Abstract
Background and Aims
Evidence continues to accumulate regarding the association between health related quality of life (HRQL) and survival across chronic diseases. The aims of the present study were to investigate the prognostic value of HRQL in patients with hepatocellular carcinoma and cholangio carcinoma after adjusting for sociodemographic, disease-, and treatment-related factors.
Methods
A total of 321 patients diagnosed with hepatocellular or cholangio carcinoma were administered the Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) instrument. Cox regression and Kaplan Meier survival analyses were performed to test the association between the five domains of HRQL and survival.
Results
Using Cox regression, overall HRQL was found to be significantly associated with survival (p=0.003), after adjusting for demographic, disease-specific factors and treatment. Subscales of the FACT-Hepatobiliary, including the physical well-being (p=0.02) and the Symptoms and Side Effects subscale (p=0.05), were also found to be significantly associated with survival after adjusting for demographic, disease specific factors, and treatment.
Conclusion
Health related quality of life was found to be prognostic of survival in patients with hepatocellular and cholangio carcinoma while covarying for demographic, disease-specific factors, and treatment. Stratifyng patients based on HRQL when testing novel treatments may be recommended.
Introduction
Evidence is accumulating in regard to the prognostic value of health related quality of life (HRQL) across chronic illnesses.1–25 The value of HRQL in predicting survival has been most extensively studied in oncology.6–25 With a single published exception,1 studies consistently show that higher HRQL is associated with longer survival across cancer types.3–24 In addition to overall HRQL, specific symptoms such as anorexia, nausea, weakness, and appetite have been found to be significant predictors of survival in advanced cancer.2–4
It is unclear whether HRQL holds similar prognostic value for survival in patients diagnosed with advanced cancer such as hepatocellular (HCC) and cholangio carcinoma (CCC) as these cancer types present at diagnosis at advanced stages and often with a poor prognosis. Poon and colleagues began to address the association between HRQL and survival in patients with resectable HCC, which have a better prognosis than those who cannot undergo surgical interventions.5 The majority of cases of HCC and CCC in the U.S. present at advanced stages and are unresectable (≥80%). No study to date has tested the link between HRQL and survival in patients who are unresectable and who do undergo surgical intervention to treat HCC and CCC. This is an important omission in the literature, as it is expected that the incidence of hepatocellular carcinoma will double in the next decade due to the increased incidence of hepatitis C and the majority of patients will be those who cannot undergo surgical resection or transplantation.6 Moreover, quality of life is of particular importance in this cancer population due to the modest survival benefits reported with available treatments.7, 8
Limitations of previous research concerning the HRQL as a prognostic indicator for survival in cancer has (1) assumed that the relationship between HRQL and survival is linear, (2) has not included all the data in the analyses (e.g., only used data in upper and lower quartiles of overall HRQL scores), (3) utilized general HRQL instruments rather than disease-specific instruments,2–4,9–14 and/or (4) specific to this patient population only included patients who were eligible for surgical intervention and, as a result, had better prognosis. The study will also examine whether each of the domains of a disease-specific instrument measuring HRQL is associated with survival. It was expected that overall HRQL would remain a prognostic indicator of survival, despite the poor prognosis associated with HCC and CCC. The symptom and side effect domain of the FACT-Hep was also expected to be associated with survival. All available data were analyzed and linear and non-linear relationships between HRQL subscale scores and survival were explored.
Methods
Design
The study was prospective in design and data was collected between August 2000 and September 2009.
Participants
Three hundred and twenty-one patients diagnosed with hepatocellular (HCC) or cholangio carcinoma (CCC) were recruited from the University of Pittsburgh Medical Center. Inclusion and exclusion criterion specified that (1) the patient was diagnosed with radiographic or biopsy proven HCC or CCC, (2) was 18 years or older, and (3) fluent in English; (4) no evidence of suicidal or homicidal ideation, hallucinations, or delusions; or (5) patient did not undergo orthotopic liver transplant. Patients included in this study were recruited as part of ongoing psychosocial research (NCIK07CA118576; NCIR21CA127046; American Cancer Society) and inclusion was not based on treatment.
Sociodemographic, Disease-Specific Factors, and Treatment
Sociodemographic, disease, and treatment-specific information was collected from medical records. Survival was assessed from the data of diagnosis until death and then recorded in days. If the patient was lost to follow-up, the patient’s date of death was obtained through the Social Security Death Index.
Instruments/Assessment
The Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep)15 was used to assess changes in symptoms and side effects of treatment. The FACT-Hep includes both the FACT-General16 (a 27-item instrument that measures four dimensions of quality of life) and a module with 18 items specific to hepatobiliary disease15. The FACT-G has four subscales including a physical (PWB), social and family (SFWB), emotional (EWB), and functional well-being (FWB). The hepatobiliary module includes items that pertain to symptoms of the disease as well as side effects of the treatment. The FACT is one of the most widely utilized quality of life questionnaires in clinical trials for new cancer treatments and both the FACT-G, as well as the FACT-Hep, have been demonstrated to be valid and reliable instruments.15,16
Procedure
The study received Institutional Review Board approval and patients provided written informed consent prior to their participation. Upon receiving written consent, patients were administered a battery of questionnaires including the FACT-Hepatobiliary by a clinical psychologist. The patient’s HRQL was assessed at the time of diagnosis and patients were followed until death or until lost to follow-up.
Data Analysis
The data were analyzed using SPSS v.21. Descriptive statistics were performed to describe sociodemographic, disease, and treatment specific characteristics of the sample. Cox Regression analyses was employed and all available sociodemographic (i.e., age, gender); disease-specific variables (i.e., Model End Stage Liver Disease [MELD] score, tumor size, number of lesions, vascularity of lesion, vascular invasion); and treatment were entered into the equation followed by the overall HRQL score, as well as the five domains of the FACT-Hep (e.g., physical well-being, emotional well-being).
Results
Three hundred and forty-five patients were approached for participation in the present study. Of the 345 patients, 321 (93%) patients agreed to participate in the study. There were no significant differences between those patients who agreed to participate versus those who refused to participate on sociodemographic, disease or treatment-related factors. The majority of the sample was male (76%), which is relatively consistent with the 2:1 ratio often observed in this cancer type in North America and Europe.30 The mean age was 65 years (range 27 to 94 years). The median survival was 268 days and 71% of the patients had died at the time of analyses. Further details of the sociodemographic, disease, and treatment-related characteristics of this sample can be found in Table 1.
Table 1.
Sociodemographic, disease-, and treatment-related characteristics of sample
| Male (n, %) | 217 (76) |
| Age (years) | |
| Mean (range) | 65 (27–94) |
| Model for End Stage Disease (MELD) | |
| Mean (range) | 10 (6–26) |
| Tumor Size (cm) | |
| Mean (range) | 6.6 (1–20) |
| Number of Lesions | |
| Mean (range) | 3.4 (1–6) |
| Vascular invasion (n, %) | 104 (41) |
| Vascularity (n, %) | |
| Hypovascular | 46 (20) |
| Hypervascular | 177 (76) |
| Mixed | 11 ( 5) |
| Treatment | |
| No Treatment | 15 (5) |
| TACE | 153 (54) |
| 90-Yttrium | 82 (29) |
| Radiofrequency Ablation | 9 (3) |
| Resection | 26 (9) |
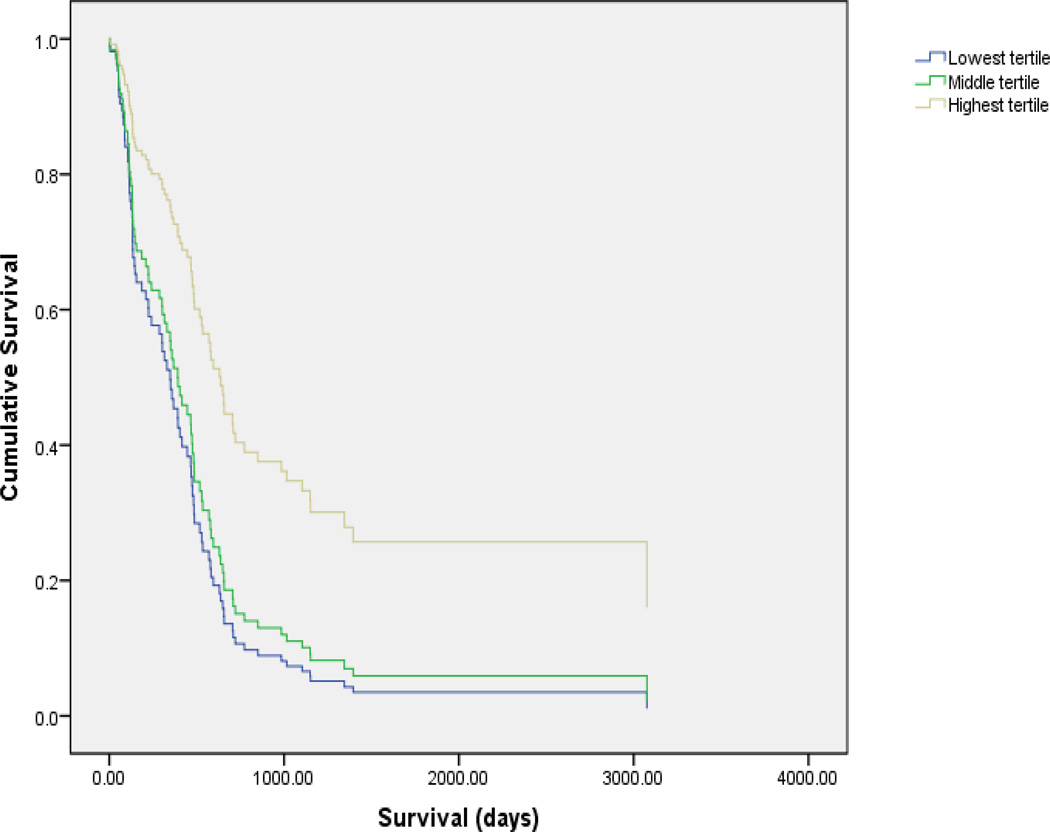
Cox regression analyses were performed to test a model that included all available sociodemographic, disease-specific variables, treatment type, and HRQL on survival. None of these significantly predicted survival in the present study. Overall HRQL was found to be significantly associated with survival after adjusting for demographic, disease-related, and treatment type (p=0.03). See Table 2 and Figure 1.
Table 2.
Cox regression analyses of health related quality of life and survival after adjusting for demographic, disease-, and treatment -related predictors
| B | p-value | Hazard Ratio |
95% Confidence Interval | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| AGE | |||||
| Ref: <50 years | .055 | .892 | 1.056 | .480 | 2.325 |
| GENDER | |||||
| Ref: male | .451 | .164 | 1.570 | .832 | 2.962 |
| MELD | .020 | .504 | 1.020 | .963 | 1.080 |
| TUMOR SIZE | |||||
| Ref: <5cm | .507 | .035 | 1.660 | 1.036 | 2.660 |
| LESION NUMBER | |||||
| Ref: <2 | -1.010 | .000 | .364 | .225 | .590 |
| VASCULARITY | |||||
| Ref: hyper | .214 | ||||
| Mixed | −.963 | .080 | .382 | .130 | 1.122 |
| Hypo | −.935 | .121 | .393 | .120 | 1.281 |
| VASCULAR INVASION | .817 | .001 | 2.264 | 1.380 | 3.713 |
| TREATMENT | |||||
| Ref: TACE | .626 | ||||
| 90 Yttrium | .071 | .897 | 1.074 | .365 | 3.157 |
| No treatment | −.242 | .671 | .785 | .257 | 2.397 |
| RFA | 1.145 | .329 | 3.143 | .315 | 31.371 |
| Resection | −.098 | .904 | .906 | .184 | 4.457 |
| HRQL | |||||
| Ref: Lowest tertile | .009 | ||||
| Middle tertile | .904 | .003 | 2.469 | 1.351 | 4.514 |
| Highest tertile | .734 | .018 | 2.084 | 1.135 | 3.824 |
Figure 1.
Additional subscales of the FACT-Hepatobiliary were also found to be associated with survival including the physical well-being (p=0.02) and symptoms and side effects (HepCS) subscale (p=0.03) after adjusting for demographic and disease specific factors. See Table 3–4.
Table 3.
Cox regression analyses of Physical Well-Being Scale of the FACT-Hepatobiliary and survival after adjusting for demographic, disease-, and treatment-related predictors
| Variable | B | p-value | Hazard Ratio |
95% Confidence Interval |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| AGE | |||||
| Ref:<50 years | −.097 | .800 | .907 | .428 | 1.926 |
| GENDER | |||||
| Ref: male | .448 | .123 | 1.566 | .886 | 2.768 |
| MELD | .035 | .199 | 1.036 | .982 | 1.093 |
| TUMOR SIZE | |||||
| Ref: <5cm | .580 | .014 | 1.787 | 1.124 | 2.840 |
| LESION NUMBER | |||||
| Ref: <2 | −.857 | .000 | .424 | .265 | .678 |
| VASCULARITY | |||||
| Ref: hyper | .267 | ||||
| Hyper | −.894 | .116 | .409 | .134 | 1.245 |
| Mixed | −.958 | .117 | .384 | .116 | 1.270 |
| VASCULAR INVASION | .688 | .005 | 1.991 | 1.225 | 3.236 |
| TREATMENT | |||||
| Ref: TACE | .245 | ||||
| 90 Yttrium | .275 | .575 | 1.316 | .503 | 3.443 |
| No treatment | −.216 | .688 | .806 | .280 | 2.316 |
| RFA | −1.083 | .338 | .338 | .037 | 3.105 |
| Resection | −.459 | .564 | .632 | .133 | 3.011 |
| Physical Well-Being | |||||
| Ref: lowest tertile | .015 | ||||
| Middle tertile | .730 | .008 | 2.075 | 1.210 | 3.560 |
| Highest tertile | .068 | .809 | 1.070 | .616 | 1.859 |
Table 4.
Cox regression analyses of Hepatobiliary Module (symptoms and side effects) of the FACT-Hepatobiliary and survival after adjusting for demographic, disease-, and treatment-related predictors
| Variable | B | p-value | Hazard Ratio |
95% Confidence Interval |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| AGE | |||||
| Ref: <50 years | .037 | .925 | 1.038 | .479 | 2.249 |
| GENDER | |||||
| Ref: male | .406 | .177 | 1.501 | .833 | 2.706 |
| MELD | .006 | .849 | 1.006 | .949 | 1.066 |
| TUMOR SIZE | |||||
| Ref: <5cm | .481 | .043 | 1.617 | 1.016 | 2.574 |
| LESION NUMBER | |||||
| Ref: <2 | −.786 | .001 | .456 | .289 | .719 |
| VASCULARITY | |||||
| Ref: hypervascular | .248 | ||||
| Mixed vascularity | −.854 | .120 | .426 | .145 | 1.251 |
| Hypovascular | −.966 | .099 | .381 | .121 | 1.201 |
| VASCULAR INVASION | .815 | .001 | 2.259 | 1.402 | 3.641 |
| TREATMENT | |||||
| Ref: TACE | .244 | ||||
| 90 Yttrium | .655 | .189 | 1.924 | .725 | 5.107 |
| No treatment | .240 | .659 | 1.271 | .439 | 3.679 |
| Radiofrequency Ablation | 1.661 | .160 | 5.265 | .518 | 53.492 |
| Resection | .145 | .857 | 1.156 | .238 | 5.622 |
| HepCS | |||||
| Ref: lowest tertile | .043 | ||||
| Middle tertile | .686 | .028 | 1.986 | 1.075 | 3.668 |
| Highest tertile | .678 | .025 | 1.970 | 1.088 | 3.568 |
Discussion
Consistent with prior research, overall HRQL was found to be associated with survival in patients diagnosed with HCC. Using all available data, a linear relationship was found between overall HRQL and survival. Patients reporting the highest level of overall HRQL were found to have the longest survival followed by those in the middle and lowest tertile of overall HRQL. A similar pattern was observed between symptoms and side effects subscale of the FACT-Hepatobiliary with high levels of symptoms and/or side effects being associated with decreased survival. Recent research has begun to explore the association between symptom clusters (e.g., sickness behavior) and associated biomarkers such as cytokines.17 Although further research is needed to understand the possible association between symptoms, underlying biological mechanisms and disease progression; the results of the present study reflect a possible association between symptoms and side effects of treatment for HCC and survival.
As would be expected, trends toward significance were observed, where patients who had low levels of physical and functional well-being also experienced increased mortality. Consistent with prior research including studies performed our team, 28,31,42–47 a trend toward significance was observed between emotional well-being and survival, in which low levels of reported emotional well-being were found to be associated with increased mortality. One possible explanation to explain the association between depression and survival may include immune system dysregulation, which has been previously reported by this team.45
The present study addressed the limitations of prior research including adjusting for sociodemographic (e.g., age, gender), disease (e.g., tumor size, vascular invasion), and treatment-related predictors in the model that tested the association between HRQL and survival. The investigators used all available data and analyzed linear and non-linear relationships between HRQL and survival. A disease-specific HRQL instrument was also employed in the present study and the sample included patients who were not recommended for treatment as well as those who received surgical and nonsurgical intervention for hepatocellular carcinoma.
The limitations of the present study included the small number of patients treated with surgical interventions. However, Poon and colleagues only included patients who underwent resection and reported similar results in that HRQL was predictive of survival in a cohort of resectable HCC patients.24 The large confidence intervals observed in the Cox regression analyses for the treatment variables may reflect a less precise hazard ratio. However, secondary to the multiple studies across cancer types as well as the results reported by Poon and colleagues, we have confidence that the association between HRQL and survival is likely accurate across treatment types.24 The MELD score was included in the model as this is the staging system used at our center. If a different staging system was included in the model (e.g., TNM, CLIP) it may have resulted in differential findings. Finally, we did not include those patients who underwent liver transplantation due to the small number of patients (<10%) and significant difference in survival rates between those who undergo liver transplantation versus those who undergo resection, radiofrequency ablation and/or regional chemotherapy or radiation. It is possible that the findings may be different if these patients were included in the analyses.
Research continues to mount regarding the prognostic value of HRQL. Accordingly, future research in the area of HRQL and survival should explore possible mediating and moderating variables between HRQL and survival. Only until recently have randomized controlled trials become possible due to the modest benefits many previous treatments offered to this patient population. With the advent of Nexavar and combinations of drugs using TACE, the increase in randomized controlled trials testing the efficacy of novel treatments in patients with hepatocellular carcinoma may rise. Baseline HRQL may facilitate the stratification of patients to treatment arms in clinical trials or the interpretation of results of clinical trials testing the efficacy of novel treatment strategies for this patient population. Outside the context of a clinical trial, baseline HRQL may also facilitate clinical decisions regarding treatment options for patients diagnosed with hepatocellular carcinoma.
Acknowledgments
Grant Support: NCIK07CA118576; NCIR21CA127046
Contributor Information
Jennifer Steel, University of Pittsburgh, Department of Surgery, Psychiatry, and Psychology.
David Geller, University of Pittsburgh, Department of Surgery.
Tiana Robinson, University of Pittsburgh, Department of Surgery.
Alexandra Savkova, University of Pittsburgh, Department of Surgery.
Deborah Brower, University of Pittsburgh, Department of Surgery.
J. Wallis Marsh, University of Pittsburgh, Department of Surgery.
Allan Tsung, University of Pittsburgh, Department of Surgery.
References
- 1.Addington-Hall JM, MacDonald LD, Anderson HR. Can the Spitzer Quality of Life Index help to reduce prognostic uncertainty in terminal care? British Journal of Cancer. 1990;62:695–699. doi: 10.1038/bjc.1990.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lis CG, Gupta D, Granick J, Grutsch JF. Can patient satisfaction with quality of life predict survival in advanced colorectal cancer?[see comment] Supportive Care in Cancer. 2006;14:1104–1110. doi: 10.1007/s00520-006-0100-3. [DOI] [PubMed] [Google Scholar]
- 3.Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. Journal of Clinical Oncology. 2000;18:646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 4.Park SM, Park MH, Won JH, et al. EuroQol and survival prediction in terminal cancer patients: a multicenter prospective study in hospice-palliative care units. Supportive Care in Cancer. 2006;14:329–333. doi: 10.1007/s00520-005-0889-1. [DOI] [PubMed] [Google Scholar]
- 5.Poon RT, Fan ST, Yu WC, Lam BK, Chan FY, Wong J. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg. 2001;136:693–699. doi: 10.1001/archsurg.136.6.693. [DOI] [PubMed] [Google Scholar]
- 6.el-Serag HB, Kunik M, Richardson P, Rabeneck L. Psychiatric disorders among veterans with hepatitis C infection. Gastroenterology. 2002;123:476–482. doi: 10.1053/gast.2002.34750. [DOI] [PubMed] [Google Scholar]
- 7.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 8.Narkush RE, Schwab JJ, Farris P, Present PA, Holzer CE., 3rd Mortality and community mental health. The Alachua County, Florida, mortality study. Archives of General Psychiatry. 1977;34:1393–1401. doi: 10.1001/archpsyc.1977.01770240019001. [DOI] [PubMed] [Google Scholar]
- 9.Coates A, Forbes J, Simes RJ. Prognostic value of performance status and quality-of-life scores during chemotherapy for advanced breast cancer. The Australian New Zealand Breast Cancer Trials Group.[comment] Journal of Clinical Oncology. 1993;11:2050. [PubMed] [Google Scholar]
- 10.Coates A, Gebski V, Signorini D, et al. Prognostic value of quality-of-life scores during chemotherapy for advanced breast cancer. Australian New Zealand Breast Cancer Trials Group.[see comment] Journal of Clinical Oncology. 1992;10:1833–1838. doi: 10.1200/JCO.1992.10.12.1833. [DOI] [PubMed] [Google Scholar]
- 11.Kramer JA, Curran D, Piccart M, et al. Identification and interpretation of clinical and quality of life prognostic factors for survival and response to treatment in first-line chemotherapy in advanced breast cancer. European Journal of Cancer. 2000;36:1498–1506. doi: 10.1016/s0959-8049(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan PW, Nelson JB, Mulani PM, Sleep D. Quality of life as a potential predictor for morbidity and mortality in patients with metastatic hormone-refractory prostate cancer. Quality of Life Research. 2006;15:1297–1306. doi: 10.1007/s11136-006-0003-2. [DOI] [PubMed] [Google Scholar]
- 13.Dancey J, Zee B, Osoba D, et al. Quality of life scores: an independent prognostic variable in a general population of cancer patients receiving chemotherapy. The National Cancer Institute of Canada Clinical Trials Group. Quality of Life Research. 1997;6:151–158. doi: 10.1023/a:1026442201191. [DOI] [PubMed] [Google Scholar]
- 14.Ringdal GI, Ringdal K, Kvinnsland S, Gotestam KG. Quality of life of cancer patients with different prognoses. Quality of Life Research. 1994;3:143–154. doi: 10.1007/BF00435257. [DOI] [PubMed] [Google Scholar]
- 15.Heffernan N, Cella D, Webster K, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. Journal of Clinical Oncology. 2002;20:2229–2239. doi: 10.1200/JCO.2002.07.093. [DOI] [PubMed] [Google Scholar]
- 16.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. Journal of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan GA, Reynolds P. Depression and cancer mortality and morbidity: prospective evidence from the Alameda County study. J Behav Med. 1988;11:1–13. doi: 10.1007/BF00846165. [DOI] [PubMed] [Google Scholar]
- 18.Bruce ML, Leaf PJ. Psychiatric disorders and 15-month mortality in a community sample of older adults. Am J Public Health. 1989;79:727–730. doi: 10.2105/ajph.79.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]; 18 Cunningham WE, Crystal S, Bozzette S, Hays RD. The association of health-related quality of life with survival among persons with HIV infection in the United States. Journal of General Internal Medicine. 2005;20:21–27. doi: 10.1111/j.1525-1497.2005.30402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valdes C, Garcia-Mendoza M, Rebollo P, Ortega T, Ortega F. Mental health at the third month of haemodialysis as a predictor of short-term survival. Nephrol Dial Transplant. 2006;21:3223–3230. doi: 10.1093/ndt/gfl392. [DOI] [PubMed] [Google Scholar]
- 20.Squier HC, Ries AL, Kaplan RM, et al. Quality of well-being predicts survival in lung transplantation candidates. American Journal of Respiratory & Critical Care Medicine. 1995;152:2032–2036. doi: 10.1164/ajrccm.152.6.8520772. [DOI] [PubMed] [Google Scholar]
- 21.Kaasa S, Mastekaasa A, Lund E. Prognostic factors for patients with inoperable non-small cell lung cancer, limited disease. The importance of patients' subjective experience of disease and psychosocial well-being. Radiother Oncol. 1989;15:235–242. doi: 10.1016/0167-8140(89)90091-1. [DOI] [PubMed] [Google Scholar]
- 22.Rumsfeld JS, MaWhinney S, McCarthy M, Jr, et al. Health-related quality of life as a predictor of mortality following coronary artery bypass graft surgery. Participants of the Department of Veterans Affairs Cooperative Study Group on Processes, Structures, and Outcomes of Care in Cardiac Surgery. JAMA. 1999;281:1298–1303. doi: 10.1001/jama.281.14.1298. [DOI] [PubMed] [Google Scholar]
- 23.Langendijk H, Aaronson NK, de Jong JM, ten Velde GP, Muller MJ, Wouters M. The prognostic impact of quality of life assessed with the EORTC QLQ-C30 in inoperable non-small cell lung carcinoma treated with radiotherapy. Radiother Oncol. 2000;55:19–25. doi: 10.1016/s0167-8140(00)00158-4. [DOI] [PubMed] [Google Scholar]
- 24.Dharma-Wardene M, Au HJ, Hanson J, Dupere D, Hewitt J, Feeny D. Baseline FACT-G score is a predictor of survival for advanced lung cancer. Quality of Life Research. 2004;13:1209–1216. doi: 10.1023/B:QURE.0000037481.36604.eb. [DOI] [PubMed] [Google Scholar]
- 25.Herndon JE, 2nd, Fleishman S, Kornblith AB, Kosty M, Green MR, Holland J. Is quality of life predictive of the survival of patients with advanced nonsmall cell lung carcinoma? Cancer. 1999;85:333–340. doi: 10.1002/(sici)1097-0142(19990115)85:2<333::aid-cncr10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR. Quality of life in lung cancer patients: as an important prognostic factor. Lung Cancer. 2001;31:233–240. doi: 10.1016/s0169-5002(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 27.Addington-Hall JM, MacDonald LD, Anderson HR. Can the Spitzer Quality of Life Index help to reduce prognostic uncertainty in terminal care? British Journal of Cancer. 1990;62:695–699. doi: 10.1038/bjc.1990.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coates A, Forbes J, Simes RJ. Prognostic value of performance status and quality-of-life scores during chemotherapy for advanced breast cancer. The Australian New Zealand Breast Cancer Trials Group.[comment] Journal of Clinical Oncology. 1993;11:2050. [PubMed] [Google Scholar]
- 29.Coates A, Gebski V, Signorini D, et al. Prognostic value of quality-of-life scores during chemotherapy for advanced breast cancer. Australian New Zealand Breast Cancer Trials Group.[see comment] Journal of Clinical Oncology. 1992;10:1833–1838. doi: 10.1200/JCO.1992.10.12.1833. [DOI] [PubMed] [Google Scholar]
- 30.Kramer JA, Curran D, Piccart M, et al. Identification and interpretation of clinical and quality of life prognostic factors for survival and response to treatment in first-line chemotherapy in advanced breast cancer. European Journal of Cancer. 2000;36:1498–1506. doi: 10.1016/s0959-8049(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 31.Lis CG, Gupta D, Granick J, Grutsch JF. Can patient satisfaction with quality of life predict survival in advanced colorectal cancer?[see comment] Supportive Care in Cancer. 2006;14:1104–1110. doi: 10.1007/s00520-006-0100-3. [DOI] [PubMed] [Google Scholar]
- 32.Ramsey SD, Andersen MR, Etzioni R, et al. Quality of life in survivors of colorectal carcinoma. Cancer. 2000;88:1294–1303. [PubMed] [Google Scholar]
- 33.Blazeby JM, Brookes ST, Alderson D. The prognostic value of quality of life scores during treatment for oesophageal cancer. Gut. 2001;49:227–230. doi: 10.1136/gut.49.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan PW, Nelson JB, Mulani PM, Sleep D. Quality of life as a potential predictor for morbidity and mortality in patients with metastatic hormone-refractory prostate cancer. Quality of Life Research. 2006;15:1297–1306. doi: 10.1007/s11136-006-0003-2. [DOI] [PubMed] [Google Scholar]
- 35.Meyers CA, Hess KR, Yung WK, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. Journal of Clinical Oncology. 2000;18:646–650. doi: 10.1200/JCO.2000.18.3.646. [DOI] [PubMed] [Google Scholar]
- 36.Dancey J, Zee B, Osoba D, et al. Quality of life scores: an independent prognostic variable in a general population of cancer patients receiving chemotherapy. The National Cancer Institute of Canada Clinical Trials Group. Quality of Life Research. 1997;6:151–158. doi: 10.1023/a:1026442201191. [DOI] [PubMed] [Google Scholar]
- 37.Park SM, Park MH, Won JH, et al. EuroQol and survival prediction in terminal cancer patients: a multicenter prospective study in hospice-palliative care units. Supportive Care in Cancer. 2006;14:329–333. doi: 10.1007/s00520-005-0889-1. [DOI] [PubMed] [Google Scholar]
- 38.Ringdal GI, Ringdal K, Kvinnsland S, Gotestam KG. Quality of life of cancer patients with different prognoses. Quality of Life Research. 1994;3:143–154. doi: 10.1007/BF00435257. [DOI] [PubMed] [Google Scholar]
- 39.Tamburini M, Brunelli C, Rosso S, Ventafridda V. Prognostic value of quality of life scores in terminal cancer patients. Journal of Pain & Symptom Management. 1996;11:32–41. doi: 10.1016/0885-3924(95)00135-2. [DOI] [PubMed] [Google Scholar]
- 40.Vigano A, Donaldson N, Higginson IJ, Bruera E, Mahmud S, Suarez-Almazor M. Quality of life and survival prediction in terminal cancer patients: a multicenter study. Cancer. 2004;101:1090–1098. doi: 10.1002/cncr.20472. [DOI] [PubMed] [Google Scholar]
- 41.Poon RT, Fan ST, Yu WC, Lam BK, Chan FY, Wong J. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg. 2001;136:693–699. doi: 10.1001/archsurg.136.6.693. [DOI] [PubMed] [Google Scholar]
- 42.Heffernan N, Cella D, Webster K, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. Journal of Clinical Oncology. 2002;20:2229–2239. doi: 10.1200/JCO.2002.07.093. [DOI] [PubMed] [Google Scholar]
- 43.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 44.Narkush RE, Schwab JJ, Farris P, Present PA, Holzer CE., 3rd Mortality and community mental health. The Alachua County, Florida, mortality study. Archives of General Psychiatry. 1977;34:1393–1401. doi: 10.1001/archpsyc.1977.01770240019001. [DOI] [PubMed] [Google Scholar]
- 45.Steel JL, Geller DA, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. Journal of Clinical Oncology. 2007;25:2397–2405. doi: 10.1200/JCO.2006.06.4592. [DOI] [PubMed] [Google Scholar]
- 46.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. Journal of Clinical Oncology. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 47.Di Bisceglie AM, Carithers RL, Jr, Gores GJ. Hepatocellular carcinoma. Hepatology. 1998;28(4):1161–1165. doi: 10.1002/hep.510280436. [DOI] [PubMed] [Google Scholar]
- 48.Kaplan GA, Reynolds P. Depression and cancer mortality and morbidity: prospective evidence from the Alameda County study. J Behav Med. 1988;11:1–13. doi: 10.1007/BF00846165. [DOI] [PubMed] [Google Scholar]
- 49.Bruce ML, Leaf PJ. Psychiatric disorders and 15-month mortality in a community sample of older adults. Am J Public Health. 1989;79:727–730. doi: 10.2105/ajph.79.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy JM, Olivier DC, Sobol AM, Monson RR, Leighton AH. Diagnosis and outcome: depression and anxiety in a general population. Psychological Medicine. 1986;16:117–126. doi: 10.1017/s0033291700057809. [DOI] [PubMed] [Google Scholar]
- 51.Bruce ML, Leaf PJ, Rozal GP, Florio L, Hoff RA. Psychiatric status and 9-year mortality data in the New Haven Epidemiologic Catchment Area Study.[see comment] Am J Psychiatry. 1994;151:716–721. doi: 10.1176/ajp.151.5.716. [DOI] [PubMed] [Google Scholar]
- 52.Huppert FA, Whittington JE. Symptoms of psychological distress predict 7-year mortality. Psychological Medicine. 1995;25:1073–1086. doi: 10.1017/s0033291700037569. [DOI] [PubMed] [Google Scholar]
- 53.Somervell PD, Kaplan BH, Heiss G, Tyroler HA, Kleinbaum DG, Obrist PA. Psychologic distress as a predictor of mortality. Am J Epidemiol. 1989;130:1013–1023. doi: 10.1093/oxfordjournals.aje.a115402. [DOI] [PubMed] [Google Scholar]
- 54.Simonsick EM, Wallace RB, Blazer DG, Berkman LF. Depressive symptomatology and hypertension-associated morbidity and mortality in older adults.[see comment] Psychosomatic Medicine. 1995;57:427–435. doi: 10.1097/00006842-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Peveler R, Carson A, Rodin G. Depression in medical patients.[see comment] BMJ. 2002;325:149–152. doi: 10.1136/bmj.325.7356.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carney RM, Blumenthal JA, Freedland KE, et al. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosomatic Medicine. 2004;66:466–474. doi: 10.1097/01.psy.0000133362.75075.a6. [DOI] [PubMed] [Google Scholar]
- 57.Yaffe K, Edwards ER, Covinsky KE, Lui LY, Eng C. Depressive symptoms and risk of mortality in frail, community-living elderly persons. Am J Geriatr Psychiatry. 2003;11:561–567. [PubMed] [Google Scholar]
- 58.Harris EC, Barraclough B. Excess mortality of mental disorder. British Journal of Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- 59.Shekelle RB, Raynor WJ, Jr, Ostfeld AM, et al. Psychological depression and 17-year risk of death from cancer. Psychosom Med. 1981;43:117–125. doi: 10.1097/00006842-198104000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Hoodin F, Kalbfleisch KR, Thornton J, Ratanatharathorn V. Psychosocial influences on 305 adults’ survival after bone marrow transplantation; depression, smoking, and behavioral self-regulation. Journal of Psychosomatic Research. 2004;57:145–154. doi: 10.1016/S0022-3999(03)00599-3. [DOI] [PubMed] [Google Scholar]
- 61.Faller H, Schmidt M. Prognostic value of depressive coping and depression in survival of lung cancer patients. Psychooncology. 2004;13:359–363. doi: 10.1002/pon.783. [DOI] [PubMed] [Google Scholar]
- 62.Hjerl K, Andersen EW, Keiding N, Mouridsen HT, Mortensen PB, Jorgensen T. Depression as a prognostic factor for breast cancer mortality. Psychosomatics. 2003;44:24–30. doi: 10.1176/appi.psy.44.1.24. [DOI] [PubMed] [Google Scholar]
- 63.Goodwin JS, Zhang DD, Ostir GV. Effect of depression on diagnosis, treatment, and survival of older women with breast cancer. J Am Geriatr Soc. 2004;52:106–111. doi: 10.1111/j.1532-5415.2004.52018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Loberiza FR, Jr, Rizzo JD, Bredeson CN, et al. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. Journal of Clinical Oncology. 2002;20:2118–2126. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- 65.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The Continuing Increase in the Incidence of Hepatocellular Carcinoma in the United States: An Update. Annals of Internal Medicine. 2003;139:817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 66.Chau I, Norman AR, Cunningham D, Waters JS, Oates J, Ross PJ. Multivariate prognostic factor analysis in locally advanced and metastatic esophago-gastric cancer--pooled analysis from three multicenter, randomized, controlled trials using individual patient data. Journal of Clinical Oncology. 2004;22:2395–2403. doi: 10.1200/JCO.2004.08.154. [DOI] [PubMed] [Google Scholar]
- 67.Yeo W, Mo FK, Koh J, et al. Quality of life is predictive of survival in patients with unresectable hepatocellular carcinoma.[see comment] Annals of Oncology. 2006;17:1083–1089. doi: 10.1093/annonc/mdl065. [DOI] [PubMed] [Google Scholar]
- 68.de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. European Journal of Cancer. 2001;37:332–339. doi: 10.1016/s0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 69.Efficace F, Biganzoli L, Piccart M, et al. Baseline health-related quality-of-life data as prognostic factors in a phase III multicentre study of women with metastatic breast cancer. European Journal of Cancer. 2004;40:1021–1030. doi: 10.1016/j.ejca.2004.01.014. [DOI] [PubMed] [Google Scholar]