Abstract
Purpose
Men with major comorbidities are at risk for overtreatment of prostate cancer due to uncertainty regarding life expectancy. We sought to characterize life expectancy and treatment in a population-based cohort of men with differing ages and comorbidity burdens at diagnosis.
Materials and Methods
We sampled 96,032 men aged 66 and older with early-stage prostate cancer with Gleason scores ≤7 diagnosed during 1991–2007 from the SEER-Medicare database. We calculated cumulative incidence of other-cause mortality and determined treatment patterns among subgroups defined by age and Charlson comorbidity index scores.
Results
Overall, life expectancy was less than 10 years (>50% 10-year other-cause mortality) for 50,049 of 96,032 men (52%). Life expectancy differed by age and comorbidity and was less than 10 years for: men aged 66–69 with Charlson scores 2 and greater; men aged 70–74 with Charlson scores 1 or greater; and all men aged 75–79 and 80+. Among those with less than 10-year life expectancies aged 66–69, 70–74, 75–79, and 80+, treatment was aggressive (surgery, radiation, or brachytherapy) 68%, 69%, 57%, and 24% of the time, respectively. Among these men, aggressive treatment was predominantly radiation therapy (50%, 53%, 63%, 69%) and less frequently surgery (30%, 25%, 13%, 9%). Multivariate models revealed little variation in probability of aggressive treatment by comorbidity within age subgroups, despite substantial differences in mortality.
Conclusions
Men younger than 80 years at diagnosis with <10-year life expectancies are often treated aggressively for low- and intermediate-risk prostate cancer, mostly with radiation therapy.
Keywords: life expectancy, survival, comorbidity, outcomes, prostate adenocarcinoma
Introduction
Men with major comorbidities are at risk for overtreatment of low- and intermediate-risk prostate cancer with aggressive therapies such as surgery and radiation. Given their high likelihood of other-cause mortality (1–5), low likelihood of cancer mortality (6–7), and the substantial morbidity associated with aggressive therapy (8–10), these men may be better served by conservative management. In fact, guidelines-producing bodies such as the American Urological Association, National Comprehensive Cancer Network, and European Association of Urology all endorse conservative management of early-stage disease for men with <10-year life expectancies (11–13). Despite these recommendations, life expectancy is poorly integrated into prostate cancer treatment decision making due to lack of data on 10-year survival based on both age and comorbidity in men with prostate cancer.
While studies have detailed how age (14,15, 16) and comorbidity (17) individually impact treatment in men with prostate cancer, less is known about how these factors concurrently affect 10-year life expectancy and treatment choice. In fact, there are no population-based studies that directly link life expectancy—vis-à-vis its main predictors, age and comorbidity—with treatment. For example, how do treatment patterns differ among “sick” 65-year old men and “healthy” 75-year old men with life expectancies <10 years? A previous study using SEER-Medicare data showed that comorbidity had little influence on aggressiveness of treatment in men older than 75, but it did not characterize life expectancy across comorbidity subgroups and did not include men younger than 75 (18). It is especially important to consider this question in men younger than 75, since these individuals comprise the majority of those newly diagnosed with prostate cancer and many have life expectancies <10 years after adjustment for comorbidity (5).
In this study, we used a nationally representative cohort of men with T1-T2 prostate cancer with Gleason scores of 7 or less to investigate the variation in treatment for men with differing ages and comorbidity burdens at diagnosis. We sought to determine which Charlson scores within age subgroups were associated with <10-year life expectancies and then to identify treatment patterns among these men. We hypothesized that we would find little variation in treatment by comorbidity status within age groups despite substantial differences in life expectancy.
Methods
Study Population
We identified men aged 66 years or older with incident prostate adenocarcinoma (International Classification of Diseases, Ninth Revision (ICD-9) code 185.0) diagnosed between January 1, 1992 and December 31, 2007 using the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database. This database contains Medicare insurance program files linked to population-based SEER cancer registries. The Medicare database covers approximately 97% of US persons aged 65 years or older, and SEER regions encompass 14% of the US population before 2000 and 25% thereafter. Our cohort included men with well or moderately differentiated T1 or T2 tumors. We excluded men with T3, T4, and metastatic tumors as well as men with poorly differentiated disease.
Variable Definitions
Sociodemographic Data
We determined sociodemographic information including age, race, marital status, and year of diagnosis from the Patient Entitlement and Diagnosis Summary File (PEDSF) of the Medicare dataset. Geographic region of diagnosis was obtained using SEER data. Age at diagnosis was grouped by 66–69, 70–74, 75–79, and ≥80. Race was grouped by Black, White, Hispanic, or Other. Marital status was defined as married or unmarried.
Comorbidity
Comorbidity burden at diagnosis was ascertained using the Deyo-Klabunde modification of the Charlson comorbidity index, utilizing both inpatient (MEDPAR Part A and Carrier Part B) and outpatient claims for the 12-months preceding prostate cancer diagnosis (19,20). We grouped men by Charlson scores of 0, 1, 2, and 3+.
Tumor Data
Tumor stage and grade were obtained using SEER data. We used extent-of-disease (EOD) codes corresponding to AJCC 6th edition definitions to define clinical tumor stage as T1 (EOD codes 13–15), T2 (EOD codes 24–29), or T1–T2 (EOD codes 30–34,40,41,48,49). Tumor grade was defined as: well differentiated (Gleason score 2-4, International Classification of Diseases for Oncology, Third Edition (ICD-O-3) code 81403/1) or moderately differentiated (Gleason score 5-7, ICD-O-3 code 81403/2). Of note, after 2003, SEER coded men with Gleason scores of 7 to the poorly differentiated code 81403/3.
Type of Treatment
Type of treatment was identified by ICD-9 and CPT-4 codes within the MEDPAR, NCH, and Outpatient files of the Medicare dataset. Treatment was categorized as aggressive or non-aggressive. Aggressive treatment was defined as radical prostatectomy (CPT-4 codes 55801, 55810, 55812, 55821, 55831, 55840, 55842, 55845; ICD-9 codes 60.3-6, 60.62), radiation therapy (CPT-4 codes 77305, 77310, 77315, 77321, 77332-77334, 77336, 77370, 77261-77263, 77280, 77285, 77290, 77295, 77299, 77300, 77401-77431, 77520-77525; ICD-9 codes 92.21-92.24, V58.0, V66.1, V67) or brachytherapy (CPT-4 codes 55859, 55860, 55862, 55865, 77326-77328, 77331, 77750, 77751-77799, C1715-C1719, C1728, C2632-C2636, Q3001; ICD-9 codes 92.28, 92.29) within the first year after diagnosis. Non-aggressive treatment was defined as watchful waiting, active surveillance (with or without treatment), or androgen deprivation therapy (ADT).
Watchful waiting was defined as no aggressive treatment with surgery, radiation, or brachytherapy per MEDPAR claims within the first year after diagnosis and no use of PSA testing (CPT codes 84152-84154, HCPCS code G0103) or transrectal ultrasound-guided rebiopsy (CPT codes 55700, 76942, 76872, 55706) during the period of follow-up. Active Surveillance without treatment was defined by use of PSA testing or transrectal ultrasound guided rebiopsy (CPT/HCPCS codes as above) following diagnosis without aggressive treatment with surgery, radiation, or brachytherapy per MEDPAR claims over the period of follow up. Active Surveillance with treatment was defined similarly except that men received aggressive treatment with surgery, radiation, or brachytherapy per MEDPAR claims more than one year after diagnosis. ADT was defined as treatment with androgen deprivation (CPT-4 codes 11980, C9216, C9430, J141, J0970, J1000, J1056, J1380, J1390, J1950, J3315, J9202, J9217, J9218, J9219, S0165, S9560; ICD-9 code 99.24) or bilateral orchiectomy (CPT-4 codes 54250, 54251, 54522, 54530, 54535; ICD-9 codes 62.3, 62.4, 62.41, 62.42) within/after 1 year of diagnosis per MEDPAR claims.
Survival and Cause of Death
Overall survival was defined as the date of diagnosis to the date of death as determined by the PEDSF file. Other-cause and cancer-specific mortality were defined by their designations in SEER.
Statistical Analysis
We compared characteristics of our sample across Charlson scores using the chi-squared test.
We used competing risks regression analysis as described by Fine and Gray (21) to determine cumulative incidence of other-cause mortality by Charlson score within age subgroups. For this analysis, our primary predictor was Charlson score, the failure event was other-cause mortality, and the competing event was prostate cancer mortality. Our models adjusted for tumor grade, stage, race, marital status, year of diagnosis, SEER site, and aggressive/non-aggressive treatment type. We then plotted cumulative incidence of non-prostate cancer mortality by Charlson score for each age subgroup.
We estimated life expectancy for each age/Charlson subgroup as the number of years after diagnosis at which the cumulative incidence of other-cause mortality reached 50%. Men with a cumulative incidence of other-cause mortality >50% at ten years were identified as having a life expectancy <10 years. This estimation may slightly differ from conventional calculation of life expectancy since it relies on median rather than mean survival.
We then stratified men by age and comorbidity and determined frequencies of treatment within age/comorbidity subgroups. We then calculated the proportion of each subgroup comprised by each treatment type, and then plotted these proportions as stacked columns.
We used multivariate logistic regression models to calculate predicted probabilities of aggressive treatment by Charlson score within each age subgroup. Covariates included tumor stage, grade, race, marital status, SEER site, and individual year of diagnosis. Models were repeated after re-categorizing year of diagnosis by 1992–1997, 1998–2002, and 2003–2007.
We used p<0.05 to denote statistical significance, and all tests were two-sided. All statistical analyses were performed in Stata 11.0 (Stata Inc., College Station, TX).
Results
Sample characteristics by Charlson score are shown in Table 1. In this sample comprised of men with stage T1–T2, grade ≤ 7 tumors, men with higher Charlson scores were more often older, Black, unmarried, diagnosed within 2002–2007, and resided in the Detroit, New Jersey, and Louisiana SEER regions. Men with higher Charlson scores also tended to have lower grade and stage tumors.
Table 1. Patient characteristics by aggressive and non-aggressive treatment (No of patients, %).
| All Patients (N=96,032) | Charlson 0 | Charlson 1 | Charlson 2 | Charlson 3+ | P-Value |
|---|---|---|---|---|---|
| Age at Diagnosis | <0.001 | ||||
| 66–69 | 19,903 (81%) | 3,357 (14%) | 789 (3%) | 411 (2%) | |
| 70–74 | 24,369 (78%) | 5,132 (16%) | 1,259 (4%) | 636 (2%) | |
| 76–80 | 17,629 (75%) | 4,157 (18%) | 1,224 (5%) | 652 (3%) | |
| >80 | 10,805 (33%) | 15,753 (47%) | 4,310 (13%) | 2,372 (7%) | |
| Race/Ethnicity | <0.001 | ||||
| White | 61,119 (78%) | 12,460 (16%) | 3,324 (4%) | 1,715 (2%) | |
| Black | 5,597 (68%) | 1,673 (20%) | 578 (7%) | 390 (5%) | |
| Hispanic | 3,124 (73%) | 792 (19%) | 221 (5%) | 144 (3%) | |
| Other | 3,629 (74%) | 911 (19%) | 219 (4%) | 136 (3%) | |
| Survival Outcomes | <0.001 | ||||
| Alive | 42,295 (83%) | 6,912 (14%) | 1,459 (3%) | 519 (1%) | |
| Other Causes | 27,857 (68%) | 8,345 (21%) | 2,725 (7%) | 1,763 (4%) | |
| Prostate Cancer | 3,117 (79%) | 579 (15%) | 158 (4%) | 103 (3%) | |
| Marital Status | <0.001 | ||||
| Married | 4,529 (74%) | 1,080 (18%) | 328 (5%) | 201 (3%) | |
| Unmarried | 68,940 (77%) | 14,756 (16%) | 4,014 (4%) | 2,184 (2%) | |
| Tumor Grade | <0.001 | ||||
| Well differentiated | 7,084 (75%) | 1,586 (17%) | 474 (5%) | 286 (3%) | |
| Moderately differentiated | 66,385 (77%) | 14,250 (16%) | 3,868 (4%) | 2,099 (2%) | |
| Tumor Stage | <0.001 | ||||
| T1 | 24,736 (75%) | 5,610 (17%) | 1,644 (5%) | 909 (3%) | |
| T2 | 31,596 (78%) | 6,496 (16%) | 1,676 (4%) | 965 (2%) | |
| T1/T2 | 17,137 (77%) | 3,730 (17%) | 1,022 (5%) | 511 (2%) | |
| Year of Diagnosis | <0.001 | ||||
| 1992–1997 | 24,972 (79%) | 4,788 (15%) | 1,266 (4%) | 655 (2%) | |
| 1998–2002 | 31,918 (76%) | 7,163 (17%) | 2,032 (5%) | 1,022 (2%) | |
| 2003–2007 | 16,579 (75%) | 3,865 (17%) | 1,044 (5%) | 708 (3%) | |
| SEER Region | <0.001 | ||||
| Connecticut | 6,915 (78%) | 1,359 (15%) | 382 (4%) | 188 (2%) | |
| Detroit | 9,406 (70%) | 2,757 (21%) | 764 (6%) | 511 (4%) | |
| Hawaii | 1,139 (77%) | 236 (16%) | 66 (4%) | 29 (2%) | |
| Iowa | 7,679 (80%) | 1,429 (15%) | 354 (4%) | 175 (2%) | |
| New Mexico | 3,232 (82%) | 538 (14%) | 130 (3%) | 56 (1%) | |
| Seattle | 5,950 (82%) | 936 (13%) | 241 (3%) | 110 (2%) | |
| Utah | 3,651 (83%) | 566 (13%) | 149 (3%) | 58 (1%) | |
| Atlanta | 2,872 (78%) | 571 (16%) | 163 (4%) | 69 (2%) | |
| Kentucky/Georgia* | 3,335 (66%) | 1,418 (28%) | 230 (5%) | 99 (2%) | |
| Louisiana | 2,682 (73%) | 629 (17%) | 196 (5%) | 143 (4%) | |
| New Jersey | 7,091 (72%) | 1,860 (19%) | 550 (6%) | 317 (3%) | |
| California | 19,517 (77%) | 4,113 (16%) | 1,117 (4%) | 630 (2%) |
Cells are combined due to SEER-Medicare rules regarding suppression of cell sizes less than 11.
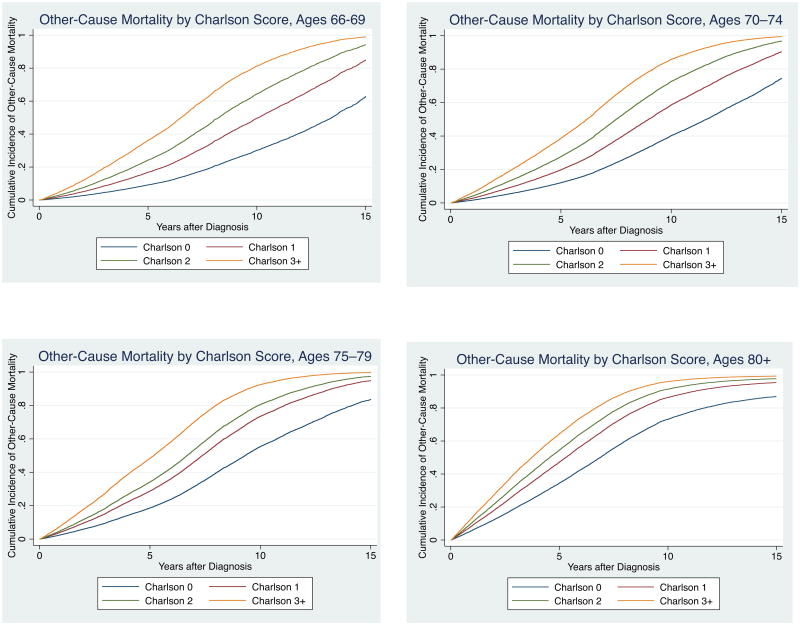
Multivariate competing risks regression analysis revealed that cumulative incidence of other-cause mortality by Charlson score differed substantially by age at diagnosis (Figure 1a-d). Life expectancy was <10 years (i.e. >50% cumulative incidence of other-cause mortality at ten years) for: men aged 66–69 with Charlson scores 2 and greater; men aged 70–74 with Charlson scores 1 or greater; and men aged 75–79 and 80+ regardless of comorbidity status (Table 2). Overall, 52% of our cohort (50,049 of 96,032 men) had life expectancies of <10 years.
Figure 1. Cumulative Incidence of Other-Cause Mortality by Charlson Score at Ages (a) 66–69, (b) 70–74, (c) 75–79, and (d) 80+.
Table 2. 10-Year Cumulative Incidence of Other-Cause Mortality by Charlson Score, Stratified by Age at Diagnosis.
| 10-Year Cumulative Incidence of Other-Cause Mortality | ||||
|---|---|---|---|---|
| Age 66–69 | Age 70–74 | Age 75–79 | Age 80+ | |
| Charlson 0 | 30% | 40% | 55% | 73% |
| Charlson 1 | 50% | 59% | 73% | 86% |
| Charlson 2 | 64% | 73% | 81% | 91% |
| Charlson 3+ | 81% | 86% | 92% | 96% |
Footnote: Cumulative incidence estimates from competing risks regression corrected for tumor grade, tumor stage, race/ethnicity, marital status, SEER region, and year of diagnosis.
Stacked column graphs showing unadjusted frequencies of treatment type by age/comorbidity subgroups are shown in Figure 2a-d. The proportion of subjects treated aggressively (with surgery, radiation, or brachytherapy) slightly decreased with increasing comorbidity within each age subgroup. However, men with <10-year life expectancies were frequently treated aggressively. Among men aged 66–69, 70–74, 75–79, and 80+ with <10-year life expectancies, treatment was aggressive 68%, 69%, 57%, and 24% of the time, respectively.
Figure 2. a-d. Type of Treatment by Charlson Score at Ages (a) 66–69, (b) 70–74, (c) 75–79, and (d) 80+.
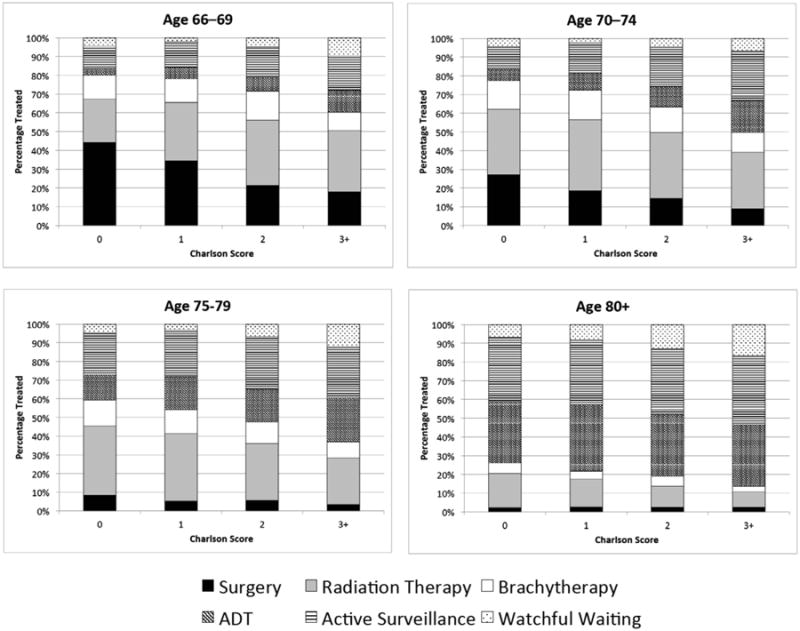
Among men with limited life expectancies who were treated aggressively, treatment type was most often radiation therapy and was less frequently surgery. Among men aged 66–69, 70–74, 75–79, and 80+ with <10-year life expectancies, radiation comprised 50%, 53%, 63%, and 69% of aggressive treatment, respectively, while surgery comprised only 30%, 25%, 13%, and 9%. Among men with limited life expectancies who were treated non-aggressively, watchful waiting was less common than active surveillance and ADT. Among men aged 66–69, 70–74, 75–79, and 80+ with <10-year life expectancies: active surveillance comprised 52%, 57%, 55%, 45% of non-aggressive treatment; ADT comprised 27%, 32%, 34%, and 44%; and watchful waiting comprised only 20%, 10%, 11%, and 11%, respectively.
There was little variation in multivariate predicted probability of aggressive treatment by comorbidity status within age subgroups, despite substantial differences in other-cause mortality. The absolute differences in probability of receiving aggressive treatment between the healthiest and sickest men within each age subgroup were 20%, 25%, 22%, and 12% for ages 66–69, 70–74, 75–79, and 80+, respectively (Figure 3), while differences in cumulative incidence of 10-year other-cause mortality were 51%, 46%, 37%, and 26%, respectively (Table 2). The probabilities of aggressive treatment for the sickest men in the younger age groups were also surprisingly high; men with Charlson scores of 3+ aged 66–69 and 70–74 had probabilities of aggressive treatment of 60% and 50%, respectively. Results did not change with categorical vs. individual correction for year of diagnosis or in subgroups defined by year of diagnosis (Figure 3).
Figure 3. Multivariate Predicted Probabilities of Aggressive Treatment by Age and Charlson Comorbidity Index Score Across Entire Cohort and Stratified by Year of Diagnosis.
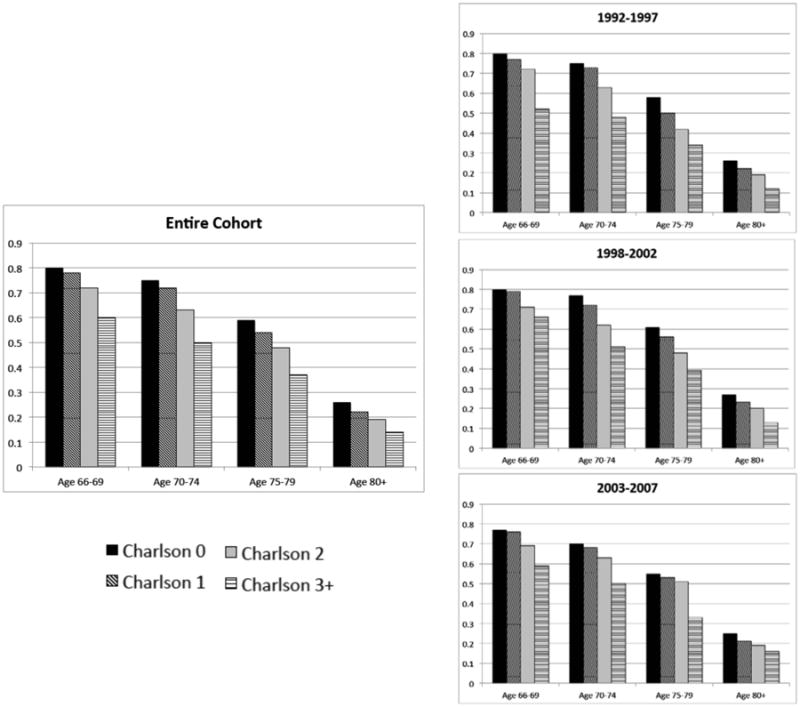
Footnote: Predicted probability estimates from a multivariate logistic regression model adjusted for tumor grade, tumor stage, race/ethnicity, martial status, SEER region, and year of diagnosis.
Discussion
Our study characterizes how the main drivers of life expectancy—age and comorbidity at diagnosis—jointly affect survival and treatment choice in men with low- and intermediate-grade, early-stage prostate cancer. Though there is a trend toward lower probability of aggressive treatment with both advancing age and worse comorbidity, our data revealed surprisingly high probabilities of aggressive treatment among men with life expectancies <10 years. We chose a 10-year life expectancy as our benchmark for defining overtreatment because randomized controlled trial evidence suggests that significant differences in survival between watchful waiting and aggressive treatment do not develop until ten years after treatment (22) and because guidelines universally agree that men with life expectancies of <10 years should not be treated aggressively (11–13).
Overtreatment of men with limited life expectancy appears to be driven by overestimation of life expectancy among men with comorbid disease burdens. Even men with the heaviest comorbid disease burden were often treated aggressively; those aged 66–69 and 70–74 with Charlson scores of 3+ had probabilities of aggressive treatment of 60% and 50%, respectively, despite other-cause mortality of 81% and 86% at 10 years after diagnosis. Furthermore, there was relatively little variation in probability of aggressive treatment by comorbidity status within age subgroups, out of proportion to substantial differences in mortality. For example, among men aged 66–69, the difference in probability of aggressive treatment between the healthiest men and the sickest men was only 20%, while the absolute difference in 10-year survival between these two groups was 51%.
Among both older and sicker men with limited life expectancies who were treated aggressively, treatment was most often radiation therapy and was only infrequently surgery. Among men aged 66–69, 70–74, 75–79, and 80+ with <10-year life expectancies, the ratios of radiation therapy to surgery were approximately 2:1, 2:1, 5:1, and 8:1, respectively. The disparity of treatment with radiation over surgery increased with both advancing age and comorbidity. That surgery is less common than radiation therapy in older and sicker men is not surprising, since these men are often poor surgical candidates; the lack of a similar inherent check on treatment with radiation therapy may help to enable overtreatment of older and sicker men with radiation. This emphasizes the importance of interventions targeted at reducing overtreatment in older and sicker men considering non-surgical treatment options.
We also found that among men with limited life expectancies, watchful waiting is underutilized compared with active surveillance (AS) and ADT. Although it is a preferable strategy over aggressive treatment, AS is unlikely to improve cancer outcomes for men with limited life expectancies given their outsize likelihood of dying of other causes and low likelihood of cancer mortality. Our data also revealed that a surprisingly high proportion of men with limited life expectancies receive ADT as primary treatment (27–44% of non-aggressive treatment). Because ADT is known to increase risk of cardiac morbidity and mortality (23,24), this treatment approach is both dangerous and unnecessary, especially for men with multiple comorbid conditions.
There are many reasons why men with limited life expectancies may choose aggressive local therapy over conservative management. First, although life tables have long been available to assist clinicians with prediction of life expectancy by age alone, there is no widely accepted method for determination of life expectancy that incorporates both age and health status. Second, diagnoses of cancer carry substantial emotional weight that may motivate strong risk aversion among patients and physicians alike (25, 26). Last, many men may willfully (and understandably) overestimate their life expectancy despite strong evidence to the contrary due to hopefulness that they will live longer than the average person of their age and health status.
Our study contributes to the discussion surrounding this dilemma in two ways. First, our population-based estimates of long-term, other-cause mortality by Charlson score and age provide benchmark data for estimation of life expectancy in men considering treatment for prostate cancer. Second, our data provide a context for discussion about what is the appropriate treatment mix for a given life expectancy. While we acknowledge that the cutpoint for what is a meaningful likelihood of treatment benefit may differ by individual, men with <10-year life expectancies should not be treated aggressively over half the time for low- and intermediate-risk disease and would be better served by higher rates of watchful waiting.
This study is subject to limitations that may affect generalizability of our findings. First, because our method of comorbidity determination was based on claims, mortality estimates by Charlson score and age may differ from those based on more granular comorbidity assessments. However, retrospective studies have suggested that chart- and claims-based comorbidity designations are similar (27). Second, because our cohort included men from a broad range of years, there may be secular trends affecting treatment choice that we have not detected, namely a recent trend toward less aggressive therapy. However, even after correcting for year of diagnosis in our multivariate model, probabilities of aggressive treatment did not substantially vary from unadjusted data. Third, lack of PSA data and inconsistency in definitions of tumor grade in SEER-Medicare do not allow for full characterization of tumor risk according to D'Amico (28) or NCCN (12) criteria. Fourth, since treatment paid for through other insurers is not captured in SEER-Medicare, the frequency of aggressive treatment may be underestimated and watchful waiting/active surveillance may be overestimated.
Conclusions
Our study provides population-based estimates of other-cause mortality and corresponding treatment trends for men with low- and intermediate-grade, early-stage prostate cancer across differing levels of age and comorbidity. We found that over half of men aged 66 or older had life expectancies of <10 years and that nearly half of them were treated aggressively, mostly with radiation therapy. Because of their low likelihood of ten-year survival, these men are unlikely to live long enough to substantially benefit from aggressive treatment but still incur its associated side effects and financial burden. We hope that this information will promote greater awareness of the role of life expectancy in treatment decision making for men with low- and intermediate-risk prostate cancer.
Acknowledgments
This work was supported by grants from the American Cancer Society (124225-PF-13-014-01-CPHPS, to TJD), Urology Care Foundation (to TJD), and National Institutes of Diabetes and Digestive and Kidney Diseases (HHSN276201200016C, to MSL). The funding sources had no role in the design, conduct, analysis, or decision to publish the manuscript. Dr. Saigal is the co-founder of WiserCare, LLC.
Research Support: This work was supported by grants from the American Cancer Society (124225-PF-13-014-01-CPHPS, to TJD), Urology Care Foundation (to TJD), and National Institutes of Diabetes and Digestive and Kidney Diseases (HHSN276201200016C, to MSL).
Footnotes
Other authors have no conflicts of interest to report.
References
- 1.Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171:1513–9. doi: 10.1097/01.ju.0000117975.40782.95. [DOI] [PubMed] [Google Scholar]
- 2.Daskivich TJ, Chamie K, Kwan L, et al. Comorbidity and competing risks for mortality in men with prostate cancer. Cancer. 2011;20:4642–50. doi: 10.1002/cncr.26104. [DOI] [PubMed] [Google Scholar]
- 3.Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29:1335–41. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berglund A, Garmo H, Tishelman C, Holmberg L, Stattin P, Lambe M. Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol. 2011;18:833–9. doi: 10.1016/j.juro.2010.10.061. [DOI] [PubMed] [Google Scholar]
- 5.Daskivich TJ, Kang-Hsien F, Koyama T, et al. Impact of Age, Tumor Risk, and Comorbidity on Competing Risks for Survival in a U.S. Population-Based Cohort of Men with Prostate Cancer. Ann Intern Med. 2013;158(10):709–17. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu-Yao GL, Albertsen PC, Moore DF. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–9. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 8.Gore JL, Kwan L, Lee SP, Reiter RE, Litwin MS. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101:888–92. doi: 10.1093/jnci/djp114. [DOI] [PubMed] [Google Scholar]
- 9.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250–61. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 10.Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, Hoffman RM, Potosky AL, Stanford JL, Stroup AM, Van Horn RL, Penson DF. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436–45. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich A, Bellmunt J, Bolla M, et al. EAU Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Treatment of Clinically Localized Disease. European Urology. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Cooperberg MR, Broering JM, Carroll PR. Time Trends and Local Variation in Primary Treatment of Localized Prostate Cancer. J Clin Oncol. 2010;28(7):1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller DC, Gruber SB, Hollenbeck BK, Montie JE, Wei JT. Incidence of Initial Local Therapy Among Men with Lower-Risk Prostate Cancer in the United States. J Natl Cancer Inst. 2006;98(16):1134–41. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 16.Konety BR, Cowan JE, Carroll PR the CaPSURE investigators. Patterns of Priamry and Secondary Therapy for Prostate Cancer in Elderly Men: Analysis of Data from CaPSURE. J Urol. 2008;179(5):1797–1803. doi: 10.1016/j.juro.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 17.Daskivich TJ, Chamie K, Kwan L, Labo J, Palvolgyi R, Dash A, Greenfield S, Litwin MS. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(10):2058–66. doi: 10.1002/cncr.25751. [DOI] [PubMed] [Google Scholar]
- 18.Roberts CB, Albertsen PC, SHao YH, Moore DF, Mehta AR, Stein MN, Lu-Yao GL. Patterns and correlates of prostate cancer treatment in older men. Am J Med. 2011;124(3):235–43. doi: 10.1016/j.amjmed.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 22.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708–17. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 23.Saigal CS, Gore JL, Krupski TL, Hanley J, Schonlau M, Litwin MS the Urologic Diseases in America Project. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110(7):1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 24.Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99(20):1516–24. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 25.Esserman L, Shieh Y, Thompson I. Rethinking Screening for Breast Cancer and Prostate Cancer. JAMA. 2009;302(15):1685–92. doi: 10.1001/jama.2009.1498. [DOI] [PubMed] [Google Scholar]
- 26.Han PK, Klein WM, Lehman TC, Massett H, Lee SC, Freedman AN. Laypersons' responses to the communication of uncertainty regarding cancer risk estimates. Med Decis Making. 2009;29(3):391–403. doi: 10.1177/0272989X08327396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care. 2006;44:921–8. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 28.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]