Abstract
Objective
Hb α and eNOS form a macromolecular complex at myoendothelial junctions; the functional role of this interaction remains undefined. To test if coupling of eNOS and Hb α regulates NO signaling, vascular reactivity and blood pressure using a mimetic peptide of Hb α to disrupt this interaction.
Approach and Results
In silico modeling of Hb α and eNOS identified a conserved sequence of interaction. By mutating portions of Hb α, we identified a specific sequence that binds eNOS. A mimetic peptide of the Hb α sequence (Hb α X) was generated to disrupt this complex. Utilizing in vitro binding assays with purified Hb α and eNOS and ex vivo proximity ligation assays on resistance arteries, we have demonstrated that Hb α X significantly decreased interaction between eNOS and Hb α. FITC-labeling of Hb α X revealed localization to holes in the internal elastic lamina (i.e., myoendothelial junctions). To test the functional effects of Hb α X, we measured cGMP and vascular reactivity. Our results reveal augmented cGMP production and altered vasoconstriction with Hb α X. To test the in vivo effects of these peptides on blood pressure, normotensive and hypertensive mice were injected with Hb α X which caused a significant decrease in blood pressure; injection of Hb α X into eNOS−/− mice had no effect.
Conclusion
These results identify a novel sequence on Hb α that is important for Hb α / eNOS complex formation and is critical for nitric oxide signaling at myoendothelial junctions.
Keywords: Endothelial cells, hemoglobin α, endothelial nitric oxide synthase, nitric oxide, myoendothelial junction
INTRODUCTION
Peripheral vascular resistance, an essential component of blood pressure regulation, is tightly governed by arterial blood vessel tone. The regulation of vascular tone involves a complex set of cell-cell signaling mechanisms between the endothelium and vascular smooth muscle, and it is well documented that molecules released from the endothelium (e.g. nitric oxide (NO), endothelium derived hyperpolarizing factor, prostaglandins) profoundly influence this process1–5. For example, signals originating from vascular smooth muscle stimulate the release of endothelium-derived NO to modulate the contractile response during α1-adrenergic-mediated vasoconstriction6, 7. Thus, it is clear that a critical balance between contractile and dilatory signaling events is tightly regulated for proper maintenance of vascular tone.
Several reports have now indicated that myoendothelial junctions (MEJs) could be a key player in regulating the balance between constriction and dilation of small resistance arteries.8–10 The MEJs are anatomical hallmarks where primarily endothelium (depending on vascular bed) breaks through the internal elastic lamina (IEL) and comes into close apposition with the overlying smooth muscle cells, forming gap junctions for direct cell-cell communication (reviewed in11). The MEJs are found in resistance arteries down to terminal arterioles, with little to no MEJs identified in conduit arteries.11 These cellular structures provide a distinct microenvironment at the interface between smooth muscle and endothelium where a number of proteins have been shown to be localized and enriched to influence heterocellular cross talk in the arterial blood vessel wall. Indeed, we have found that eNOS is polarized across vascular beds at MEJs7, and more recently have demonstrated that endothelial cells in resistance arteries synthesize and express hemoglobin α (Hb α) which co-localizes with eNOS at MEJs where it functions to regulate NO diffusion to vascular smooth muscle during α1-adrenergic-dependent vasoconstriction10.
Detailed biochemical analysis of the Hb α heme iron oxidation state, which is controlled by MEJ localized cytochrome B5 reductase 3, has indicated that the oxidation state of the heme iron dictates permissive NO diffusion or NO scavenging10. From these studies, the importance of Hb α at the MEJ both in small arteries and in a vascular cell co-culture (VCCC) was elucidated. Of particular interest, we observed that Hb α and eNOS form a macromolecular protein complex at the MEJ where they participate in protein-protein interaction as shown in small arteries, the VCCC and purified proteins10. These data provide a potential mechanism by which Hb α/eNOS protein-protein interaction may regulate NO signaling, acting to balance the overall constriction with relaxation to ensure tone is maintained. However, the mechanisms describing how native Hb α and eNOS associate and identification of specific protein sequences critical for this interaction remain unknown. Therefore, we hypothesized that possible disruption of the Hb α/eNOS complex at the MEJ would also disrupt the amount of NO available to smooth muscle cells.
A common and powerful method used to disrupt protein-protein interaction has been the use of mimetic peptides directed against a particular sequence where binding between two different proteins occurs. For example, the use of a caveolin-1 disrupting peptide has been demonstrated to have potent effects on reactivity and blood pressure.12–14 We hypothesized that the Hb α/eNOS complex, being unique to resistance arteries, would be an especially attractive target. After in silico modeling of the Hb α/eNOS macromolecular complex, our results demonstrate a highly probable and distinct region of overlap, confirmed via mutational analysis, from which a mimetic peptide with a tat tag to pass through the plasma membrane was derived (termed Hb α X). We show this peptide disrupts Hb α/eNOS complexes from purified proteins and in the actual arterial wall, ex vivo. In addition, we found the peptide localizes to holes in the IEL (i.e., MEJs) and inhibits PE-induced constriction, that is only restored when eNOS is inhibited. Further we show the peptide has no effect on conduit artery constriction or on constriction and blood pressure of eNOS−/− mice, again demonstrating the possible strong ability of the peptide to localize to MEJs in resistance arteries. To our knowledge, this data demonstrates for the first time that targeting a protein enriched at the MEJ can alter vascular reactivity, and possibly blood pressure.
METHODS AND MATERIALS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Hb α X peptide disrupts the interaction between eNOS and Hb α
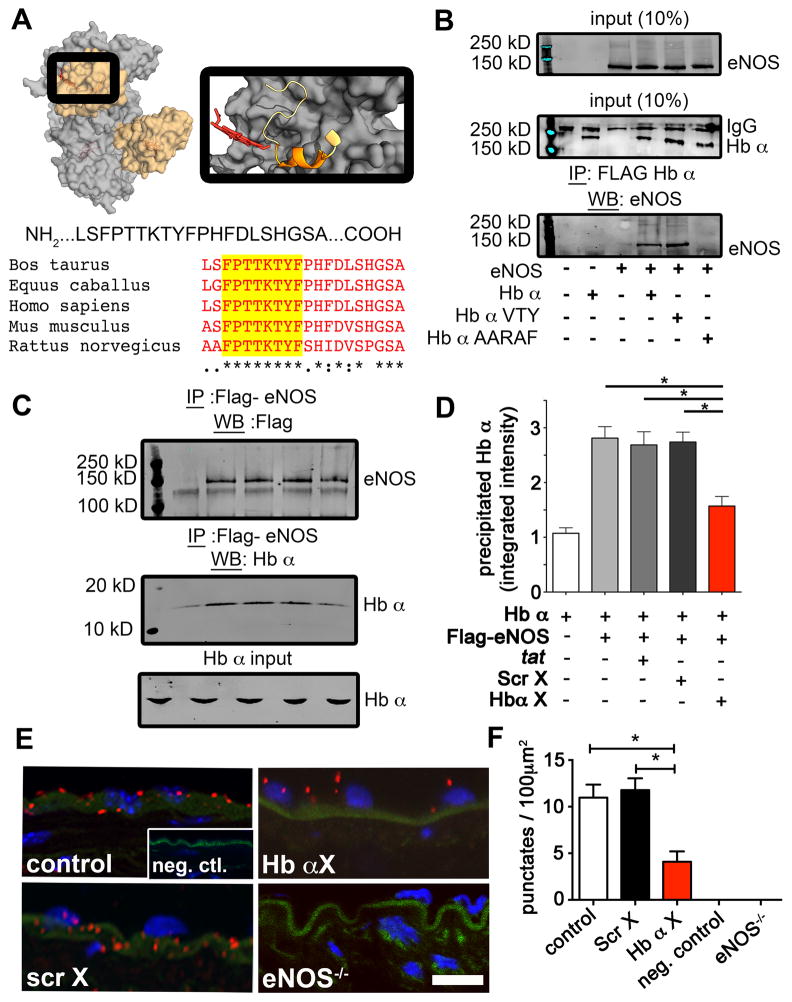
Previous work demonstrated that eNOS and Hb α form a macromolecular complex and can interact10. Therefore, we used in silico modeling of the known crystal structures for the oxygenase domain of eNOS and Hb α to determine potential interactions based on geometric, electrostatic and hydrophobic indices. From this analysis, we found a discreet Hb α sequence (LSFPTTKTYFPHFDLSHGSA) that interacted with eNOS (Fig. 1a). Sequences were subjected to homology analysis among several mammalian species revealing a conserved peptide fragment (Fig. 1a). To confirm if this sequence specifically can bind eNOS, we performed mutational analysis followed by co-immunoprecipitation. Since the proline in position 38 and the phenylalanine in position 44 have been show to be unstable, we mutated two portions of Hb α, LSF and TTKTY. Our results reveal that TTKTY of Hb α is essential for binding to eNOS (Fig 1b). Next, we synthesized the peptide (LSFPTTKTYF) linked to an HIV tat sequence along with a scrambled control (FPYFSTKLTT). The peptides were named Hb α X and Scr X respectively.
Figure 1. Identification of a conserved sequence of Hb α that interacts with eNOS.
a, In silico modeling of the PDB crystal structures for eNOS (gray; 3NOS) and Hb α (orange; 1Y01) using GRAMMX server. The magnified image on right shows the region of Hb α (ribbon structure) that is modeled to interact with eNOS (dark gray region). The identified Hb α sequence is depicted below and was blasted against other mammalian species showing conserved sequences highlighted in yellow. b, Western blot analysis of co-immunoprecipitations experiments from HEK 293 cells overexpressed with eNOS, Flag-Hb α or Flag-Hb α mutants. c, Western blot analysis of Flag-eNOS input, Hb α input and Hb α precipitated with Flag-eNOS (n=3 separate runs). d, Quantification of precipitated Hb α with p<0.05; all error bars represent s.e.m.. e, Proximity ligation assay for eNOS and Hb α (red punctuates) on transverse sections of a mouse thoracodorsal artery. Green shows internal elastic lamina autofluorescence, blue is DAPI indicating nuclei. Scale bar is 10 μm. f, The graph on right shows quantification of red punctates from the proximity ligation assay with p<0.05; all error bars represent s.e.m. (n=3 mice).
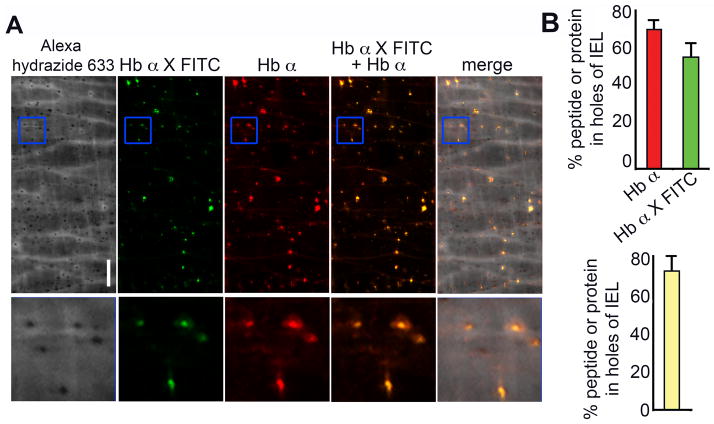
To determine if these peptides competitively inhibited eNOS and Hb α binding, we incubated Flag-eNOS with tat only, Scr X or Hb α X, followed by the addition of purified Hb α chains. Complexes were precipitated and subjected to Western blot analysis, demonstrating that only Hb α X peptide significantly disrupted the eNOS/Hb α interaction (Fig. 1c) and quantified in Fig. 1d). To test this ex vivo, we incubated TD arteries with peptides and measured colocalization of eNOS and Hb α on transverse sections using a proximity ligation assay (Fig. 1e). These studies demonstrate a significant loss of protein-protein association between eNOS and Hb α in the presence of Hb α X (Fig 1e–f). Next, we perfused FITC-tagged Hb α X peptide into the lumen of pressurized TD arteries followed by fixation and immunolabeling for Hb α (Fig. 2a). Analysis of the holes in the IEL revealed that both the FITC-tagged Hb α X peptide and Hb α protein localized to these distinct regions and that the peptide and the protein were found to be in the same holes the majority of the time observed (Fig. 2b). This data provided evidence at the protein level that the Hb α X peptide can localize to the MEJ and disrupt eNOS/Hb α interactions.
Figure 2. Hb α X peptide localizes to holes in the internal elastic lamina of thoracodorsal arteries.
In a, en face immunofluorescence of Alexa hydrazide 633 (grey), FITC-labeled Hb α X – FITC (green) and Hb α (red) on mouse thoracodorsal arteries. Blue boxes indicate enlarged images underneath (10 μm × 10 μm). Scale bar is 10 μm. In b graphs show quantitation of colocalized FITC-labeled Hb α X and Hb α in holes of the IEL. * indicates significance (p<0.05) between conditions and all error bars represent s.e.m.
Hb α X peptide alters NO signaling in the blood vessel wall
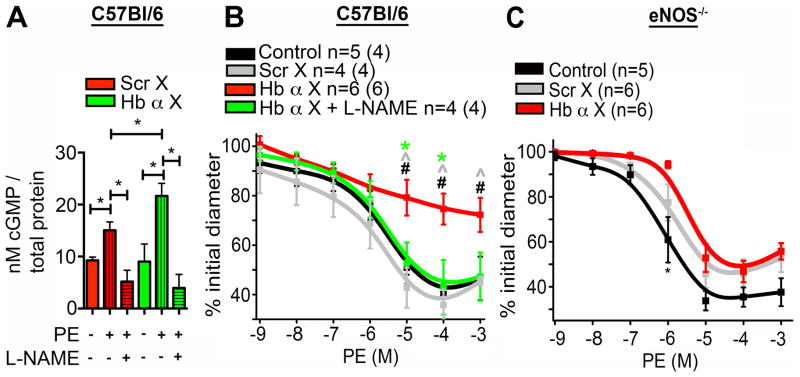
Following α1-adrenergic receptor stimulation, a rise in endothelial cell calcium and activation of eNOS occurs, resulting in increased NO production that can diffuse back to the smooth muscle cell and active soluble guanylyl cyclase resulting in increased cGMP production6, 7, 10, 15. To determine the effects of Hb α X peptide on cGMP accumulation during α1-adrenergic-dependent vasoconstriction, TD arteries were incubated with Scr X or Hb α X peptides and stimulated with the α1-adrenergic receptor agonist phenylephrine. We observed a significant increase in cGMP accumulation in arteries treated with Hb α X as compared to the Scr X peptide, which was reversed upon eNOS inhibition with the nitric oxide synthase inhibitor, L-NAME (Fig. 3a). Our previous work demonstrated that a monolayer of endothelial cells, in the absence of contact with smooth muscle cells, do not express Hb α10. Therefore, we tested whether Scr X or Hb α X altered eNOS activity in the absence of endogenous Hb α. Treatment of human microvascular coronary endothelial cell monolayers with Hb α X or Scr X showed no effect on eNOS phosphorylation at the activating serine 1177 site (Supp. Fig. I) or on the accumulation of the NO metabolite, nitrite (Supp. Fig. I). In addition, there was no change in nitrite accumulation between Scr X and Hb α X treatments following stimulation of human microvascular coronary endothelial cells with bradykinin, an agonist that stimulates eNOS activity in endothelial cells (Supp Fig. I), indicating that the functional effects of Hb α X on NO signaling are specific to the eNOS/Hb α signaling axis. Lastly, Hb α X had no effect on NO-induced dilation in aorta using acetylcholine dose-response (Supp Fig I). These results provide evidence that the Hb α X peptide applied ex vivo alters cGMP levels by disrupting the interaction between Hb α and eNOS, preventing NO scavenging by Hb α resulting in increased NO diffusion to the smooth muscle cell layer.
Figure 3. Hb α X peptide increases nitric oxide signaling in the vessel wall of wild type but not eNOS−/− mice.
a, Measurement of cGMP accumulation following phenylephrine stimulation in thoracodorsal arteries pretreated with Scr X or Hb α X peptide in the presence or absence of L-NAME (n=3). b, Dose response to phenylephrine on arteries treated with Scr X or Hb α X in the presence or absence of L-NAME. c, Cumulative dose response curve on thoracodorsal arteries from eNOS−/− mice with Scr X or Hb α X. In b and c, n indicates the number of arteries; value in parentheses shows the number of mice. In b and c, * indicates significance between Scr X vs. Hb α X, ^ indicates significance between Hb α X vs. Hb α X + L-NAME analyzed using 1-way ANOVA. All error bars represent s.e.m.
Next, we performed vasoreactivity to determine the functional effect of the Hb α X peptide on vasoconstriction to phenylephrine. In TD arteries, phenylephrine dose response curves in vessels treated with the Hb α X peptide revealed a significant decrease in constriction as compared to untreated arteries, which was reversed with L-NAME (Fig. 3b) with basal tone being unchanged (Supp Fig II). Differences are also presented as a change in initial inner diameter measured in micrometers (Supp Fig. II). The Scr X peptide showed no difference from control constriction. Both the EC50 and Emax are shown in Supp. Table I and demonstrate a significant difference only in the presence of Hb α X. Previous work from different labs has demonstrated that conduit arteries (e.g. aorta, carotid) do not express Hb α10, 16. Therefore, we examined the effect of the Hb α X peptide on isolated abdominal aortic rings by wire myography, which showed no significant change in phenylephrine dose response curves compared to untreated aortas or aortas treated with Scr X (Supp. Fig. III). Lastly, because we have shown that the Hb α X peptide disrupts Hb α/eNOS interaction, we proposed that eNOS−/− mice should not have an altered phenotype when Hb α X is applied. Indeed, when eNOS−/− mice were treated with Hb α X, there was no alteration of the magnitude of phenylephrine induced constriction in TD arteries (Fig 3c). Together, these results demonstrate that the Hb α X peptide induces significant changes in contractility due to increased NO production that is confined to the small arteries expressing Hb α, but not in conduit arteries where Hb α is absent or mice lacking eNOS.
Hb α X peptide acutely alters systemic blood pressure
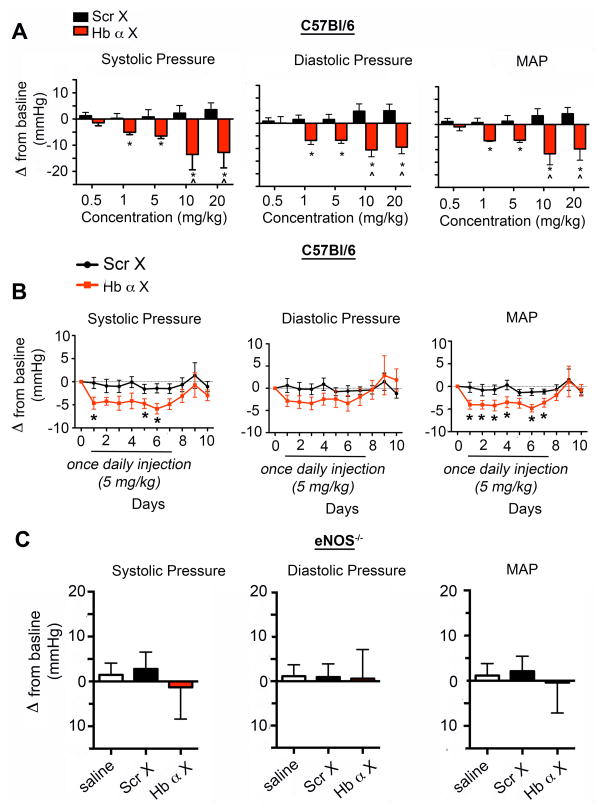
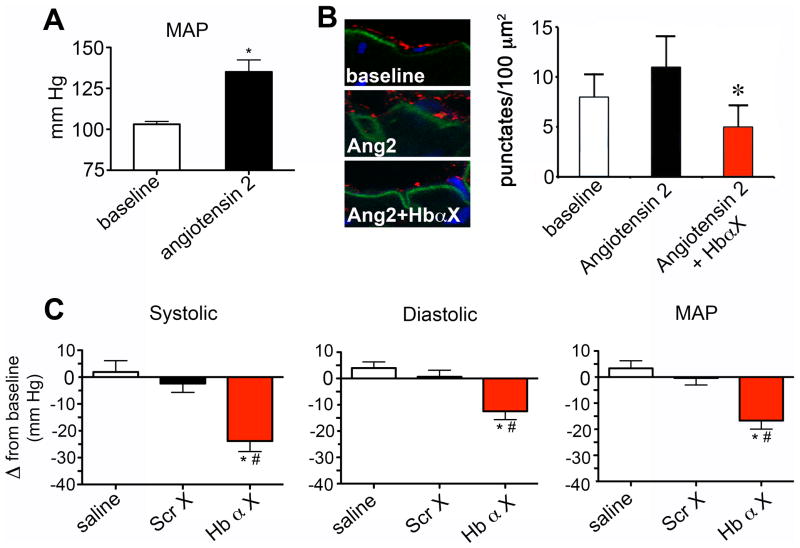
Our results above indicate that the Hb α X peptide has a confined and significant effect on small artery NO signaling, but not in conduit arteries. This provided initial evidence that the peptide could possibly also alter blood pressure through a change in peripheral vascular resistance. Therefore, we implanted radio transmitters into C57Bl/6 mice to monitor blood pressure in real time and elucidate the physiological effect of Hb α X on systemic blood pressure. Administration of bolus Hb α X peptide greater than 1 mg/kg into C57BL/6 mice induced a significant decrease in systolic, diastolic and mean arterial blood pressure, an effect that was absent in mice injected with saline or Scr X peptide (Fig. 4a). This effect was sustained following daily single bolus injections for seven continuous days (Fig 4b). A representative tracing following Scr X and Hb α X is shown in Supp. Fig. IV. There was no observable change in the heart rate of the mice (data not shown). A previous report demonstrated that eNOS is expressed in RBCs and could possibly regulate systemic blood pressure17. Following exposure to Hb α X altered NO release from RBCs showed no difference compared to Scr X (Supp Fig V). The over or under expression of eNOS protein significantly contributes to systemic blood pressure regulation18–20 and because of this we tested whether Hb α X could alter blood pressure in eNOS−/− mice. In parallel with a lack of effect on vasoreactivity in eNOS−/− mice, there was no alterations in blood pressure in eNOS −/− mice injected with saline or either of the peptides (Fig. 4c; MAP of eNOS mice was 114 mm Hg +/− 2 mm Hg). Using an angiotensin II (Ang II) mouse model hypertension (Figure 5a), we tested if an increased association between Hb α and eNOS occurred. Shown in Figure 5b, PLA demonstrates augmented association between Hb α and eNOS which was reversed with Hb α X peptide. Lastly, injection of Hb α X reversed angiotensin II-induced hypertension in C57Bl/6 mice (Fig 5c). Together, these results provide in vivo evidence that disruption of eNOS/Hb α interactions permit excessive NO diffusion implicated in vasorelaxation, hypotension and ablation of Ang II-induced hypertension.
Figure 4. Hb α X peptide decreases systemic blood pressure.
a, Radio telemetry measurements of systolic, diastolic and mean arterial blood pressure from mice injected with various doses of Scr X or Hb α X peptide (0.5–20 mg/kg) in C57BL/6. b, time course of systolic, diastolic and mean arterial blood pressure from C57BL/6 mice injected with a single bolus of 5 mg/kg Scr X or Hb α X daily. c, Systolic, diastolic and mean arterial blood pressure from eNOS−/− mice injected with saline, 5 mg/kg Scr X or Hb α X. * indicates significant differences using a t-test (a,b) and ^ shows significance using a 2-Way ANOVA (a). In c, statistical differences were analyzed using a 1-way ANOVA followed by a Bonferroni’s post-hoc test. n≥4 mice for all conditions; error bars indicate s.e.m.
Figure 5. Hb α X reverses angiotensin II-induced hypertension.
a, Mean arterial pressure measurements using radiotelemetry from C57BL/6 mice or mice continuously infusion of angiotensin II for five days via osmotic minipump. b, Proximity ligation assay for eNOS and Hb α (red punctuates) on transverse sections of a mouse thoracodorsal artery isolated from Ang-II hypertensive animals with and without Hb α X (5mg/kg). c, systolic, diastolic and mean arterial pressure measurements following injection of Scr X or Hb α X peptide (all 5 mg/kg) in angiotensin II-induced hypertensive mice. Significant differences between saline or Scr X and Hb α X analyzed by a t-test (a) and 1-way ANOVA followed by a Bonferroni’s post-hoc test (b,c). * and # indicates significance between saline and Hb α X or Scr X and Hb α X (p<0.05). n≥5 mice for all conditions; error bars indicate s.e.m.
DISCUSSION
Fluctuations in peripheral vascular resistance and systemic blood pressure are governed by highly orchestrated heterocellular signaling cascades between endothelial and smooth muscle cells comprising the arterial wall3, 21–23. The known mechanisms regulating resistance arterial tone involve a multifaceted palate of inputs including vasodilators such as endothelial derived hyperpolarizing factor, prostaglandins and nitric oxide 1, 2, 5. The recent discovery that endothelial cells in small resistance arteries express Hb α which functions as a key regulator of NO diffusion to smooth muscle has provided critical insight into how small arteries modulate NO signaling networks during vasoconstriction10. The work presented herein builds on this initial observation, including: (i) the identification of a conserved sequence in the Hb α protein that is modeled to interface with eNOS, (ii) identification of specific amino acids critical for eNOS and Hb α binding (iii) the development of a novel mimetic peptide that disrupts the eNOS/Hb α protein-protein interaction, (iv) the identification of a novel mechanism by which coupling of eNOS/Hb α is critical for NO scavenging and vascular reactivity, (v) the first line of evidence suggesting that the eNOS/Hb α interaction at the MEJ is critical for physiological blood pressure regulation, (vi) evidence that disruption of eNOS/Hb α reverses hypertension and (vii) targeting proteins polarized to the MEJ can significantly alter vascular function. The aggregate of these results offers new mechanistic insight into how Hb α regulates NO signaling in the resistance arterial wall.
Based on our previous work10, we hypothesized that the strong association and complex formation between Hb α and eNOS may be crucial for the functional role of Hb α as a NO scavenger. Our first step to test this hypothesis was to perform an in-depth protein-protein interaction analysis using in silico modeling of the known crystal structures for Hb α and eNOS. One limitation of this analysis is that eNOS, comprised of both an oxygenase and reductase domain, only has the oxygenase domain crystalized thereby constraining the modeling to this region. Despite this restriction, we identified a sequence in Hb α that is highly conserved across multiple mammalian species and models to an interaction interface between Hb α and eNOS. This predicted sequence was confirmed using site directed mutagenesis and prompted the development of a mimetic peptide to this sequence (Hb α X) for use in disrupting the eNOS/Hb α complex. Studies utilizing purified Hb α and eNOS protein as well as ex vivo resistance arteries show greater than 90% inhibition of eNOS/Hb α binding in the presence of the Hb X peptide, confirming the in silico modeling and the mutational analysis. Interestingly, there are two known human SNPs in the conserved Hb α interaction sequence: position 41, K→M 24; position 42, T→S 25. However, it remains to be determined whether these mutations play any functional role in blood pressure regulation or in the development of cardiovascular disease. Future studies will be required identify the specific amino acid(s) on eNOS that are critical for binding to Hb α.
Functionally, it was shown that Hb α Χ disrupts NO-dependent signaling as shown in cGMP and vasoreactivity studies. This work provides the first line of evidence demonstrating the importance of the protein-protein interaction between Hb α and eNOS, possibly similar to the mechanism by which caveolin-1 regulates eNOS12–14. We ruled out the possibility of non- specific effects of Hb α X assessed by basal phosphorylation of eNOS S1177, NO release measured by nitrite accumulation in basal and stimulated conditions, the lack of effect in abdominal aortas (where Hb α is not expressed) and in eNOS−/− animals. Even though the functional effects of the peptide are apparent, it is still unclear at this point how the Hb α /eNOS complex assembles. The complex may be pre-constructed and assembled similar to that of NADPH oxidase subunits26. Based on previous work12 and this study, it is tempting to speculate that caveolin-1 maintains eNOS inactive until stimulation, where eNOS then dissociates and recruits met-Hb α and possibly cytochrome B5 reductase 3 to form a macromolecular complex allowing tight NO regulation. Future work dedicated to dissection of the cell biology regulating the spatial and temporal assembly of this complex will be required to elucidate this aspect.
This paper for the first time describes a pharmacological approach to targeting proteins that are polarized to the MEJ. We show that the Hb a X peptide localizes specifically to holes in the IEL of TD arteries, the location where MEJs occur27, 28. This is an important observation as it is also the polarized location of both Hb α and eNOS, where together they form a macromolecular complex7, 10. We also show that the Hb α X peptide increases cGMP after PE induced constriction and prevents the artery from constricting. The interpretation of this work is that Hb α X, acting directly at the MEJ, can regulate delivery of NO to smooth muscle. By extension, this work also provides a first-line of evidence that MEJs could play an important role in blood pressure regulation (see below). Possibly the more immediate ramification for this work in regards to MEJs is the ability to begin to tease apart with greater confidence the functional roles for these structures. Indeed, the presented work underscores and builds upon the plethora of data pointing to a key role for the MEJ in regulation of arterial vasoreactivity (e.g., 8, 9, 27, 29–35).
Studies demonstrating a significant effect of the Hb α X peptide on physiological and pathophysiological blood pressure control places our purified protein studies and in vitro and ex vivo experiments into an important physiological context where NO signaling plays an important translational role. An exciting observation is that we can acutely lower blood pressure in normotensive and hypertensive mice by disrupting this complex at the MEJ. Once more, we can sustain a significant drop in blood pressure with Hb α X over a seven day period with a washout over three days. This could have important translational implications for blood pressure control, especially since this work was done in normotensive animals, where blood pressure is tightly controlled. In the hypertensive mice, the effect was even more dramatic. With the Hb α X peptide, the observed hypotension is conceivably achieved by increasing the amount of NO available to smooth muscle cells to increase cGMP and reduce the ability of resistance arteries to constrict, lowering the overall peripheral resistance. However, more work is needed to test this hypothesis. Although the data presented is in line and correlates with our vascular reactivity results and studies of blood pressure in eNOS−/− knockout animals, it does not take into account the other cell types that regulate blood pressure. These other cell types, known to express somatic Hb, include neurons in the brain36 and renal mesangial cells37. Of note, there was no increase in NO when applied to red blood cells; this does not indicate a lack of eNOS in the cells17, merely that in these cells, eNOS and Hbα do not likely interact. In addition, although there was no effect on the heart rate, we cannot at this point rule out the acute effect of the Hb α X peptide on changes in cardiac output. Blood pressure regulation is multifaceted, but the sum of the results demonstrated here both in terms of vasoreactivity and blood pressure provide a basis for further work on the potential role of this peptide in blood pressure regulation.
Lastly, this discovery of a conserved Hb α sequence and the development of a novel Hb α mimetic inhibitor provide important initial steps for understanding the basic physiological mechanisms that arterial blood vessels use to regulate NO signaling. These observations provide a basis for future studies to dissect the molecular and cellular mechanisms of Hb α /eNOS biology in the endothelium. This work may provide a platform for strategic development of small molecule inhibitors to treat hypertension and possibly other related cardiovascular diseases.
Supplementary Material
SIGNIFICANCE.
Hemoglobin alpha (Hbα) and eNOS are in macromolecular complexes at myoendothelial junctions, locations in resistance arteries where endothlelium and smooth muscle make contact. Hbα can regulate NO in resistance arteries, and we hypothesized that interfering with the Hbα/eNOS complex would allow greater NO in the vasculature and translate to whole animal decreases in blood pressure. We designed a mimetic peptide against Hbα that was conserved across species. The peptide prevented phenylephrine-induced constriction, presumably due to excessive NO release. Injection of the peptide disrupted Hbα/eNOS complexes in the vasculature, and lowered blood pressure acutely and chronically, both in normotensive and hypertensive mice. This work demonstrates that a therapeutic-type molecule can be targeted to sites of protein-protein interaction at the myoendothelial junctions.
Acknowledgments
We thank the Histology core at the University of Virginia and Alexander Lohman and Lauren Biwer for helpful discussion during the course of this project; Dr. Sruti Shiva and Yanna Wang for nitrite measurements; and Michael P. Bauer for the eNOS construct at the University of Pittsburgh.
SOURCE OF FUNDING
This work was supported by the National Institutes of Health grants HL088554 (B.E.I), HL107963 (B.E.I), HL112904 (A.C.S), GM087828 (L.C.), DK088905 (A.V.S.) and GM086457 (A.V.S.).
Nonstandard Abbreviations
- Hb α
hemoglobin alpha
- NO
nitric oxide
- eNOS
endothelial nitric oxide synthase
- cGMP
cyclic guanosine monophosphate
- FITC
fluorescein isothiocyanate
- DAPI
4′-6-Diamidino-2-phenylindole
- PLA
proximity ligation assay
- L-NAME
L-NG-Nitroarginine Methyl Ester
- EGTA
ethylene glycol tetraacetic acid
- TDA
thoracodorsal artery
- MEJ
myoendothelial junction
Footnotes
DISCLOSURE
The authors have nothing to disclose.
Contributor Information
Adam C. Straub, Email: astraub@pitt.edu.
Joshua T. Butcher, Email: jtb3a@virginia.edu.
Marie Billaud, Email: mb7bf@virginia.edu.
Stephanie M. Mutchler, Email: smmutchler@email.wm.edu.
Mykhaylo V. Artamonov, Email: mva3m@virginia.edu.
Anh T. Nguyen, Email: atn17@pitt.edu.
Tyler Johnson, Email: tjj4k@virginia.edu.
Angela K. Best, Email: akb9c@virginia.edu.
Megan P. Miller, Email: mpl8@pitt.edu.
Lisa A. Palmer, Email: lap5w@virginia.edu.
Linda Columbus, Email: columbus@virginia.edu.
Avril V. Somlyo, Email: avs5u@virginia.edu.
Thu H. Le, Email: thl4t@virginia.edu.
Brant E. Isakson, Email: brant@virginia.edu.
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Bunting S, Gryglewski R, Moncada S, Vane JR. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976;12:897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- 3.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–70. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- 4.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circulation research. 2000;87:474–9. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 5.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–72. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 6.Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:399–407. doi: 10.1161/ATVBAHA.110.215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18174–9. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin alpha regulates nitric oxide signalling. Nature. 2012;491:473–7. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straub AC, Zeigler AC, Isakson BE. The myoendotheila junction: connections that deliver the message. Physiology (Bethesda) 2013 doi: 10.1152/physiol.00042.2013. In Press. [DOI] [PMC free article] [PubMed]
- 12.Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. The Journal of biological chemistry. 1997;272:25437–40. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 13.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. The Journal of biological chemistry. 1997;272:15583–6. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 14.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. The Journal of biological chemistry. 1997;272:18522–5. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 15.Dora KA, Hinton JM, Walker SD, Garland CJ. An indirect influence of phenylephrine on the release of endothelium-derived vasodilators in rat small mesenteric artery. British journal of pharmacology. 2000;129:381–7. doi: 10.1038/sj.bjp.0703052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgoyne JR, Prysyazhna O, Rudyk O, Eaton P. cGMP-dependent activation of protein kinase G precludes disulfide activation: implications for blood pressure control. Hypertension. 2012;60:1301–8. doi: 10.1161/HYPERTENSIONAHA.112.198754. [DOI] [PubMed] [Google Scholar]
- 17.Wood KC, Cortese-Krott MM, Kovacic JC, Noguchi A, Liu VB, Wang X, Raghavachari N, Boehm M, Kato GJ, Kelm M, Gladwin MT. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1861–71. doi: 10.1161/ATVBAHA.112.301068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13176–81. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol. 2003;549:313–25. doi: 10.1113/jphysiol.2003.041897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, Sakoda T, Kurihara H, Yazaki Y, Yokoyama M. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. The Journal of clinical investigation. 1998;102:2061–71. doi: 10.1172/JCI4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budel S, Bartlett IS, Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: unmasking an NO wave. Circ Res. 2003;93:61–68. doi: 10.1161/01.RES.0000080318.81205.FD. [DOI] [PubMed] [Google Scholar]
- 22.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 23.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation. 2012;19:403–15. doi: 10.1111/j.1549-8719.2011.00146.x. [DOI] [PubMed] [Google Scholar]
- 24.Miyashita H, Hashimoto K, Mohri H, Ohokubo T, Harano T, Harano K, Imai K. Hb Kanagawa [alpha 40(C5)Lys----Met]: a new alpha chain variant with an increased oxygen affinity. Hemoglobin. 1992;16:1–10. doi: 10.3109/03630269209005670. [DOI] [PubMed] [Google Scholar]
- 25.Ohba Y, Imai K, Uenaka R, Ami M, Fujisawa K, Itoh K, Hirakawa K, Miyaji T. Hb Miyano or alpha 41(C6)Thr----Ser: a new high oxygen affinity alpha chain variant found in an erythremic blood donor. Hemoglobin. 1989;13:637–47. doi: 10.3109/03630268908998841. [DOI] [PubMed] [Google Scholar]
- 26.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circulation research. 2012;110:1364–90. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. CircRes. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 28.Kirby BS, Bruhl A, Sullivan MN, Francis M, Dinenno FA, Earley S. Robust internal elastic lamina fenestration in skeletal muscle arteries. PLoS One. 2013;8:e54849. doi: 10.1371/journal.pone.0054849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca(2+) events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008 doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of Endothelial Cell KCa3.1 Channels During Endothelium-Derived Hyperpolarizing Factor Signaling in Mesenteric Resistance Arteries. CircRes. 2008 doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. CircRes. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 33.Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. AmJPhysiol. 1998;274:H178–H186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- 34.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? JAnat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. CircRes. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 36.Schelshorn DW, Schneider A, Kuschinsky W, Weber D, Kruger C, Dittgen T, Burgers HF, Sabouri F, Gassler N, Bach A, Maurer MH. Expression of hemoglobin in rodent neurons. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:585–95. doi: 10.1038/jcbfm.2008.152. [DOI] [PubMed] [Google Scholar]
- 37.Nishi H, Inagi R, Kato H, Tanemoto M, Kojima I, Son D, Fujita T, Nangaku M. Hemoglobin is expressed by mesangial cells and reduces oxidant stress. Journal of the American Society of Nephrology: JASN. 2008;19:1500–8. doi: 10.1681/ASN.2007101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.