Abstract
Objective
Mounting evidence links osteoprotegerin (OPG) with cardiovascular disease. Elevated serum and aortic tissue OPG are associated with the presence and growth of abdominal aortic aneurysm (AAA) in humans, however, a role for OPG in AAA pathogenesis remains to be shown. We examined the functional significance of OPG in aortic aneurysm using an Opg-deficient mouse model and in vitro investigations.
Approach/Results
Homozygous deletion of Opg in apolipoprotein E-deficient mice (ApoE−/−Opg−/−) inhibited AngII-induced aortic dilatation. Survival free from aortic rupture was increased from 67% in ApoE−/−Opg+/+ controls to 94% in ApoE−/−Opg−/− mice (P=0.040). Serum concentrations of pro-inflammatory cytokines/chemokines, and aortic expression for cathepsin S (CTSS), matrix metalloproteinase (MMP) 2 and MMP9 after seven days (early-phase) AngII infusion were significantly reduced in ApoE−/−Opg−/− mice compared to ApoE−/−Opg+/+ controls. In addition, aortic expression of markers for an inflammatory phenotype in aortic vascular smooth muscle cells (AoSMC) in response to early-phase AngII infusion were significantly lower in Opg-deficient mice. In vitro, human AAA vascular smooth muscle cells (AASMC) produced more CTSS and exhibited increased CTSS-derived elastolytic activity than healthy AoSMC; while recombinant human OPG stimulated CTSS-dependent elastase activity in AoSMC.
Conclusions
These findings support a role for OPG in aortic aneurysm through up-regulation of CTSS, MMP2, and MMP9 within the aorta, and promoting an inflammatory phenotype in AoSMC in response to AngII.
Keywords: aneurysm, apolipoprotein E-null mouse, osteoprotegerin, matrix metalloproteinase, cathepsin S, vascular smooth muscle cells
Introduction
Abdominal aortic aneurysm (AAA) is the degenerative weakening and dilatation of the abdominal aorta. The main complication of AAA isaortic rupture which is associated with a mortality of up to 80% [1]. There is no currently accepted drug treatment to limit AAA rupture [2].
Despite advances in our understanding of its pathophysiology, precise mechanisms involved in AAA are uncertain. It is commonly accepted that an unknown inciting event results in injury of the aortic wall and recruitment of leukocytes to the site. Release of proteolytic enzymes from these cells and resident vascular cells (AoSMC) in response to local pro-inflammatory cytokine production contribute to the destruction of the ECM. Apoptosis of AoSMC results in the loss of cells primarily responsible for the synthesis of ECM proteins which limits ECM repair [3].
Osteoprotegerin (OPG; TNFRSF11B; TR1; OCIF) is a secreted glycoprotein member of the tumour necrosis factor receptor superfamily. Since its initial discovery as a key regulator of bone metabolism, OPG has attracted interest for its role in vascular disease [4]. High concentrations of serum OPG have been identified as a marker and risk factor for cardiovascular disease, as well as a predictor for future incident cardiovascular events and mortality [5–8]. In line with this, we have previously reported that AAA patients have elevated aortic and serum OPG concentrations that are positively associated with aneurysm diameter and growth [9,10], and independently associated with AAA after adjusting for other risk factors such as coronary heart disease [11]. In vitro, OPG stimulates production of elastolytic enzymes in human monocyte/macrophages and AoSMC [9,10], and promotes an aneurysm phenotype in healthy AoSMC through limiting cell proliferation and inducing apoptosis [10]. The in vivo role of OPG in AAA pathogenesis remains unclear.
We positively correlated human AAA concentrations of OPG with aneurysm diameter in a recent study [9]. Historically, matrix metalloproteinases (MMP) have been the main proteolytic enzymes implicated in ECM degradation in AAA [12]. More recently, other proteolytic enzymes such as the cysteine cathepsin proteases have been implicated in AAA pathogenesis [12,13]. Cathepsin S (CTSS) is a potent elastolytic/collagenolytic protease for which elevated expression and activity has been demonstrated in human AAA tissue [14]. Plasma levels of total, pro- and active CTSS have been positively associated with aortic diameter in patients with AAA [15,16]. Experimentally, aortic aneurysm induction in mice is dependent on AoSMC-expression of CTSS [17] and inhibited by Ctss deficiency [18].
The aim of this study was to assess the effect of Opg-deficiency on aortic aneurysm development in a mouse model. Using an Opg-deficient angiotensin II (AngII)-infused apolipoprotein E-deficient (ApoE−/−) mouse model, we provide in vivo evidence for the involvement of OPG in structural (ECM) and cellular (AoSMC) destabilisation within the aortic wall. A novel functional association between OPG and CTSS is reported.
Material and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Opg deficiency inhibits AngII-induced aortic dilatation and rupture in a mouse model of aortic aneurysm
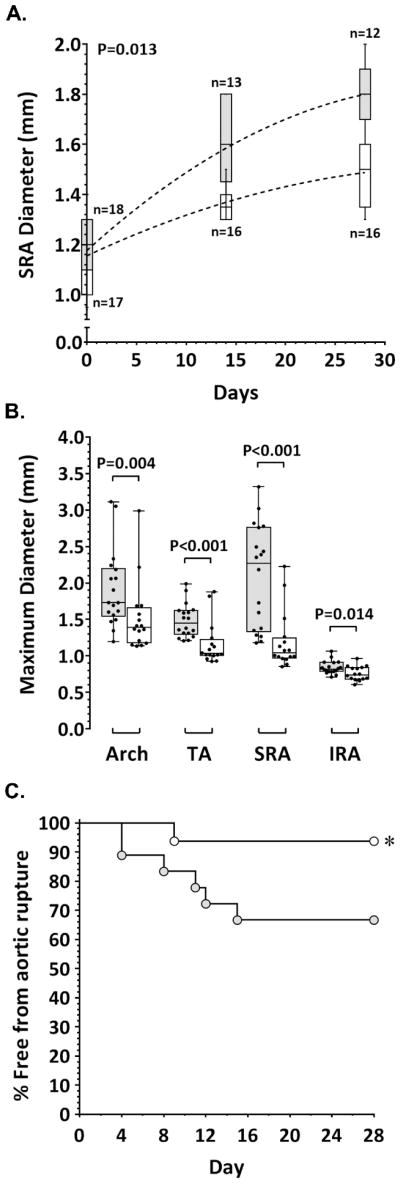
Aortic and serum OPG concentrations are elevated in patients with AAA and positively associated with AAA expansion in humans [9,10]. The in vivo importance of OPG in the development of aortic aneurysm was investigated in Opg-deficient mice infused with AngII. A preliminary study using C57BL/6 (ApoE+/+) mice demonstrated that Opg deficiency limited AngII-induced aortic dilatation in these mice compared to wild-type controls, most significantly within the aortic arch and thoracic aorta (Supplementary Table I). We subsequently investigated the effect of Opg deficiency on AngII-induced aortic aneurysm in the ApoE−/− mouse model. A time-dependent increase in SRA diameter in response to AngII infusion over the the 28-day experimental period was observed by ultrasound in both ApoE−/−Opg+/+ control (P<0.001) and ApoE−/−Opg−/− experimental (P<0.001) mice (Figure 1A). The rate of SRA expansion in response to AngII was, however, markedly less in ApoE−/−Opg−/− mice (P<0.001; Figure 1A). Aortas from all mice were harvested following fatality or sacrifice at study end and regional maximum aortic diameters determined by morphometric analysis (Supplementary Figure II). ApoE−/−Opg−/− mice exhibited significantly smaller median maximum diameter of aortic arch, thoracic aorta (TA), suprarenal aorta (SRA), and infrarenal aorta (IRA) compared to ApoE−/−Opg+/+ controls (Figure 1B; Supplementary Figure III). An important complication associated with the infusion of AngII in ApoE−/− mice is fatality due to aortic rupture. Opg deficiency markedly improved survival from aortic rupture, increasing survival rate from 67% in ApoE−/−Opg+/+ mice to 94% in ApoE−/−Opg−/− mice (P=0.040; Figure 1C).
Figure 1. Effect of Opg deficiency on AngII-induced aortic dilatation in ApoE−/− mice.
A. SRA diameter measured in ApoE−/−Opg+/+ (grey) and ApoE−/−Opg−/− (white) mice by ultrasound at baseline (Day 0) then at 14 day intervals after the start of AngII infusion. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for maximum diameter (mm). P-value calculated for difference between groups by mixed-effects linear regression. B. Regional aortic diameter in ApoE−/−Opg+/+ (grey; n=18) and ApoE−/−Opg−/− (white; n=16) mice determined by morphometry at sacrifice (day 28) or sudden death (aortic rupture). Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for maximum diameters (mm) which were compared by Mann-Whitney U test. TA, thoracic aorta; SRA, suprarenal aorta; IRA, infrarenal aorta. C. Kaplan-Meier curves of survival free from aneurysm rupture in AngII-infused ApoE−/−Opg+/+ (grey), and ApoE−/−Opg−/− (white) mice; *P=0.040 (Mantel-Cox (Log-rank) test).
The effect of Opg deficiency on AngII-induced aortic dilatation is blood pressure-independent and associated with a reduced initial inflammatory response to AngII
Infusion of AngII in ApoE−/− mice resulted in a time-dependent increase in blood pressure over the 28-day infusion period (P<0.001; Table 1). The increase in blood pressure induced by AngII was not affected by Opg-deficiency. Median systolic, diastolic, and mean arterial blood pressures obtained at baseline (day 0), day 14, and day 28 remained comparable between ApoE−/−Opg+/+ and ApoE−/−Opg−/− mice (Table 1). The concentration of a range of cytokines and chemokines was measured in the serum of ApoE−/−Opg+/+ and ApoE−/−Opg−/− mice during a seven-day infusion of AngII (Table 2). Median concentrations of interleukin (IL)1α, IL1β, IL2, IL3, IL6, IL12, IL17, monocyte chemotactic protein (MCP)-1, tumor necrosis factor (TNF)α, macrophage inflammatory protein-1α, granulocyte-macrophage colony-stimulating factor, and regulated on activation, normal T cell expressed-and-secreted were significantly increased in ApoE−/−Opg+/+ control mice after seven days of AngII infusion compared to baseline concentrations (Table 2). In contrast, levels of these cytokines were not markedly elevated in serum from ApoE−/−Opg−/− mice after the same period of AngII infusion (Table 2). Notably, baseline concentrations of IL6, MCP-1, and TNFα were significantly lower in ApoE−/−Opg−/− mice compared to ApoE−/−Opg+/+ controls, and median fold-increase in all markers other than IL1β and MCP-1 in response to AngII infusion remained significantly lower in ApoE−/−Opg−/− mice (Table 2). The presence of MOMA-2 positive (monocyte/macrophage) cells was demonstrated within the suprarenal aortic adventitia of AngII-infused ApoE−/−Opg−/− mice at 28 days (Supplementary Figure IV).
Table 1.
Blood pressure in ApoE−/−Opg+/+ and ApoE−/−Opg−/− mice over a 28-day AngII infusion period
| ApoE−/−Opg+/+ | ApoE−/−Opg−/− | P2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Baseline | Day 14 | Day 28 | P1 | Baseline | Day 14 | Day 28 | P1 | ||
| SBP | 94 (89–101) n=19 |
118 (99–125) n=13 |
128 (117–142) n=12 |
<0.001 | 89 (84–103) n=17 |
110 (100–125) n=16 |
130 (112–137) n=16 |
<0.001 | 0.853 |
| DBP | 70 (62–76) n=17 |
85 (76–89) n=16 |
91 (81–105) n=16 |
<0.001 | 69 (60–76) n=17 |
89 (74–94) n=16 |
92 (84–101) n=16 |
<0.001 | 0.257 |
| MAP | 77 (72–84) n=19 |
96 (90–105) n=13 |
107 (98–113) n=12 |
<0.001 | 74 (68–83) n=17 |
98 (87–105) n=16 |
101 (94–115) n=16 |
<0.001 | 0.236 |
Data presented as median (interquartile range) pressure presented as mmHg; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; P1, two-sided p-value for comparison of pressure over time within each group by repeat measures one-way ANOVA; P2, two-sided p-value for comparison between change in pressure over time in ApoE−/−Opg+/+ mice and change in pressure over time in ApoE−/−Opg−/− mice by mixed-effects linear regression.
Table 2.
Serum cytokine concentration in ApoE−/−Opg+/+ and ApoE−/−Opg−/− mice following seven days of AngII infusion
| ApoE−/−Opg+/+ (n=12) | ApoE−/−Opg−/− (n=16) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Day 0 | Day 7 | MFI | P1 | Day 0 | Day 7 | MFI | P1 | P2 | |
| IL-1α | 72.1 (31.2–107.0) | 168.5 (158.1–174.2) | 2.4 | <0.001 | 70.4 (54.4–103.0) | 84.8 (60.9–105.8) | 1.1 | 0.492 | <0.001 |
| IL-1β | 146.4 (114.2–191.8) | 270.7 (244.6–275.1) | 1.7 | <0.001 | 144.9 (89.1–196.7) | 171.6 (108.2–246.6) | 1.2 | 0.301 | 0.081 |
| IL-2 | 105.4 (48.5–174.8) | 165.5 (121.9–190.7) | 1.6 | 0.033 | 90.2 (66.2–115.2) | 95.8 (60.4–131.3) | 1.1 | 0.661 | 0.002 |
| IL-3 | 29.5 (22.7–58.3) | 46.5 (37.2–75.8) | 1.5 | 0.038 | 34.6 (18.2–58.6) | 33.9 (27.1–52.2) | 1.1 | 0.975 | 0.013 |
| IL-6 | 135.5 (100.8–146.2) | 180.6 (165.5–189.4) | 1.4 | <0.001 | 105.8 (74.3–118.0)† | 121.0 (73.9–143.3) | 1.1 | 0.397 | <0.001 |
| IL-12 | 227.4 (174.3–380.1) | 578.8 (479.1–671.0) | 2.5 | <0.001 | 259.7 (194.0–312.3) | 279.5 (127.7–419.1) | 1.1 | 0.583 | <0.001 |
| IL-17 | 146.4 (106.0–190.8) | 228.4 (190.6–271.4) | 1.6 | 0.001 | 117.3 (93.5–170.3) | 156.0 (67.6–206.7) | 1.1 | 0.635 | <0.001 |
| MCP-1 | 93.6 (90.1–98.3) | 103.9 (102.7–106.7) | 1.1 | <0.001 | 74.4 (64.2–79.7)† | 81.3 (70.3–90.9) | 1.1 | 0.085 | 0.937 |
| TNFα | 47.9 (41.3–51.9) | 65.8 (63.1–67.8) | 1.4 | <0.001 | 30.3 (20.2–40.2)† | 35.7 (30.7–47.9) | 1.2 | 0.085 | 0.041 |
| MIP-1α | 30.8 (26.1–44.6) | 50.4 (47.1–53.5) | 1.6 | <0.001 | 29.4 (20.7–36.1) | 34.90 (27.32–44.72) | 1.3 | 0.158 | 0.053 |
| GMCSF | 117.0 (93.0–155.7) | 160.4 (144.7–196.6) | 1.4 | 0.007 | 137.8 (86.3–188.5) | 145.3 (103.1–195.3) | 1.1 | 0.377 | 0.013 |
| RANTES | 114.4 (86.7–133.8) | 133.3 (126.8–139.2) | 1.2 | 0.020 | 103.1 (63.6–116.4) | 101.8 (89.9–113.0) | 1.0 | 0.858 | 0.059 |
Data expressed as median (interquartile range) concentration presented as pg/ml; MFI, median fold-increase; P1, two-sided p-value for comparison between concentrations at Day 0 and concentrations Day 7 within each group by paired t test; P2, two-sided p-value for MFI in ApoE−/−Opg+/+ mice versus MFI in ApoE−/−Opg−/− mice by Mann-Whitney U test;
P<0.05 compared to ApoE−/−Opg+/+ baseline by Mann-Whitney U test.
Reduced proteolysis in aortas of AngII-infused ApoE−/−Opg−/− mice
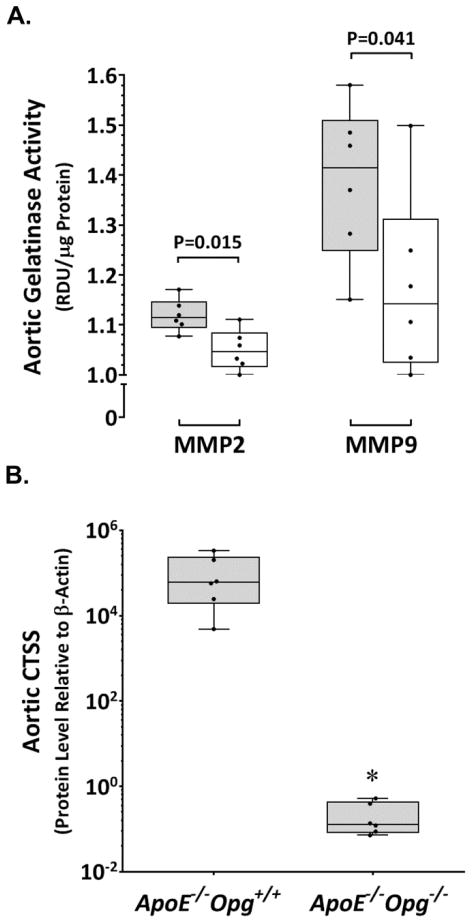
Based upon reported associations between OPG and metallo/cysteine proteinases in promoting AAA [9,10,19], we examinedMMP2, MMP9, and CTSS expression within aortas of ApoE−/−Opg+/+ (n=6) and ApoE−/−Opg−/−(n=6) mice after seven days of AngII infusion. Median relative expression of Mmp2, Mmp9, and Ctss within the aortas of ApoE−/−Opg−/− mice were reduced by 2-, 3-, and 7-fold reductions, respectively, compared to ApoE−/−Opg+/+ controls (Table 3). The lower mRNA expression translated to reduced aortic levels of these proteases. Median levels of MMP2 and MMP9 (P=0.001, P=0.028; Figure 2A; Supplementary Figure V), and CTSS (P=0.002; Figure 2B) were significantly lower within aortic tissue from ApoE−/−Opg−/− mice compared to ApoE−/−Opg+/+ controls after the seven-day AngII infusion period. Interestingly, median activity of CTSS within aortic tissue of ApoE−/−Opg−/− mice (n=16), measured using a substrate-specific assay, was 2-foldlowerthan in ApoE−/−Opg+/+ controls (n=12) after 28 days of AngII infusion (3.27, 2.17–4.44, vs 6.89, 5.02–8.99 ΔFU/min/μg protein; P=0.009).
Table 3.
Relative expression of selected genes within the aorta of ApoE−/−Opg+/+ and ApoE−/−Opg−/− mice following seven days of AngII infusion
| Gene | ApoE−/−Opg+/+ | ApoE−/−Opg−/− | P |
|---|---|---|---|
| Cnn1 | 1.50 (0.65–1.83) | 15.29 (13.14–22.29) | 0.002 |
| Bax/Bcl2* | 0.69 (0.58–0.75) | 0.37 (0.34–0.51) | 0.032 |
| Mapk1/3 (Erk1/2) | 1.66 (0.98–8.45) | 12.12 (8.55–16.58) | 0.008 |
| Mapk14 (p38 Mapk) | 6.25 (5.00–7.46) | 3.02 (1.82–4.39) | 0.041 |
| Pparg | 4.90 (2.62–8.03) | 16.05 (9.87–32.00) | 0.026 |
| Nfkb | 1.80 (0.52–2.63) | 5.98 (4.30–8.74) | 0.004 |
| Mmp2 | 0.58 (0.48–0.73) | 0.26 (0.03–0.33) | 0.002 |
| Mmp9 | 0.39 (0.36–0.41) | 0.13 (0.11–0.16) | 0.002 |
| Ctss | 0.67 (0.52–0.94) | 0.09 (0.06–0.17) | 0.002 |
Data expressed as median (interquartile range) mRNA expression relative to ‘house-keeping’ gene Gapdh;
ratio of Bax mRNA expression relative to Gapdh to Bcl2 mRNA expression relative to Gapdh; P, two-sided p-value for comparison by Mann-Whitney U test.
Figure 2. Down-regulation of MMP2, MMP9, and CTSS in the aortas of ApoE−/−Opg−/− mice assessed after seven days AngII infusion.
A. Decreased aortic MMP2 and MMP9 in ApoE−/−Opg−/− mice (white; n=6) compared to ApoE−/−Opg+/+controls (grey; n=6) determined by zymographic analysis. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for relative density units (RDU) per μg protein (P values calculated by Mann-Whitney U test). B. Reduced CTSS in aortas from ApoE−/−Opg−/− mice (n=8) compared to ApoE−/−Opg+/+controls (n=8) determined by TaqMan® Protein Assay. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for expression relative to aortic β-actin protein; *P=0.002 (P values calculated by Mann-Whitney U test).
Elevated production of CTSS by human AAA vascular smooth muscle cells (AASMC) and stimulation of CTSS-derived elastolytic activity in healthy AoSMC by OPG in vitro
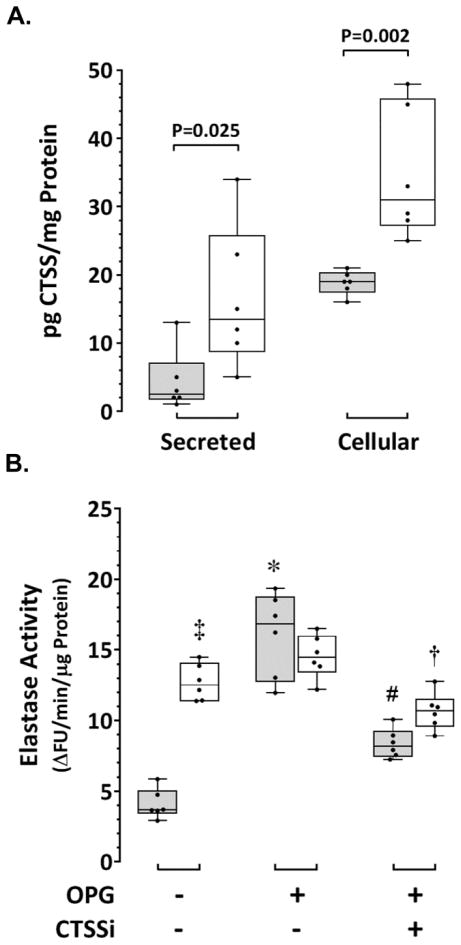
Serum concentrations of OPG and CTSS are elevated in patients with AAA and positively associated with aneurysm diameter [10,11,15,16]. Production of OPG and CTSS within AAA tissue may contribute to circulating levels of these proteins in AAA patients. We have previously demonstrated a positive correlation between tissue concentrations of OPG and CTSS within human AAA biopsies, and that OPG upregulates CTSS in healthy human AoSMC in vitro [9]. The production of CTSS within smooth muscle cells isolated from AASMC compared to AoSMC, and the ability of OPG to stimulate CTSS-dependent elastolytic activity in AoSMC was assessed in vitro. The concentration of total CTSS in supernatants (secreted) and cell lysates (cellular) from human AASMC (n=6 cultures) and AoSMC (n=6 cultures) was determined by ELISA. The median concentration of secreted CTSS from AASMC was 5-fold higher than that of AoSMC (P=0.025; Figure 3A). Similarly, median cellular CTSS was 1.6-fold higher in AASMC than that of AoSMC (P=0.002; Figure 3A). CTSS-derived elastase activity in AoSMC and AASMC was measured using a commercial elastin degradation assay that we initially evaluated using AngII-activated AoSMC (Supplementary Figure VI). Cells were co-incubated with the CTSS inhibitor Z-FL-COCHO (20 nM) to assess the contribution of CTSS to total elastase activity (Supplementary Figure VI). CTSS-derived elastase activity was three-fold higher in unstimulated AASMC than in unstimulated healthy AoSMC (P=0.002; Figure 3B). Incubation of AASMC in the presence of recombinant human (rh) OPG (50 nM; n=6) over 36 hours did not significantly affect elastase activity in these cells. In contrast, the median level of elastase activity in AoSMC cultured in the presence of rhOPG was 4.5-fold higher than that measured in control AoSMC (P=0.002; Figure 3B). In the presence of the CTSS inhibitor, median elastase activity stimulated by rhOPG, while remaining elevated compared to control cultures, was reduced in both AASMC and AoSMC by 1.5-fold (P=0.004) and 2-fold (P=0.002), respectively (Figure 3B).
Figure 3. Elevated CTSS and CTSS-derived elastase activity in human AASMC and stimulation of CTSS-derived elastase activity in human AoSMC by rhOPG.
A. Concentration of CTSS measured in the supernatant (secreted) and cell lysate (cellular) of healthy human aortic smooth muscle cells (AoSMC; grey) and aortic smooth muscle cells derived from human AAA biopsies (AASMC; white) after 96 hours in culture. Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for pg CTSS per mg protein from six repeat cultures (P values calculated by Mann-Whitney U test). B. CTSS-derived elastase activity measured in AASMC (white) and AoSMC (grey) cultured over 36 hours in control media, in the presence of rhOPG (50 nM), or in the presence of rhOPG + CTSS inhibitor (CTSSi; 20 nM). Data expressed as median and interquartile range with maximum and minimum data points (whiskers) for change in fluorescence units (ΔFU) per minute per μg protein from six repeat cultures (P values calculated by Mann-Whitney U test); ‡P=0.002 vs AoSMC control; *P=0.002 vs AoSMC control; †P=0.004 vs AASMC in OPG alone; #P=0.002 vs AoSMC in OPG alone.
Differential expression of mRNA for factors regulating AoSMC function within aortas of AngII-infused ApoE−/−Opg+/+ and ApoE−/−Opg−/− mice
The contribution of AoSMC loss to structural degeneration of the aortic media has been suggested through studies in human AAA tissue and animals models of aortic aneurysm [18,20,21]. We have shown previously the ability of OPG to limit AoSMC growth and survival in vitro [10]. Aortic mRNA expression for eight selected genes associated with AoSMC function was compared between ApoE−/−Opg+/+ (n=6) and ApoE−/−Opg−/−(n=6) mice by qPCR after seven days of AngII infusion. Median relative expression of transcripts for the AoSMC differentiation (contractile phenotype) marker scalponin 1 (Cnn1), mitogen-activated protein kinases 1/3 (Mapk1/3) and 14 (Mapk14), and nuclear transcription factors peroxisome proliferator-activated receptor-γ (Pparg) and nuclear factor kappa-light-chain-enhancer of activated B cells (Nfkb), were significantly higher in ApoE−/−Opg−/− mice compared to ApoE−/−Opg+/+ controls. Conversely, the median ratio of mRNA expression for apoptosis promoter Bcl2-like protein 4 (Bax) to apoptosis inhibitor B-cell lymphoma 2 (Bcl2) was reduced 2-fold (Table 3). Expression of aortic mRNA for the serine/threonine-protein kinaseAkt1 remained comparable between ApoE−/−Opg−/− mice and ApoE−/−Opg+/+ controls.
Discussion
The presence and growth of AAA in humans is independently associated with serum concentrations of OPG [10,11,22]. Expression of OPG within AAA biopsies is positively correlated with AAA diameter [8,9]. These association studies implicate OPG in AAA, however previously in vivo evidence for a role of OPG in AAA pathogenesis has been absent. Here we report that Opg deficiency in the ApoE−/− mouse limits AngII-induced aortic dilatation and rupture, and present additional in vivo and in vitro data to support a contributing role for OPG in aortic wall weakening.
Maximum aortic diameter is commonly presented as the primary outcome measure in experimental AAA studies [23,24,25]. Aneurysm diameter is an important clinical indicator of rupture risk for AAA in humans and routinely used in selected patients for surgical intervention [2]. Ultimately any medical treatment for aortic aneurysm aims to limit the main complication of aneurysm rupture. We utilised the AngII-infused ApoE−/− mouse model of aortic aneurysm in which aortic dilatation results from breaks in medial elastic lamellae and bleeding into the artery wall, a process that leads to acute aortic rupture in approximately 30% of mice [26]. This model allowed the assessment of both survival free from aortic rupture and maximum aortic diameter. Aortic diameter was assessed by two techniques, namely ultrasound performed in live mice and morphometry of harvested aortas. Opg-deficiency consistently limited AngII-induced aortic dilatation and rupture as assessed by all these outcome measures. The five-fold lower incidence of fatality due to aortic rupture in Opg-deficient ApoE−/− mice was particularly noteworthy. These findings suggest the importance of OPG in promoting aortic wall degeneration in this model.
Consistent with previous reports, we observed a time-dependent increase in blood pressure in ApoE−/−Opg+/+ mice [23,27]. Higher levels of serum and aortic OPG are measured in AngII-infused ApoE−/−Opg+/+ mice that develop more severe aortic dilatations [19,28] indicating a potential link between elevated blood pressure and increased OPG contributing to aneurysm formation in this model. Previous studies however suggest that AngII induces aortic aneurysm by mechanisms independent of blood pressure elevation [23,29]. Opg deficiency did not affect the hypertensive response to AngII infusion suggesting that Opg promotes aortic aneurysm by blood pressure independent mechanisms.
Leukocyte infiltration of the aortic wall is a prominent early response to AngII infusion that has been strongly implicated in aneurysm formation in this model [24,26,33]. Human and animal studies suggest that cytokines and chemokines, such as TNFα, IL-6, and MCP-1, play a key role in aortic leukocyte recruitment and activation [30,31]. In the current study, a lower incidence of AngII-induced aortic dilatation in Opg-deficient ApoE−/− mice corresponded with decreased levels of a range of circulating pro-inflammatory cytokine and chemokines. The most notable of these were TNFα, IL-6, and MCP-1. Baseline concentrations of these proteins were significantly lower in ApoE−/−Opg−/− mice compared to controls. Importantly, the concentrations of these proteins did not significantly increase in ApoE−/−Opg−/− mice following AngII infusion unlike the situation in ApoE−/−Opg+/+ controls in which a significant increase in the concentrations of all three proteins was identified. These findings suggest that Opg deficiency acted to suppress the inflammatory response to AngII infusion.
Infusion of AngII results in the overexpression of OPG, CTSS, MMP2, and MMP9 in the aorta of ApoE−/− mice [19,28]. In the present study, although detection of MOMA-2 in SRA of ApoE−/−Opg−/− mice indicated the presence of monocyte/macrophages within the aortic wall, reduced aortic dilatation in these mice was associated with down-regulation of aortic CTSS, MMP2, and MMP9. A recent study reported that reduced aortic aneurysm in Ctss-null AngII-infused ApoE−/− mice was associated with decreased aortic activity of MMP2 and MMP9, implicating CTSS in the regulation of these proteases [18]. These findings suggest a functional association between OPG, CTSS, MMP2 and MMP9 in aortic aneurysm. Indeed, aortic concentrations of OPG correlate with those of CTSS, MMP2 and MMP9, and increasing infrarenal aorta diameter in AAA patients [9]. Vascular smooth muscle cells have been identified as a major source of OPG within human AAA tissue [9], and MMP production in healthy AoSMC is stimulated by OPG in vitro [10]. Here, we show AASMC also produce large quantities of CTSS in vitro. Interestingly, incubation of AASMC in the presence of OPG had no significant effect to increase CTSS in these cells. Cellular and secreted levels of OPG [10] and CTSS are markedly elevated in AASMC in vitro compared to healthy human AoSMC. Thus it is plausible that exogenous OPG had little effect on already maximally stimulated cells from advanced-stage AAA. Nonetheless, our observation that OPG stimulated CTSS and CTSS-derived elastase-activity in healthy human AoSMC in vitro provides evidence of an association between OPG and upregulation of protease activity within the aneurysmal aortic wall.
Previous human and animal studies suggest that AASMC exhibit a phenotype that favours aortic ECM degeneration [18,20,25,32,33]. In particular, they have reduced ability to proliferate, increased apoptosis tendency, and promote inflammation and matrix degradation [18,20,25,32,33]. Exposure of AoSMC to OPG in vitro results in inhibition of cell growth and induction of apoptosis [10]. De-differentiation (phenotype modulation) of vascular smooth muscle cells to an inflammatory phenotype in response to vascular injury involves down regulation of genes involved in the regulation and modulation of smooth muscle contraction [34]. Phenotypic modulation is associated with reduction in MAPK1/3 activation resulting in decreased myosin light chain phosphorylation and loss of contractile properties [35]. CNN1 facilitates signal transduction by MAPK1/3 [36], and Cnn1 deletion in differentiated vascular smooth muscle cells results in impaired MAPK1/3 activity and cell contractility [37]. Evidence also suggests that MAPK1/3 possesses actin-binding properties and that an intact actin cytoskeleton, degraded during apoptosis, is required for MAPK1/3 signalling [38]. Our comparison of selected genes in the aortas of ApoE−/−Opg−/− and ApoE−/−Opg+/+ mice after seven days of AngII infusion showed a 10- and 7-fold greater expression of Cnn1 and Mapk1/3, respectively, within the aortas of ApoE−/−Opg−/− mice. These findings suggest that Opg deficiency promotes a more stable AoSMC phenotype within the aortic wall, a theory supported by the two-fold lower expression ratio of the apoptosis markers Bax and Bcl2 also observed within aortas of ApoE−/−Opg−/− mice. Thus, it is postulated that the inhibition of aortic dilatation and rupture in ApoE−/−Opg−/− mice is contributed to by an AoSMC phenotype that is less responsive to functional change following AngII infusion.
Activation of PPARγ down regulates AngII-type 1 receptor (ATr1) and OPG expression in vascular smooth muscle cells [39,40], while its own expression is negatively regulated by AngII via activation of ATr1 [41,42]. We have previously shown that PPARγ activation blocks OPG-induced up-regulation of ATr1 in human AAA explant and AoSMC [19]. A protective role for PPARγ in aortic aneurysm has recently been demonstrated in two independent mouse models of AAA incorporating vascular smooth muscle cell-selective Pparg deletion [17,43]. Importantly, the absence of vascular smooth muscle cell PPARγ rendered mice more susceptible to CTSS-associated degeneration of medial elastin [17]. PPARγ binds to a peroxisome proliferator-activated receptor response element upstream of the CTSS gene in AoSMC and knockdown and overexpression of PPARγ results in the increase and decrease, respectively, of CTSS mRNA and activity [17]. Here, we report increased expression of Pparg and decreased expression of Ctss in aortas of ApoE−/−Opg−/− mice associated with reduced incidence of AngII-induced aortic aneurysm. The upregulation of PPARγ in Opg-deficient mice may act to negatively regulate ATr1 and/or CTSS in AoSMC and contribute to the relative resistance of these mice to AngII-induced aortic aneurysm. AngII induced aortic aneurysm in ApoE−/− mice involves the activation of NFκB with increases in both p52 and p65 NFκB subunits within the aortic wall [41]. The three-fold increase in NFκB mRNA observed within aortas of ApoE−/−Opg−/− mice appears inconsistent with the inhibition of AngII-induced aortic dilatation in these mice. An explanation might be in the concurrent increases of aortic Mapk1/3 and Pparg reported above. Phosphorylation of PPARγ via active MAPK1/3 leads to the physical association of PPARγ with the NFκB p65 subunit and inhibition of NFκB activity [44]. It is possible that the increase in Nfkb expression in Opg-deficient mice is a compensatory response. However, why Nfkb expression is increased in aortas of AngII-infused ApoE−/−Opg−/− mice compared to ApoE−/−Opg+/+ controls remains to be elucidated.
Several study limitations are acknowledged. First, only the AngII-induced mouse model was used to examine the effect of Opg deficiency on aneurysm formation. This model was employed as it simulates both aortic expansion and rupture seen in human aortic aneurysm. We have also previously linked OPG and AngII in aortic aneurysm pathogenesis [19,28]. Examination of the effect of OPG in other mouse models is needed. Second, we focused on CTSS in the current study as a potential effector of OPG in promoting aortic aneurysm based on previous studies implicating it in aneurysm pathogenesis [17,18]. Other cysteine protease cathepsins (e.g. B, K, and L) and the endogenous cathepsin inhibitor, cystatin C, have also been implicated in experimental and human AAA [13]. Whether the inhibition of aortic aneurysm resulting from OPG deficiency involved modulation of these proteases was not investigated. Finally, the mechanistic focus of the present study was on OPG-mediated phenotype switching in AoSMC. We did not study the potential immune-regulatory actions of OPG and CTSS that may contribute to human aortic aneurysm [45,46]. Expression of OPG and CTSS in advanced-stage human AAA is not limited to vascular cells, but present also in leukocytes, lymphocytes, and plasma cells [9,10,47]. The effect of Opg deficiency on these cells requires further study.
In summary, the current study suggests a role for OPG in aortic aneurysm formation and rupture. In vitro, OPG stimulated CTSS and CTSS-derived elastase activity in AoSMC. In vivo, Opg deficiency was associated with down-regulation of CTSS, MMP2, and MMP9 within aortas of AngII-infused ApoE−/− mice, and an aortic gene expression profile suggesting AoSMC differentiation and viability. It is postulated that Opg-deficiency reduced aortic proteolysis and stimulation of an AoSMC inflammatory phenotype in response to AngII, thus reducing aortic dilatation and rupture.
Supplementary Material
Significance.
Abdominal aortic aneurysm (AAA) is an important cause of mortality and requirement for surgical intervention in older adults. There is a need to identify effective medical therapies that can slow or halt AAA growth. The work presented in this report suggests that OPG is important in the development and rupture of AAA. The authors demonstrate that Opg deficiency inhibits aortic expression of the proteolytic enzymes CTSS, MMP2, and MMP9, limiting vessel dilatation and rupture within a mouse model. These molecules are considered to be among the key proteases responsible for negative remodeling of the aortic wall associated with AAA pathogenesis in humans.
Acknowledgments
Sources of Funding
Funding from the National Institute of Health, National Health and Medical Research Council (1022752, 1021416, 1020955, 1003707 and 1000967) and Queensland Government supported this work. JG holds a Practitioner Fellowships from the National Health and Medical Research Council, Australia (1019921). JG holds a Senior Clinical Research Fellowship from the Queensland Government, Australia. CM holds a Smart Futures Fellowship from the Queensland Government, Australia. The funding bodies played no role in the production of this publication.
Abbreviations
- AAA
abdominal aortic aneurysm
- AASMC
aortic aneurysm smooth muscle cells
- AngII
angiotensin II
- AoSMC
aortic smooth muscle cells
- ApoE
apolipoprotein E gene
- ATr1
angiotensin II type 1 receptor
- CNN1
calponin 1 (gene = Cnn1)
- CTSS
cathepsin S (gene = Ctss)
- ECM
extracellular matrix
- IL
Interleukin
- MAPK
mitogen-activated protein kinase (gene = Mapk)
- MMP
metalloproteinase (gene = Mmp)
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells (gene = Nfkb)
- OPG
osteoprotegerin (gene = Opg)
- PPARγ
peroxisome proliferator-activated receptor gamma (gene = Pparg)
- Bax
Bcl2-like protein 4 (gene = Bax)
- Bcl2
B-cell lymphoma 2 (gene = Bcl2)
Footnotes
Disclosures
None
The authors have no conflict of interests relevant to this article.
References
- 1.Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, van Herwaarden JA, Holt PJ, van Keulen JW, Rantner B, Schlösser FJ, Setacci F, Ricco JB European Society for Vascular Surgery. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41 (Suppl 1):S1–S58. doi: 10.1016/j.ejvs.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Lederle FA. The natural history of abdominal aortic aneurysm. Acta Chir Belg. 2009;109:7–12. doi: 10.1080/00015458.2009.11680364. [DOI] [PubMed] [Google Scholar]
- 3.Nataatmadja M, west M, West J, Summers K, Walker P, Nagata M, Watanabe T. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(Suppl 1):II329–II334. doi: 10.1161/01.cir.0000087660.82721.15. [DOI] [PubMed] [Google Scholar]
- 4.Van Campenhout A, Golledge J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis. 2009;204:321–329. doi: 10.1016/j.atherosclerosis.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, Nishizawa Y. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106:1192–1194. doi: 10.1161/01.cir.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- 6.Ueland T, Jemtland R, Godang K, Kjekshus J, Hognestad A, Omland T, Squire IB, Gullestad L, Bollerslev J, Dickstein K, Aukrust P. Prognostic value of osteoprotegerin in heart failure after acute myocardial infarction. J Am Coll Cardiol. 2004;44:1970–1976. doi: 10.1016/j.jacc.2004.06.076. [DOI] [PubMed] [Google Scholar]
- 7.Golledge J, McCann M, Mangan S, Lam A, Karan M. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke. 2004;35:1636–1641. doi: 10.1161/01.STR.0000129790.00318.a3. [DOI] [PubMed] [Google Scholar]
- 8.Vik A, Mathiesen EB, Brox J, Wilsgaard T, Njølstad I, Jørgensen L, Hansen JB. Serum osteoprotegerin is a predictor for incident cardiovascular disease and mortality in a general population: the Tromsø Study. J Thromb Haemost. 2011;9:638–644. doi: 10.1111/j.1538-7836.2011.04222.x. [DOI] [PubMed] [Google Scholar]
- 9.Koole D, Hurks R, Schoneveld A, Vink A, Golledge J, Moran CS, de Kleijn DP, van Herwaarden JA, de Vries JP, Laman JD, Huizinga R, Pasterkamp G, Moll FL. Osteoprotegerin is associated with aneurysm diameter and proteolysis in abdominal aortic aneurysm disease. Arterioscler Thromb Vasc Biol. 2012;32:1497–1504. doi: 10.1161/ATVBAHA.111.243592. [DOI] [PubMed] [Google Scholar]
- 10.Moran CS, McCann M, Karan M, Norman P, Ketheesan N, Golledge J. Association of osteoprotegerin with human abdominal aortic aneurysm progression. Circulation. 2005;111:3119–3125. doi: 10.1161/CIRCULATIONAHA.104.464727. [DOI] [PubMed] [Google Scholar]
- 11.Moran CS, Clancy P, Biros E, Blanco-Martin B, McCaskie P, Palmer LJ, Coomans D, Norman PE, Golledge J. Association of PPARγ allelic variation, osteoprotegerin and abdominal aortic aneurysm. Clin Endocrinol (Oxf) 2010;72:128–132. doi: 10.1111/j.1365-2265.2009.03615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdul-Hussien H, Soekhoe RG, Weber E, von der Thüsen JH, Kleemann R, Mulder A, van Bockel JH, Hanemaaijer R, Lindeman JH. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am J Pathol. 2007;170:809–817. doi: 10.2353/ajpath.2007.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin Y, Cao X, Yang Y, Shi GP. Cysteine protease cathepsins and matrix metalloproteinases in the development of abdominal aortic aneurysms. Future Cardiol. 2013;9:89–103. doi: 10.2217/fca.12.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, Ridker PM, Libby P, Chapman HA. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lv BJ, Lindholt JS, Cheng X, Wang J, Shi GP. Plasma cathepsin S and cystatin C levels and risk of abdominal aortic aneurysm: a randomized population–based study. PLoS One. 2012;7:e41813. doi: 10.1371/journal.pone.0041813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin Y, Yang Y, Liu R, Cao X, Liu O, Liu J, Wang M, Yang Y, Chen Z, Zhang H, Du J. Combined Cathepsin S and hs-CRP predicting inflammation of abdominal aortic aneurysm. Clin Biochem. 2013;46:1026–1029. doi: 10.1016/j.clinbiochem.2013.05.065. [DOI] [PubMed] [Google Scholar]
- 17.Hamblin M, Chang L, Zhang H, Yang K, Zhang J, Chen YE. Vascular smooth muscle cell peroxisome proliferator-activated receptor-γ deletion promotes abdominal aortic aneurysms. J Vasc Surg. 2010;52:984–993. doi: 10.1016/j.jvs.2010.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin Y, Cao X, Guo J, Zhang Y, Pan L, Zhang H, Li H, Tang C, Du J, Shi GP. Deficiency of cathepsin S attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Cardiovasc Res. 2012;96:401–410. doi: 10.1093/cvr/cvs263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moran CS, Cullen B, Campbell JH, Golledge J. Interaction between angiotensin II, osteoprotegerin, and peroxisome proliferator-activated receptor-γ in abdominal aortic aneurysm. J Vasc Res. 2009;46:209–217. doi: 10.1159/000163019. [DOI] [PubMed] [Google Scholar]
- 20.Satta J, Mennander A, Soini Y. Increased medial TUNEL-positive staining associated with apoptotic bodies is linked to smooth muscle cell diminution during evolution of abdominal aortic aneurysms. Ann Vasc Surg. 2002;16:462–466. doi: 10.1007/s10016-001-0071-2. [DOI] [PubMed] [Google Scholar]
- 21.Yamanouchi D, Morgan S, Stair C, Seedial S, Lengfeld J, Kent KC, Liu B. Accelerated aneurysmal dilation associated with apoptosis and inflammation in a newly developed calcium phosphate rodent abdominal aortic aneurysm model. J Vasc Surg. 2012;56:455–461. doi: 10.1016/j.jvs.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadoglou NP, Moulakakis KG, Papadakis I, Ikonomidis I, Alepaki M, Lekakis J, Liapis CD. Changes in aortic pulse wave velocity of patients undergoing endovascular repair of abdominal aortic aneurysms. J Endovasc Ther. 2012;19:661–666. doi: 10.1583/JEVT-12-3916MR.1. [DOI] [PubMed] [Google Scholar]
- 23.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1660–5. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran CS, Jose RJ, Moxon JV, Roomberg A, Norman PE, Rush C, Körner H, Golledge J. Everolimus limits aortic aneurysm in the apolipoprotein E-deficient mouse by downregulating C-C chemokine receptor 2 positive monocytes. Arterioscler Thromb Vasc Biol. 2013;33:814–821. doi: 10.1161/ATVBAHA.112.301006. [DOI] [PubMed] [Google Scholar]
- 25.Krishna SM, Seto SW, Moxon JV, Rush C, Walker PJ, Norman PE, Golledge J. Fenofibrate increases high-density lipoprotein and sphingosine 1 phosphate concentrations limiting abdominal aortic aneurysm progression in a mouse model. Am J Pathol. 2012;181:706–718. doi: 10.1016/j.ajpath.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angII-Infused apoE deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 27.Seto SW, Krishna SM, Yu H, Liu D, Khosla S, Golledge J. Impaired acetylcholine-induced endothelium-dependent aortic relaxation by caveolin-1 in angiotensin II-infused apolipoprotein-E (ApoE−/−) knockout mice. PLoS One. 2013;8:e58481. doi: 10.1371/journal.pone.0058481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rush C, Nyara M, Moxon JV, Trollope A, Cullen B, Golledge J. Whole genome expression analysis within the angiotensin II-apolipoprotein E deficient mouse model of abdominal aortic aneurysm. BMC Genomics. 2009;10:298. doi: 10.1186/1471-2164-10-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanematsu Y, Kanematsu M, Kurihara C, Tsou TL, Nuki Y, Liang EI, Makino H, Hashimoto T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension. 2010;55:1267–1274. doi: 10.1161/HYPERTENSIONAHA.109.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golledge AL, Walker P, Norman PE, Golledge J. A systematic review of studies examining inflammation associated cytokines in human abdominal aortic aneurysm samples. Dis Markers. 2009;26:181–188. doi: 10.3233/DMA-2009-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC. Phenotypic alteration of vascular smooth muscle cells precedes elastolys is in a mouse model of Marfan syndrome. Circ Res. 2001;88:37–43. doi: 10.1161/01.res.88.1.37. [DOI] [PubMed] [Google Scholar]
- 33.Pai A, Leaf EM, El-Abbadi M, Giachelli CM. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol. 2011;178:764–173. doi: 10.1016/j.ajpath.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rzucidlo EM. Signaling pathways regulating vascular smooth muscle cell differentiation. Vascular. 2009;17 (Suppl 1):S15–20. doi: 10.2310/6670.2008.00089. [DOI] [PubMed] [Google Scholar]
- 35.Carrillo-Sepúlveda MA, Barreto-Chaves ML. Phenotypic modulation of cultured vascular smooth muscle cells: a functional analysis focusing on MLC and ERK1/2 phosphorylation. Mol Cell Biochem. 2010;341:279–289. doi: 10.1007/s11010-010-0459-9. [DOI] [PubMed] [Google Scholar]
- 36.Appel S, Morgan KG. Scaffolding proteins and non-proliferative functions of ERK1/2. Commun Integr Biol. 2010;3:354–356. doi: 10.4161/cib.3.4.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Je HD, Gangopadhyay SS, Ashworth TD, Morgan KG. Calponin is required for agonist-induced signal transduction--evidence from an antisense approach in ferret smooth muscle. J Physiol. 2001;537(Pt 2):567–577. doi: 10.1111/j.1469-7793.2001.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsakiridis T, Bergman A, Somwar R, Taha C, Aktories K, Cruz TF, Klip A, Downey GP. Actin filaments facilitate insulin activation of the src and collagen homologous/mitogen-activated protein kinase pathway leading to DNA synthesis and c-fos expression. Biol Chem. 1998;273:28322–31. doi: 10.1074/jbc.273.43.28322. [DOI] [PubMed] [Google Scholar]
- 39.Sugawara A, Takeuchi K, Uruno A, Ikeda Y, Arima S, Kudo M, Sato K, Taniyama Y, Ito S. Transcriptional suppression of type 1 AngII receptor gene expression by peroxisome proliferator-activated receptor-gamma in vascular smooth muscle cells. Endocrinology. 2001;142:3125–3134. doi: 10.1210/endo.142.7.8272. [DOI] [PubMed] [Google Scholar]
- 40.Fu M, Zhang J, Lin YgYg, Zhu X, Willson TM, Chen YE. Activation of peroxisome proliferator-activated receptor gamma inhibits osteoprotegerin gene expression in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2002;294:597–601. doi: 10.1016/S0006-291X(02)00533-8. [DOI] [PubMed] [Google Scholar]
- 41.Tham DM, Martin-McNulty B, Wang YX, Wilson DW, Vergona R, Sullivan ME, Dole W, Rutledge JC. Angiotensin II is associated with activation of NF-kappaB-mediated genes and downregulation of PPARs. Physiol Genomics. 2002;11:21–30. doi: 10.1152/physiolgenomics.00062.2002. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian V, Golledge J, Heywood EB, Bruemmer D, Daugherty A. Regulation of peroxisome proliferator-activated receptor-γ by angiotensin II via transforming growth factor-β1-activated p38 mitogen-activated protein kinase in aortic smooth muscle cells. Arterioscler Thromb Vasc Biol. 2012;32:397–405. doi: 10.1161/ATVBAHA.111.239897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchesi C, Rehman A, Rautureau Y, Kasal DA, Briet M, Leibowitz A, Simeone SM, Ebrahimian T, Neves MF, Offermanns S, Gonzalez FJ, Paradis P, Schiffrin EL. Protective role of vascular smooth muscle cell PPARγ in angiotensin II-induced vascular disease. Cardiovasc Res. 2013;97:562–570. doi: 10.1093/cvr/cvs362. [DOI] [PubMed] [Google Scholar]
- 44.Chen F, Wang M, O’Connor JP, He M, Tripathi T, Harrison LE. Phosphorylation of PPARgamma via active ERK1/2 leads to its physical association with p65 and inhibition of NF-kappabeta. J Cell Biochem. 2003;90:732–744. doi: 10.1002/jcb.10668. [DOI] [PubMed] [Google Scholar]
- 45.Stolina M, Guo J, Faggioni R, Brown H, Senaldi G. Regulatory effects of osteoprotegerin on cellular and humoral immune responses. Clin Immunol. 2003;109:347–354. doi: 10.1016/j.clim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Costantino CM, Ploegh HL, Hafler DA. Cathepsin S regulates class II MHC processing in human CD4+ HLA-DR+ T cells. J Immunol. 2009;183:945–952. doi: 10.4049/jimmunol.0900921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lohoefer F, Reeps C, Lipp C, Rudelius M, Zimmermann A, Ockert S, Eckstein HH, Pelisek J. Histopathological analysis of cellular localization of cathepsins in abdominal aortic aneurysm wall. Int J Exp Path. 2012;93:252–258. doi: 10.1111/j.1365-2613.2012.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.