of 5-LO in inflammation, a number of compounds targeting the 5-LO pathway have been developed 21. To resolve the debate of the role of 5-LO in AAA, we used genetic and pharmacological approaches in two distinct mouse models of AAA, and assessed the impact of 5-LO inhibition in AAA initiation as well as in treatment of small, developing AAA. We tested the hypothesis that 5-LO is critical to aneurysm formation, and that interfering with 5-LO pathway would reduce aneurysm formation. To this end, we aimed to comprehensively investigate the involvement of the 5-LO pathway in experimental AAA progression, and to explore the relevance of the 5-LO pathway to human disease.
METHODS
Please see supplemental files.
RESULTS
Genetic loss or pharmacologic inhibition of 5-LO attenuated aneurysm formation in an elastase perfusion model
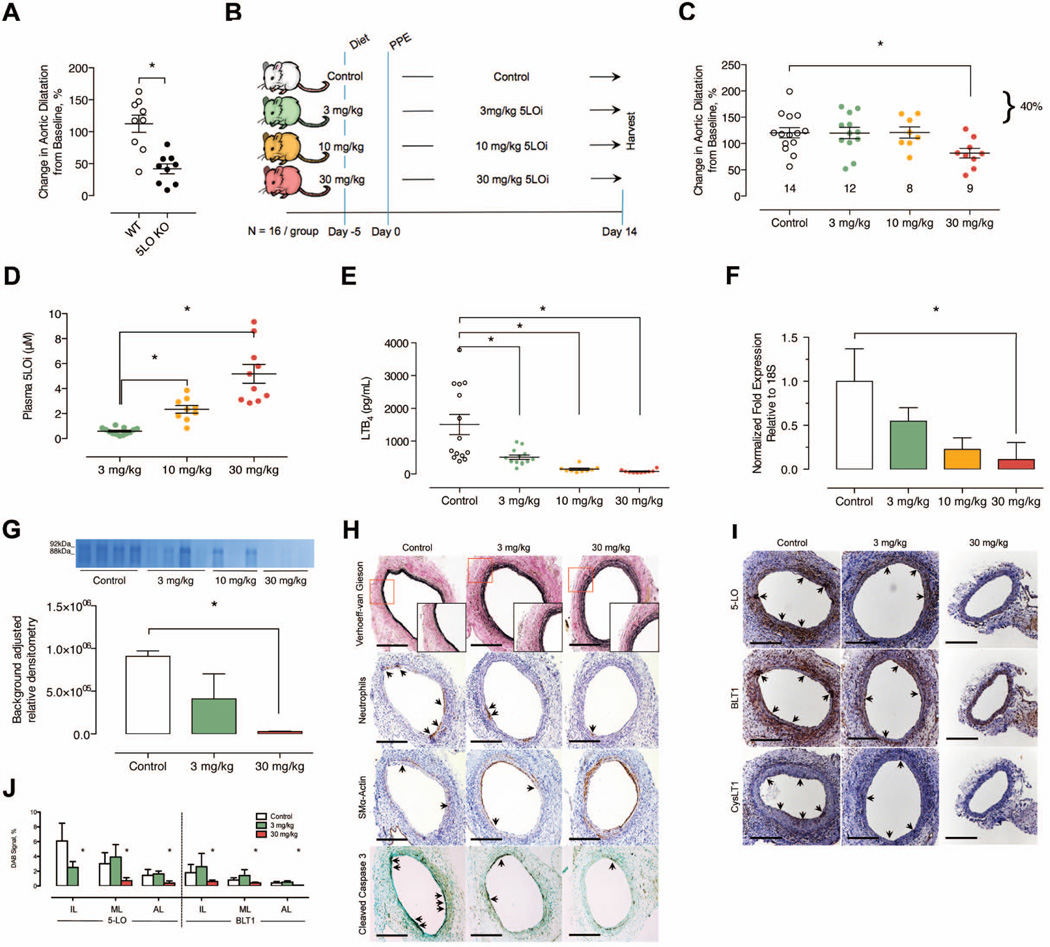
To evaluate whether 5-LO is involved in experimental aneurysm progression, we first compared aneurysm formation in WT and 5-LO−/− mice using the aortic elastase perfusion model. Genetic deletion of 5-LO resulted in a 71% reduction in aortic dilatation compared to WT controls at day 14 (WT: 113±14% [N=9] vs. 5-LO−/−: 42±8% [N=9], P < 0.05) (Figure 1A). To further assess the role of the 5-LO pathway in AAA subsequent studies were performed using an oral and highly selective 5-LO inhibitor (AZD4407, Supplementary Table I, Figure 1B). Aneurysm formation was attenuated in the highest dose group (30 mg/kg/d) indicating that a high level of 5-LO inhibition is required for a phenotypic effect (Figure 1C). A dose dependent increase in plasma compound exposure and inhibition of LTB4 production in blood was observed when mice were administered oral AZD4407 at 3, 10 and 30 mg/kg/d (Figures 1D and 1E), and was associated with a dose-dependent reduction in ALOX5 gene expression and MMP-9 enzymatic activity in aortic tissue at day 14 (Figures 1F and 1G). Consistent with findings in Figure 1C, histological examination of aortic tissue sections indicated that there was less destruction of the elastic lamellae in mice treated with 30 mg/kg/d AZD4407 compared to controls or mice treated with lower doses (Figure 1H). Immune cell infiltration was also lower at day 14 in this high dose group (Figure 1H and Supplementary Figure IA). Expression of the SMC marker smooth muscle α-Actin was dose-dependently preserved with greater 5-LO inhibition, and was associated with concomitant reductions in cleaved caspase-3 (Figure 1H). Expression of the LT receptors BLT1, and CysLT1, were also dose dependently reduced in response to 5-LO inhibition (Figures 1I and IJ). Taken together, these data suggest that 5-LO activity is involved in aneurysm formation, and in part influences elastin morphology and MMP9 protease activity through LT production.
Figure 1.
Comparison using the elastase perfusion model in WT versus 5-LO−/− mice. (A) In situ video micrometry of infrarenal aortic dilatation in mice compared to their baseline aortic diameter post perfusion (N=9 per group). *P < 0.05 versus control by ANOVA. Effects of oral (dietary) inhibition of 5-LO in the elastase perfusion model. (B) Experimental design. (C) In situ video micrometry of infrarenal aortic dilatation in mice compared to their baseline aortic diameter post perfusion (N=16 per group). *P < 0.05 versus control by ANOVA. (D) Plasma 5-LO inhibitor levels measured at day 14 by LC-MS/ MS. *P < 0.05 pair-wise comparison versus control. (E) Ex vivo whole blood stimulated LTB4 production determined by EIA at day 14. *P < 0.05 pair-wise comparison versus control. (F) mRNA transcript levels of ALOX5 in the aortic wall of elastase perfused mice. *P < 0.05 pair-wise comparison versus control. (G) Background adjusted relative densitometry of MMP9 in the aorta of elastase-perfused mice. *P < 0.05 versus control. (H) Representative histology and IHC stains exhibiting elastin fragmentation, immune cell infiltration, apoptosis, and smooth muscle cell marker expression in the aorta of elastase perfused mice. (I) Representative IHC exhibiting 5-LO pathway protein expression in the aorta of elastase perfused mice. (J) Relative quantification of 5-LO and BLT1r proteins in the intimal layer (IL), medial layer (ML), and adventitial layer (AL) of the aorta from elastase perfused mice. *P < 0.05 pair-wise comparison versus control.
5-LO inhibition attenuated aneurysm formation in Ang II infused hypercholesterolemic LDLr−/− mice
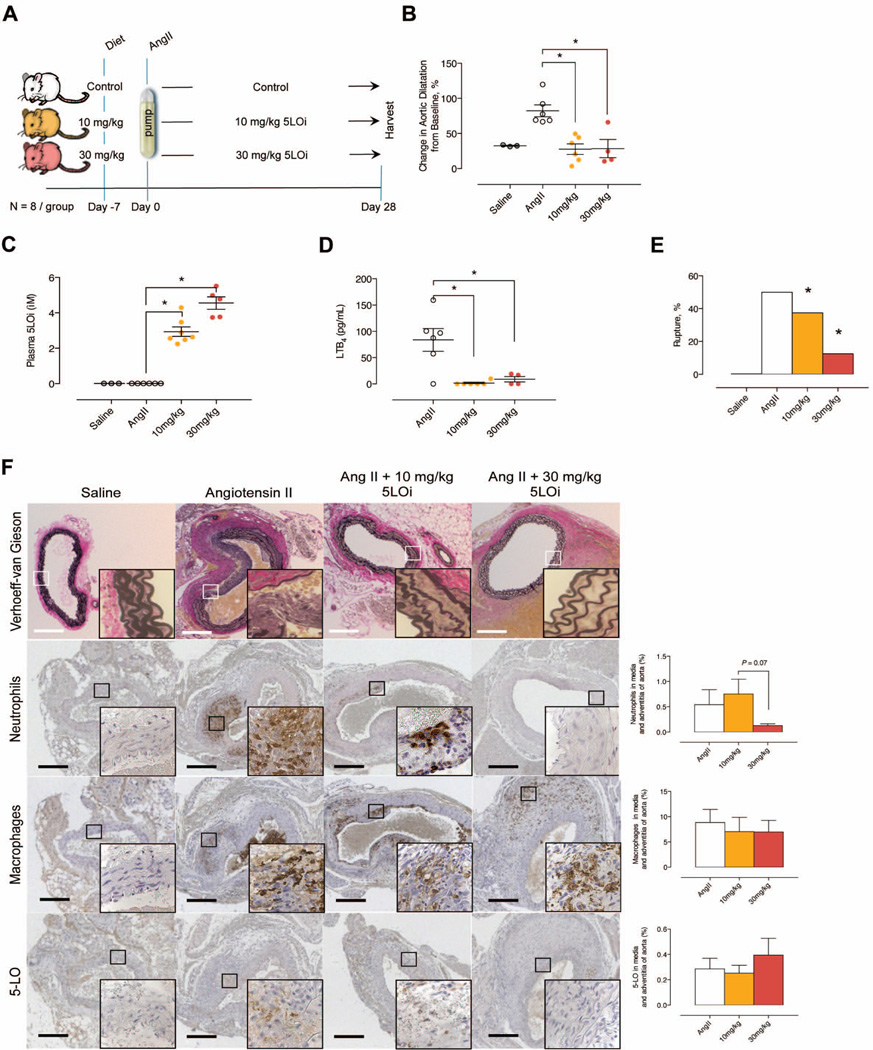
We sought to confirm our results in a second model, and therefore we investigated the effect of 5-LO inhibition using LDLr−/− mice infused with Ang II for 28 days (Figure 2A) 22–24. LDLr−/− mice infused with Ang II and fed control chow had an aortic diameter of 1.51±0.16mm, those fed 10 mg/kg/d AZD4407 diet had a diameter of 1.10±0.13mm, while mice fed 30 mg/kg/d AZD4407 diet had a diameter of 1.03±0.26mm at day 28 (Figure 2B). Administration of 10 and 30 mg/kg/d of AZD4407 in the chow for 28 days inhibited 5-LO activity in the circulation in part leading to a 54% reduction in aneurysm formation (Figures 2C and 2D). The incidence of aneurysms was 100%, 0% and 25% in control, 10 mg/kg/d AZD4407, and 30 mg/kg/d AZD4407 groups, respectively (P < 0.05, defined by >50% increase in aortic size from baseline 25). Mortality at day 28 was 5.4%, 2.6%, and 6.1% in the control, 10 mg/kg/d AZD4407, and 30 mg/kg/d AZD4407 groups. The incidence of mortality occurred principally between days 5 and 7 following Ang II infusion as has been demonstrated in other studies, and necropsy demonstrated suprarenal AAA rupture. Treatment with AZD4407 prevented aortic rupture (Figure 2E) and preserved elastin morphology in the media compared with controls (Figure 2F). In addition, 5-LO inhibition decreased PMNL in the media and adventitia of the suprarenal aorta compared to control aneurysms (Figure 2F). However, there was no difference in Mac2 or 5-LO positive cells at day 28 (Figures 2F). Plasma cytokine levels at day 28 were also similar, as were aortic wall BLT1 and CysLT1 receptor expression levels (Supplementary Figures IIE and IIF). The reduction in aneurysm size was independent of an effect on systemic blood pressure or plasma levels of cholesterol, triglycerides and HDL (Supplementary Figures IIA through IID). Collectively these data suggest that the 5-LO pathway is critical to experimental AAA formation, and that the 5-LO axis is in part regulated via aortic wall PMNLs.
Figure 2.
Effects of oral (dietary) inhibition of 5-LO in the Ang II infusion model. (A) Experimental design. (B) In situ video micrometry of suprarenal aortic dilatation in mice compared to their baseline aortic diameter (N=8 per group). *P < 0.05 pair-wise comparison versus control. (C) Plasma 5-LO inhibitor levels measured at day 28 by LC-MS/ MS. *P < 0.05 pair-wise comparison versus control. (D) Ex vivo whole blood stimulated LTB4 production determined by EIA at day 28. *P < 0.05 pair-wise comparison versus control. (E) Proportion of mice with ruptured aortic aneurysms. *P < 0.05 pair-wise comparison versus control. (F) Representative histology sections exhibiting elastin fragmentation, IHC stains and quantification exhibiting PMNL and macrophage infiltration, along with 5-LO positive cells in the aorta of Ang II infused hypercholesterolemic mice.
Bone marrow derived 5-LO contributes to aneurysm formation
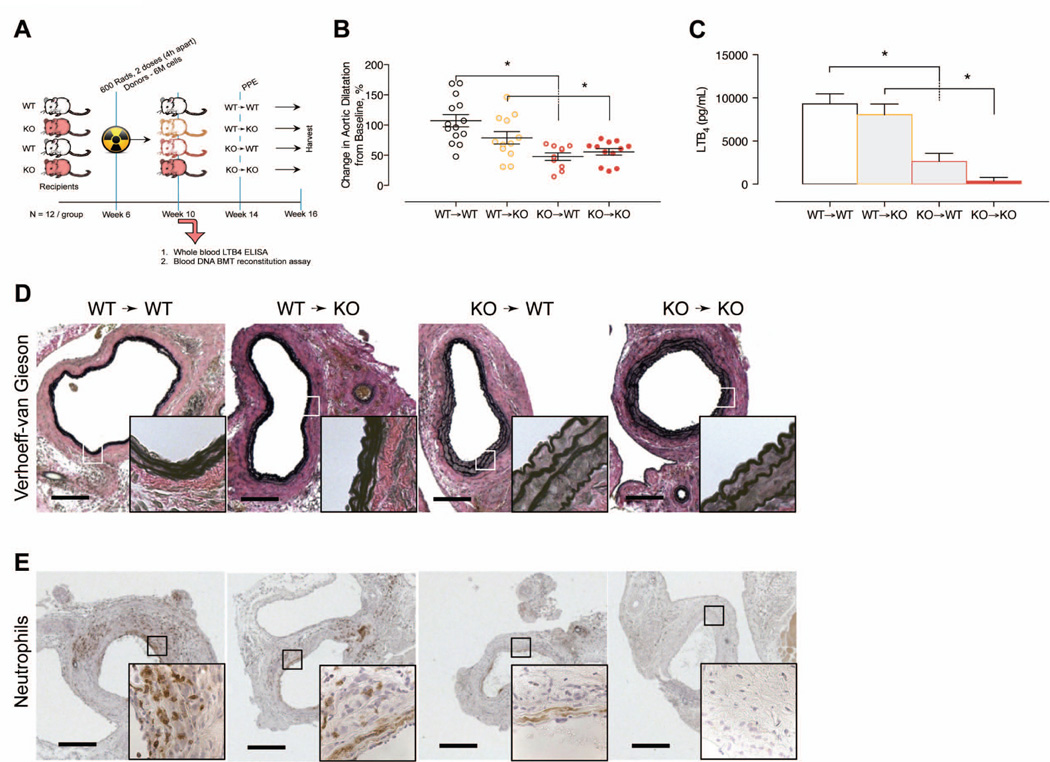
Based on the predominant expression of 5-LO in leukocytes, we postulated that 5-LO activity from bone marrow derived cells plays an important role in aneurysm formation. To address this hypothesis, we performed elastase perfusion in chimeric mice following bone marrow transplantation from 5-LO−/− or WT donors to both 5-LO−/− and WT recipients (Figure 3A). Transplantation of 5-LO−/− bone marrow cells attenuated aneurysm formation in both 5-LO−/− and WT recipients, whereas aneurysm formation was unaffected in recipients that received bone marrow cells from WT donors (Figure 3B and 3C). Breakdown of elastin in the media was significantly attenuated in mice that received 5-LO−/− bone marrow cells (Figure 3D)26. Despite similar plasma cytokine levels at day 14 among groups (Supplementary Figure IIIA), immunohistochemistry indicated that PMNL infiltration to the aorta, and aortic wall BLT1 and CysLT1 receptor expression was suppressed in mice receiving 5-LO−/− cells (Figure 3E and Supplementary Figure IIIB). Thus, these data suggest that 5-LO activity in myeloid cells plays a predominant role in aneurysm formation in experimental AAA.
Figure 3.
Effects of adoptive transfer bone marrow transplantation of 5-LO. (A) Experimental design. (B) In situ video micrometry of infrarenal aortic dilatation in mice compared to their baseline aortic diameter (N=12 per group). *P < 0.05 pair-wise comparison versus control. (C) Ex vivo whole blood stimulated LTB4 production determined by EIA 4 weeks following adoptive transfer of bone marrow cells. *P < 0.05 pair-wise comparison versus control. (D) Representative histology sections exhibiting elastin fragmentation in the aorta of elastase perfused mice. (E) Representative IHC stains exhibiting PMNL infiltration in the aorta of elastase perfused mice.
5-LO inhibition attenuates PMNL infiltration in the aortic wall early in aneurysm formation
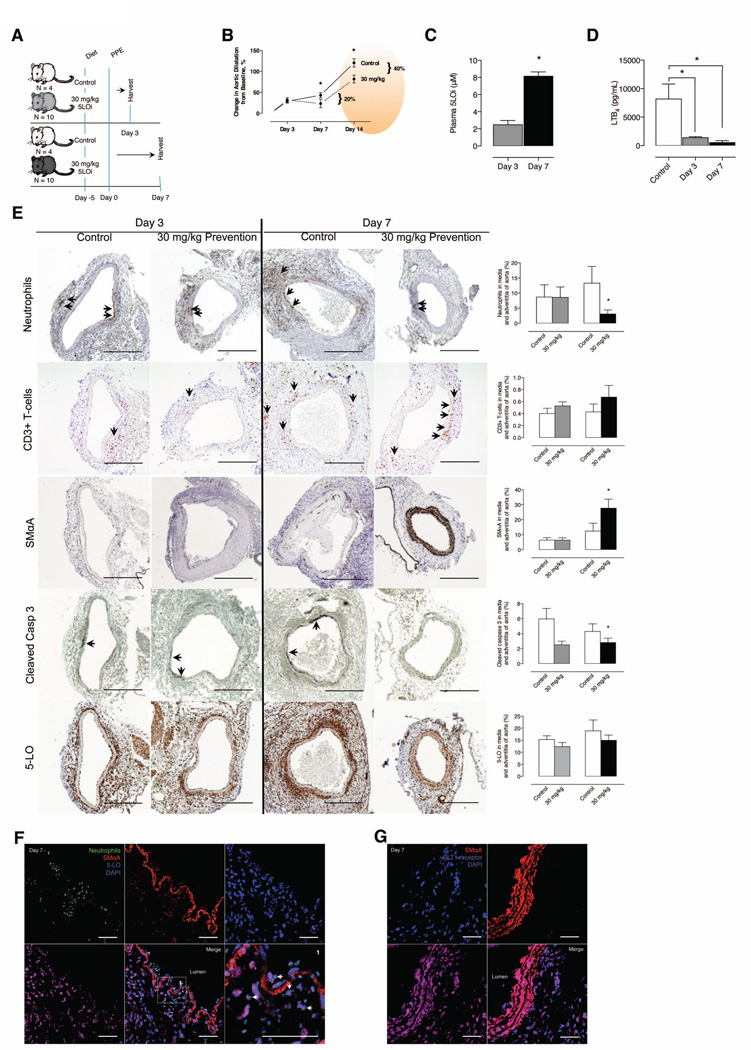
To study the contribution of 5-LO activity early in aneurysm formation, we analyzed aortas at days 3 and 7 following elastase perfusion from mice pretreated with 30 mg/kg/d AZD4407 (Figure 4A). Mice treated with AZD4407 had a 20% reduction (AZD4407 treated: 23.16% above baseline versus control: 43.18% above baseline) in aortic size at day 7 after elastase perfusion (Figure 4B). PMNL infiltration in the aortic media was similar at day 3 between controls and treated mice, but day 7 was lower in 5-LO inhibitor treated mice (Figure 4E). Mac2 positive cells increased from day 3 to day 7 but did not differ between groups (Figure 4E). SMC marker protein smooth muscle α-Actin was significantly higher in treated mice on day 7 indicating preservation of SMC phenotype (Figure 4E). Levels of cleaved caspase-3 in the media were higher in controls on day 3, suggesting more apoptosis in control mice (Figure 4E). 5-LO expression was similar in both controls and treated mice at days 3 and 7 and co-localized primarily with PMNLs (Figure 4F) but not with Mac2 positive cells (data not shown), while expression of BLT1 co-localized with the SMC rich medial elastic lamellae (Figure 4G). Expression of CysLT1 also co-localized with the SMC medial layer (data not shown). Collectively, these data indicate that 5-LO activity could have a prominent effect on PMNL infiltration and SMC phenotype in the early stages of aneurysm formation.
Figure 4.
Time course evaluation of effects of oral (dietary) inhibition of 5-LO in the elastase perfusion model. (A) Experimental design (Controls, N=4 per group and Comparison, N=10 per group). (B) In situ video micrometry of infrarenal aortic dilatation in mice compared to their baseline aortic diameter post perfusion. The day 14 data from Figure 1 (orange ellipse) is used to contextualize the continued aortic dilatation seen in mice in the elastase perfusion model. (C) Plasma 5-LO inhibitor levels measured at days 3 and 7 by LC-MS/ MS. *P < 0.05 pair-wise comparison versus control. (D) Ex vivo whole blood stimulated LTB4 production determined by EIA at days 3 and 7. *P < 0.05 pair-wise comparison versus control. (E) Representative IHC stains and quantification exhibiting immune cell infiltration, smooth muscle cell marker expression, apoptosis and 5-LO protein expression at day 3 and 7 of harvest in the aorta of elastase perfused mice. *P < 0.05 pair-wise comparison versus control. (F) Representative confocal IHC stains exhibit luminal PMNL infiltration into the aorta of control mice at day 7. (G) Representative confocal IHC stains exhibit BLT1r and smooth muscle cell colocalization in the aorta of control mice at day 7.
5-LO inhibition in small aneurysms reduced progression
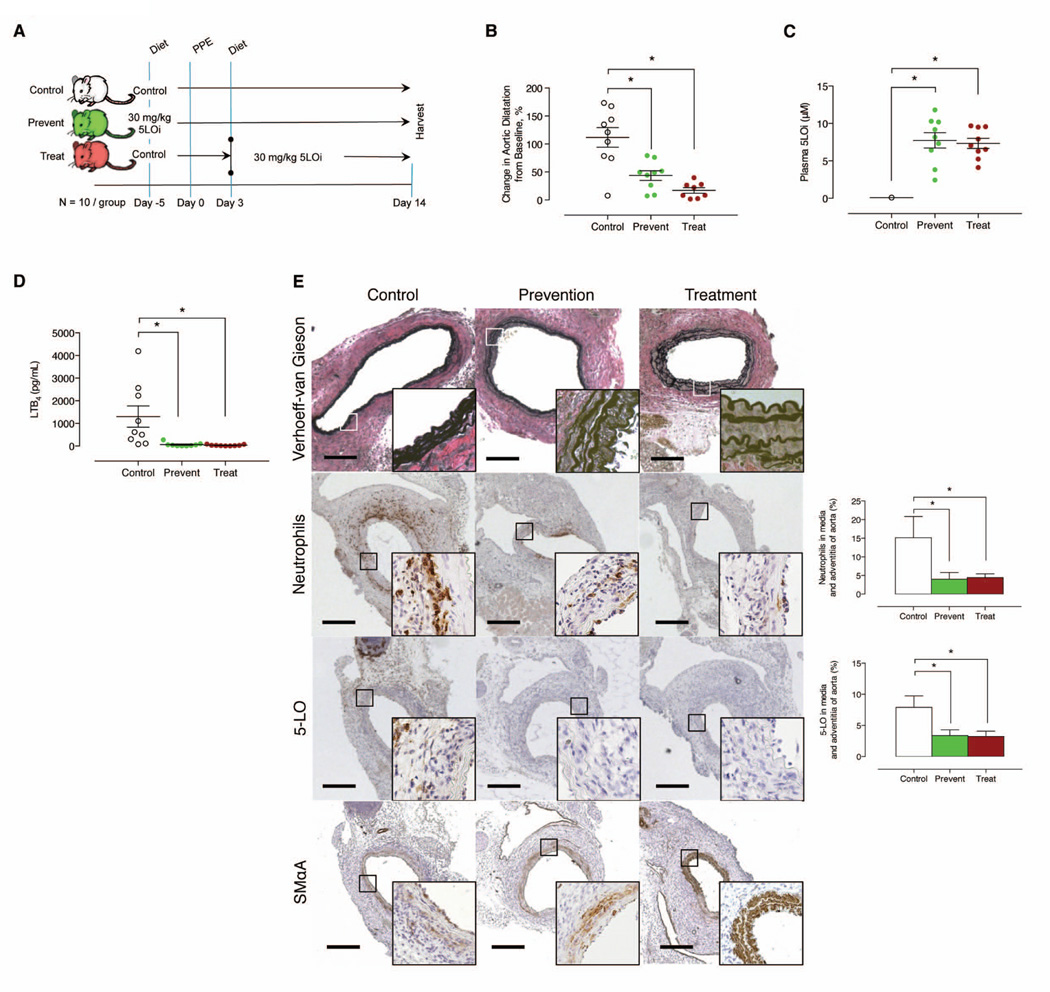
To determine if 5-LO inhibition could be used as a treatment strategy to attenuate aneurysm growth, we compared the effect of 30 mg/kg/d AZD4407 administered 5 days prior to elastase (“prevention”) to mice treated 3 days after elastase (“treatment”) (Figure 5A). AZD4407 attenuated aneurysm development in both the prevention and treatment groups by day 14 (Figure 5B), and was associated with preservation of the elastin layers in the aortic media and attenuation of PMNL infiltration (Figures 5E). Plasma cytokine IL-1β and TNFα were lower in mice that received AZD4407 (Supplementary Figure IVA). In addition, 5-LO positive cells, and aortic wall BLT1 receptor expression were lower in mice that received AZD4407 (Figure 5E and Supplementary Figure IVB). Medial layer preservation was also observed among both groups of mice that received AZD4407 (Figure 5E). While macrophage infiltration was equivalent at day 14 after elastase perfusion, cleaved caspase-3 expression was reduced in mice when 5-LO was inhibited (Supplementary Figure IVB). These data suggest that 5-LO could be targeted in small AAA and is associated with reduced PMNL infiltration and preserved SMCs.
Figure 5.
Effects of prophylactic and therapeutic oral (dietary) inhibition of 5-LO in the elastase perfusion model. (A) Experimental design. (B) In situ video micrometry of infrarenal aortic dilatation in mice compared to their baseline aortic diameter post perfusion (N=10 per group). *P < 0.05 pair-wise comparison versus control. (C) Plasma 5-LO inhibitor levels measured at day 14 by LC-MS/ MS. *P < 0.05 pair-wise comparison versus control. (D) Ex vivo whole blood stimulated LTB4 production determined by EIA at day 14. *P < 0.05 pair-wise comparison versus control. (E) Representative histology exhibiting elastin fragmentation, IHC stains and quantification exhibiting PMNL and macrophage infiltration, and smooth muscle cell marker expression in the aorta of elastase-perfused mice. *P < 0.05 pair-wise comparison versus control.
ALOX5 expression is reduced in advance stage human aneurysms
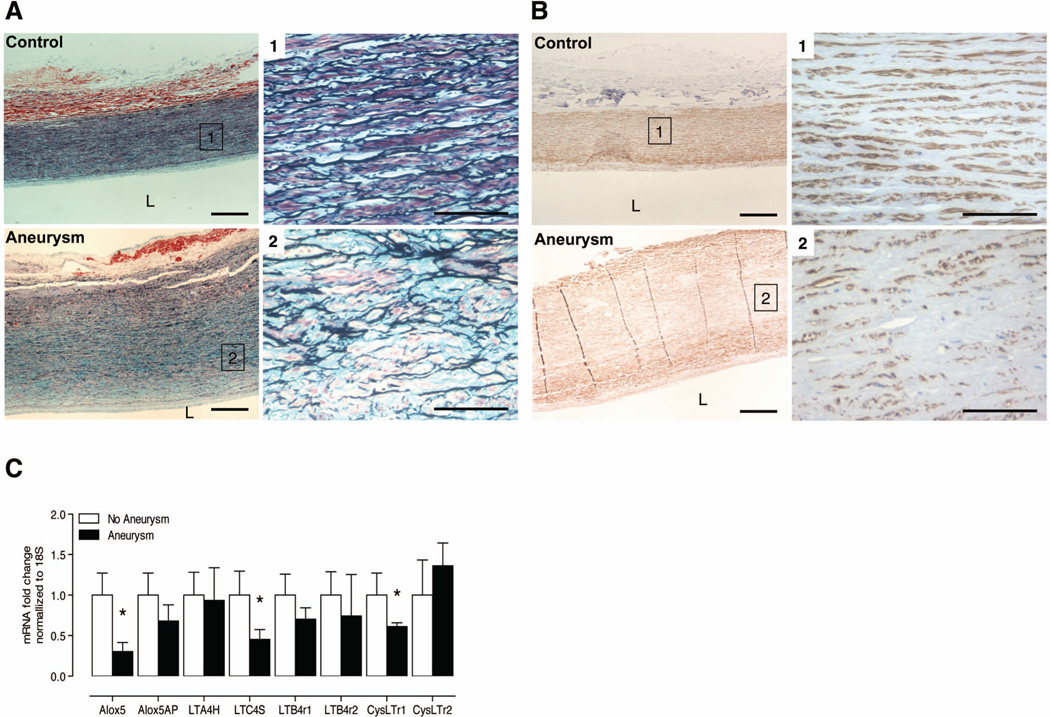
Movat staining from the intima, medial, and adventitial layers of the aortic wall in patients with AAA (Figures 6A) exhibited significant elastin loss compared to control aorta. Smooth muscle α-actin expression was also markedly lower in patients with AAA (Figures 6B). We observed very few PMNLs and Mac2 positive cells in the aortic wall of patients with AAA (data not shown). Interestingly, we observed significantly higher ALOX5 expression in healthy control aorta compared to late stage AAA (Figure 6C).
Figure 6.
Late stage human aneurysms and expression of genes encoding 5-LO pathway proteins. (A) Representative Movat staining in healthy control aorta (N=9 per group). Panels 1 and 2 are 40X magnifications of select areas from the 10X pictomicrograph. (B) Representative smooth muscle cell marker expression in human samples with clinically diagnosed AAA. Panels 1 and 2 are 40X magnifications of select areas from the 10X pictomicrograph. (C) Relative fold change of mRNA transcripts, normalized to 18S, of 5-LO pathway protein encoding genes in non-AAA and AAA human samples. *P < 0.05 versus patients without clinically diagnosed AAA.
DISCUSSION
Previous studies investigating the significance of 5-LO in aortic aneurysm formation have been equivocal. The current study is the first to comprehensively examine the 5-LO pathway by using both genetic and pharmacologic approaches to disrupt the 5-LO pathway in two complementary murine models. Our studies demonstrate this pathway is critical to experimental AAA formation and that disruption of 5-LO is associated with preservation of the elastic lamina, enhanced SMC marker smooth muscle α-Actin expression and reductions in neutrophil count and MMP9 levels in the aortic media. Bone marrow chimeric studies indicate that 5-LO in myeloid cells determine aneurysm progression while confocal studies suggest that neutrophils are an important source for 5-LO. Collectively, these data provide strong evidence that 5-LO inhibition is a potential treatment strategy for AAA disease.
Previous studies demonstrated that AAA is attenuated in ALOX5 LDLr−/−, and ALOX5 ApoE−/− fed an atherogenic diet containing cholate, an effect that was associated with a reduction in MIP-1α and MIP-2 inflammatory signaling 14. Subsequent observations reported that both BLT1 ApoE−/− mice as well as BLT receptor antagonist had attenuated AAA formation following Ang II infusion for 28 days 15, 16. In contrast, separate studies reported that AAA formation was not affected in ApoE ALOX5−/− mice nor in ApoE−/− mice treated with FLAP inhibitor (MK-0591) following AngII infusion20.
Our findings differ from previous investigations potentially due to differences in the experimental strategies. We used LDLr−/− mice fed a hyperlipidemic diet and infused Ang II at 1000 ng/kg/min, while Cao et al. used ApoE−/− mice fed a normal chow diet and infused Ang II at 500 ng/kg/min. It is possible that different genetic backgrounds could have an impact on the inflammatory response associated with aneurysm formation and the expression of the 5-LO pathway in the aorta. One consequence of using a lower dose of Ang II in the Cao study was that the incidence of aneurysm formation was significantly lower than in our study (30% versus 100% in controls). Potentially, this low incidence of AAA in their study could have hidden any effect that 5-LO deletion or FLAP inhibition had on aneurysm phenotype. With respect to the different pharmacological approaches used, we observed >90% inhibition of LTB4 production in the blood of mice treated with AZD4407 whereas Cao et al. reported 50% inhibition of LTB4 production using MK-0591. Our studies indicate that a high level of 5-LO inhibition is required to achieve a protective effect on aneurysm phenotype and it is possible that the level of inhibition achieved by others was inadequate. The high level of 5-LO inhibition in blood needed to reduce aneurysm progression may relate to adequate exposure of compound within the aorta in order to inhibit 5-LO activity. This could be particularly important since it has been observed that the potency of some non-redox 5-LO inhibitors can be impaired in tissues 27, 28. Supportive of this potential explanation is the fact that SMC marker smooth muscle α-Actin expression, MMP9 levels, and 5-LO pathway gene expression in the aorta were dose-dependently affected by 5-LO inhibition.
Myeloid cells are known to be one of the sources of the 5-LO pathway. Our bone marrow chimera experiments demonstrated that 5-LO activity in myeloid cells is critical to aneurysm formation. Moreover, we demonstrated that 5-LO expression is predominantly associated with PMNLs in the aortic wall early in AAA development. However, in contrast to earlier reports 14, 20 29, we did not demonstrate co-localization with macrophages. Prior studies have demonstrated that neutrophil counts are elevated in human AAA 17, 30, 31 and have suggested a pathological role for neutrophils in AAA 32–37. Since LTB4 is a potent chemokine for neutrophils and can also promote neutrophil survival by inhibiting apoptosis 38, the reduction in PMNL following 5-LO inhibition could be explained by reduced infiltration and/or decreased survival of neutrophils in the aortic wall. This reduction in neutrophil number between day 3 and 7 could be pivotal to the mechanism by which 5-LO inhibition attenuates aneurysm progression. SMC marker α-Actin expression was reduced on day 3 in both control mice and those treated with the 5-LO inhibitor. However, by day 7 it appeared that SMC marker α-Actin was again re-expressed in mice that had received the 5-LO inhibitor. This intriguing and novel finding suggests that SMC phenotype is reversible and that 5-LO inhibition can revert SMCs back to healthy state. Whether this effect could be explained by a direct action of leukotrienes on vascular SMCs or through an alternative mechanism linked to fewer neutrophils in the aneurysm cannot be deduced from our data but is worthy of further investigation. We also demonstrated a dose dependent reduction in MMP9 expression with 5-LO inhibition suggesting yet another mechanism by which 5-LO inhibition could attenuate aneurysm progression.
A clinically relevant finding in this study is that 5-LO inhibition could attenuate aneurysm progression of small or developing AAA. The effect of 5-LO inhibition on aneurysm phenotype was similar whether 5-LO inhibition was initiated prior to elastase perfusion or after elastase in small AAA, indicating that 5-LO plays a prominent physiological role in aneurysm progression. We initially chose day 3 to start the treatment arm based on time course studies that suggested that inflammatory changes occur prominently during this post-surgical period in the elastase model. However, significant aortic dilation does not proceed until around day 7. To improve possible translation to the clinic, it will be important to assess whether 5-LO inhibition can have therapeutic effects on established aneurysms, by evaluating treatment initiated after 14 or 28 days and analyzing the aneurysm phenotype at later time points. The rapid formation of AAA in both the elastase perfusion and Ang II models have always been a limitation in the translation to human AAA yet have provided important insights to our understanding of the disease. Since there is evidence that the inflammatory response is attenuated late in experimental and human aneurysm disease, it is not certain that 5-LO will play an active role in aneurysm progression in advanced AAA. In addition to producing pro-inflammatory leukotrienes, the 5-LO pathway is involved in the transcellular formation of lipoxins that can mediate inflammatory resolution. By targeting 5-LO activity early in experimental models, our current studies have primarily addressed the impact of the pro-inflammatory effects of this pathway on aneurysm formation. Future studies should also address whether treatment with a 5-LO inhibitor could have an impact on inflammatory resolution in experimental aneurysms and evaluate the effect that this could have on aneurysm phenotype.
The primary long-term objective of our studies is to determine if 5-LO inhibition could be a therapeutic strategy to treat AAA progression in humans. There is currently no approved medication for AAA, and the small number of clinical trials that have been performed have failed to identify significant effects of existing medications on aneurysm progression 39. We have demonstrated that the 5-LO pathway is highly relevant early in experimental AAA using two different mouse models. Moreover, myeloid derived 5-LO is critical in neutrophil recruitment in the aortic wall of early AAA. Given that the 5-LO pathway and inflammation is attenuated in late stage human and experimental AAA, our findings suggest the 5-LO pathway could be targeted early in AAA to prevent aneurysm progression.
Supplementary Material
SIGNIFICANCE.
Abdominal aortic aneurysm (AAA) is a top-20 cause of mortality overall, and is the 15th leading cause of death in men over the age of 55. Surgery is the only established treatment as there are currently no medical therapies to delay the onset or prevent AAA. To this end, the current study is the most comprehensive and first to examine the 5-LO pathway by using both genetic and pharmacologic approaches to disrupt this pathway in two complementary murine models in the study of AAA. These data demonstrate that this pathway is critical to experimental AAA formation and that disruption of 5-LO is associated with preservation of the elastic lamina, enhanced SMC marker smooth muscle α-Actin expression and reductions in neutrophil count and MMP9 levels in the aortic media. Collectively these data provide strong evidence that 5-LO inhibition could be utilized as a potential medical therapy for AAA in humans.
ACKNOWLEDGEMENTS
The authors thank Anthony J Herring and Cindy S Dodson (University of Virginia School of Medicine) for their technical assistance with the mouse colonies. The authors also thank Melissa A Marshall and Suseela R Srinivasan (University of Virginia School of Medicine) for their technical assistance with experiments. The authors thank Melissa H Bevard (Histology Core Facility, Robert M Berne Cardiovascular Research Center, University of Virginia School of Medicine) for her technical assistance with the histology and immunohistochemical preparation(s). The authors thank Daniel Karlsson (Cardiovascular Disease Section, Bioscience Department, AstraZeneca R&D, Mölndal, Sweden) for his assistance with the initial dose exposure studies.
SOURCES OF FUNDING
Research support, in part, and salary support provided to Castigliano M Bhamidipati by AstraZeneca, PLC administered by the University of Virginia School of Medicine towards investigations presented in this manuscript. Research support also in part provided to the laboratories of Gilbert R Upchurch Jr. and Gorav Ailawadi by AstraZeneca, PLC administered by the University of Virginia School of Medicine towards investigations presented in this manuscript. The University of Virginia School of Medicine and AstraZeneca, PLC are in an academic-industry cardiovascular research alliance.
NONSTANDARD ABBREVIATIONS AND ACRONYMS
- 5-LO
5-lipoxygenase
- AA
Arachidonic acid
- FLAP
5-lipoxygenase activating protein
- LT
Leukotrienes
Footnotes
DISCLOSURES
All relevant financial disclosures are described herein. The authors have no other conflicts of interest related to the current work.
REFERENCES
- 1.(WISQARS™) CDC. 20 Leading Causes of Death, United States. Injury Prevention & Control: Data & Statistics. 2007 [Google Scholar]
- 2.Murphy SL, Xu JQ, Kochanek KD. Deaths: Final Data for 2010. 2013;61(4) [PubMed] [Google Scholar]
- 3.Thompson RW, Curci JA, Ennis TL, Mao D, Pagano MB, Pham CT. Pathophysiology of abdominal aortic aneurysms: insights from the elastase-induced model in mice with different genetic backgrounds. Ann N Y Acad Sci. 2006;1085:59–73. doi: 10.1196/annals.1383.029. [DOI] [PubMed] [Google Scholar]
- 4.Ailawadi G, Eliason JL, Upchurch GR., Jr Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003;38:584–588. doi: 10.1016/s0741-5214(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 5.Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, Baxter BT. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg. 2002;35:923–929. doi: 10.1067/mva.2002.123757. [DOI] [PubMed] [Google Scholar]
- 6.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 7.Funk CD, Cao RY, Zhao L, Habenicht AJ. Is there a role for the macrophage 5-lipoxygenase pathway in aortic aneurysm development in apolipoprotein E-deficient mice? Ann N Y Acad Sci. 2006;1085:151–160. doi: 10.1196/annals.1383.012. [DOI] [PubMed] [Google Scholar]
- 8.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50:470–479. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 9.Poeckel D, Funk CD. The 5-lipoxygenase/leukotriene pathway in preclinical models of cardiovascular disease. Cardiovasc Res. 2010;86:243–253. doi: 10.1093/cvr/cvq016. [DOI] [PubMed] [Google Scholar]
- 10.Di Gennaro A, Wagsater D, Mayranpaa MI, Gabrielsen A, Swedenborg J, Hamsten A, Samuelsson B, Eriksson P, Haeggstrom JZ. Increased expression of leukotriene C4 synthase and predominant formation of cysteinyl-leukotrienes in human abdominal aortic aneurysm. Proc Natl Acad Sci U S A. 2010;107:21093–21097. doi: 10.1073/pnas.1015166107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radmark O, Samuelsson B. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun. 2010;396:105–110. doi: 10.1016/j.bbrc.2010.02.173. [DOI] [PubMed] [Google Scholar]
- 12.Funk CD. Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nat Rev Drug Discov. 2005;4:664–672. doi: 10.1038/nrd1796. [DOI] [PubMed] [Google Scholar]
- 13.Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 14.Zhao L, Moos MP, Grabner R, Pedrono F, Fan J, Kaiser B, John N, Schmidt S, Spanbroek R, Lotzer K, Huang L, Cui J, Rader DJ, Evans JF, Habenicht AJ, Funk CD. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 15.Ahluwalia N, Lin AY, Tager AM, Pruitt IE, Anderson TJ, Kristo F, Shen D, Cruz AR, Aikawa M, Luster AD, Gerszten RE. Inhibited aortic aneurysm formation in BLT1-deficient mice. J Immunol. 2007;179:691–697. doi: 10.4049/jimmunol.179.1.691. [DOI] [PubMed] [Google Scholar]
- 16.Kristo F, Hardy GJ, Anderson TJ, Sinha S, Ahluwalia N, Lin AY, Passeri J, Scherrer-Crosbie M, Gerszten RE. Pharmacological inhibition of BLT1 diminishes early abdominal aneurysm formation. Atherosclerosis. 2010;210:107–113. doi: 10.1016/j.atherosclerosis.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houard X, Ollivier V, Louedec L, Michel JB, Back M. Differential inflammatory activity across human abdominal aortic aneurysms reveals neutrophil-derived leukotriene B4 as a major chemotactic factor released from the intraluminal thrombus. FASEB J. 2009;23:1376–1383. doi: 10.1096/fj.08-116202. [DOI] [PubMed] [Google Scholar]
- 18.Houard X, Touat Z, Ollivier V, Louedec L, Philippe M, Sebbag U, Meilhac O, Rossignol P, Michel JB. Mediators of neutrophil recruitment in human abdominal aortic aneurysms. Cardiovasc Res. 2009;82:532–541. doi: 10.1093/cvr/cvp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisoendial RJ, Tanck MW, Golledge J, Broekhuizen LN, Legemate DA, Stroes ES, Norman PE. The association between the gene encoding 5-lipoxygenase activating protein and abdominal aortic aneurysms. Atherosclerosis. 2012;220:425–428. doi: 10.1016/j.atherosclerosis.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 20.Cao RY, Adams MA, Habenicht AJ, Funk CD. Angiotensin II-induced abdominal aortic aneurysm occurs independently of the 5-lipoxygenase pathway in apolipoprotein E-deficient mice. Prostaglandins Other Lipid Mediat. 2007;84:34–42. doi: 10.1016/j.prostaglandins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Whatling C, McPheat W, Herslof M. The potential link between atherosclerosis and the 5-lipoxygenase pathway: investigational agents with new implications for the cardiovascular field. Expert Opin Investig Drugs. 2007;16:1879–1893. doi: 10.1517/13543784.16.12.1879. [DOI] [PubMed] [Google Scholar]
- 22.Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor -/- mice. Ann N Y Acad Sci. 1999;892:108–118. doi: 10.1111/j.1749-6632.1999.tb07789.x. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 25.Daugherty A, Cassis L. Angiotensin II-mediated development of vascular diseases. Trends Cardiovasc Med. 2004;14:117–120. doi: 10.1016/j.tcm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 27.Fischer L, Szellas D, Radmark O, Steinhilber D, Werz O. Phosphorylation- and stimulus-dependent inhibition of cellular 5-lipoxygenase activity by nonredox-type inhibitors. FASEB J. 2003;17:949–951. doi: 10.1096/fj.02-0815fje. [DOI] [PubMed] [Google Scholar]
- 28.Werz O, Szellas D, Henseler M, Steinhilber D. Nonredox 5-lipoxygenase inhibitors require glutathione peroxidase for efficient inhibition of 5-lipoxygenase activity. Mol Pharmacol. 1998;54:445–451. doi: 10.1124/mol.54.2.445. [DOI] [PubMed] [Google Scholar]
- 29.Brock TG, Maydanski E, McNish RW, Peters-Golden M. Co-localization of leukotriene a4 hydrolase with 5-lipoxygenase in nuclei of alveolar macrophages and rat basophilic leukemia cells but not neutrophils. J Biol Chem. 2001;276:35071–35077. doi: 10.1074/jbc.M105676200. [DOI] [PubMed] [Google Scholar]
- 30.Folkesson M, Kazi M, Zhu C, Silveira A, Hemdahl AL, Hamsten A, Hedin U, Swedenborg J, Eriksson P. Presence of NGAL/MMP-9 complexes in human abdominal aortic aneurysms. Thromb Haemost. 2007;98:427–433. [PubMed] [Google Scholar]
- 31.Fontaine V, Touat Z, Mtairag el M, Vranckx R, Louedec L, Houard X, Andreassian B, Sebbag U, Palombi T, Jacob MP, Meilhac O, Michel JB. Role of leukocyte elastase in preventing cellular re-colonization of the mural thrombus. Am J Pathol. 2004;164:2077–2087. doi: 10.1016/s0002-9440(10)63766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannawa KK, Eliason JL, Woodrum DT, Pearce CG, Roelofs KJ, Grigoryants V, Eagleton MJ, Henke PK, Wakefield TW, Myers DD, Stanley JC, Upchurch GR., Jr L-selectin-mediated neutrophil recruitment in experimental rodent aneurysm formation. Circulation. 2005;112:241–247. doi: 10.1161/CIRCULATIONAHA.105.535625. [DOI] [PubMed] [Google Scholar]
- 33.Eliason JL, Hannawa KK, Ailawadi G, Sinha I, Ford JW, Deogracias MP, Roelofs KJ, Woodrum DT, Ennis TL, Henke PK, Stanley JC, Thompson RW, Upchurch GR., Jr Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation. 2005;112:232–240. doi: 10.1161/CIRCULATIONAHA.104.517391. [DOI] [PubMed] [Google Scholar]
- 34.Grigoryants V, Hannawa KK, Pearce CG, Sinha I, Roelofs KJ, Ailawadi G, Deatrick KB, Woodrum DT, Cho BS, Henke PK, Stanley JC, Eagleton MJ, Upchurch GR. Tamoxifen up-regulates catalase production, inhibits vessel wall neutrophil infiltration, and attenuates development of experimental abdominal aortic aneurysms. J Vasc Surg. 2005;41:108–114. doi: 10.1016/j.jvs.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Sinha I, Cho BS, Roelofs KJ, Stanley JC, Henke PK, Upchurch GR., Jr Female gender attenuates cytokine and chemokine expression and leukocyte recruitment in experimental rodent abdominal aortic aneurysms. Ann N Y Acad Sci. 2006;1085:367–379. doi: 10.1196/annals.1383.027. [DOI] [PubMed] [Google Scholar]
- 36.Shi GP. Role of cathepsin C in elastase-induced mouse abdominal aortic aneurysms. Future Cardiol. 2007;3:591–593. doi: 10.2217/14796678.3.6.591. [DOI] [PubMed] [Google Scholar]
- 37.Pagano MB, Zhou HF, Ennis TL, Wu X, Lambris JD, Atkinson JP, Thompson RW, Hourcade DE, Pham CT. Complement-dependent neutrophil recruitment is critical for the development of elastase-induced abdominal aortic aneurysm. Circulation. 2009;119:1805–1813. doi: 10.1161/CIRCULATIONAHA.108.832972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrin D, Turcotte S, Gilbert AK, Rola-Pleszczynski M, Stankova J. The anti-apoptotic effect of leukotriene B4 in neutrophils: a role for phosphatidylinositol 3-kinase, extracellular signal-regulated kinase and Mcl-1. Cell Signal. 2006;18:479–487. doi: 10.1016/j.cellsig.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Samson R. Can pharmacologic agents slow abdominal aortic aneurysm growth? Semin Vasc Surg. 2012;25:25–28. doi: 10.1053/j.semvascsurg.2012.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.