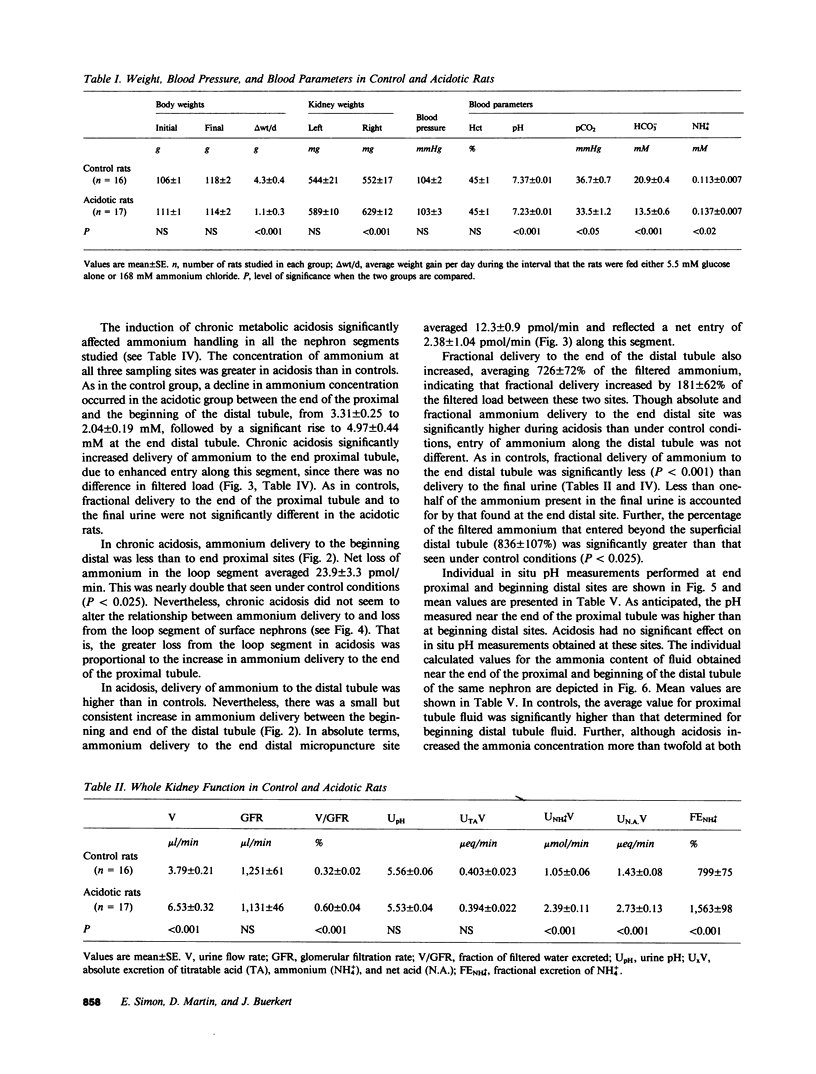
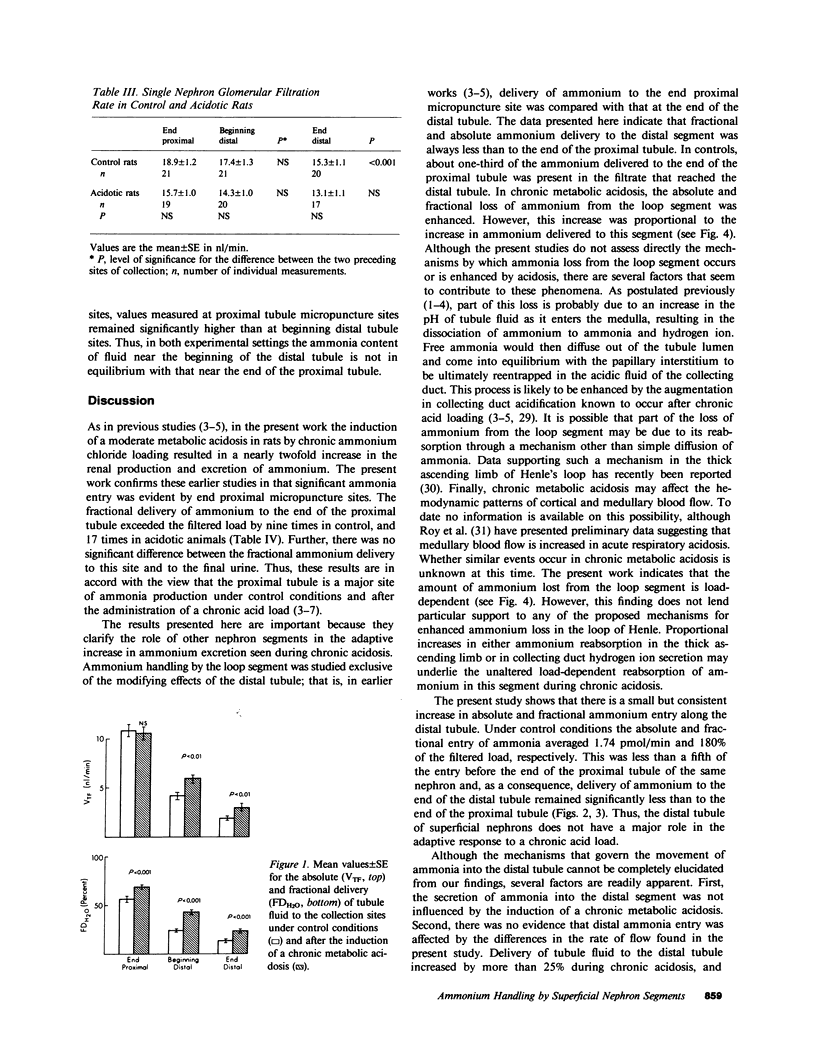
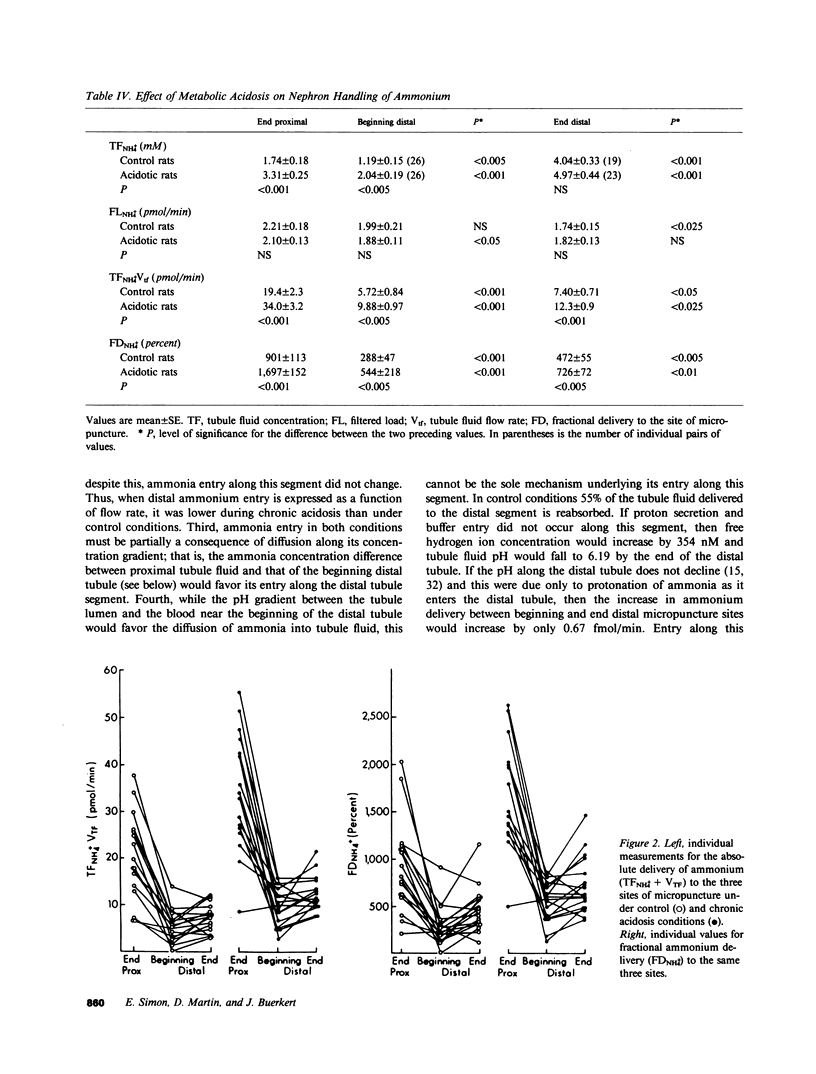
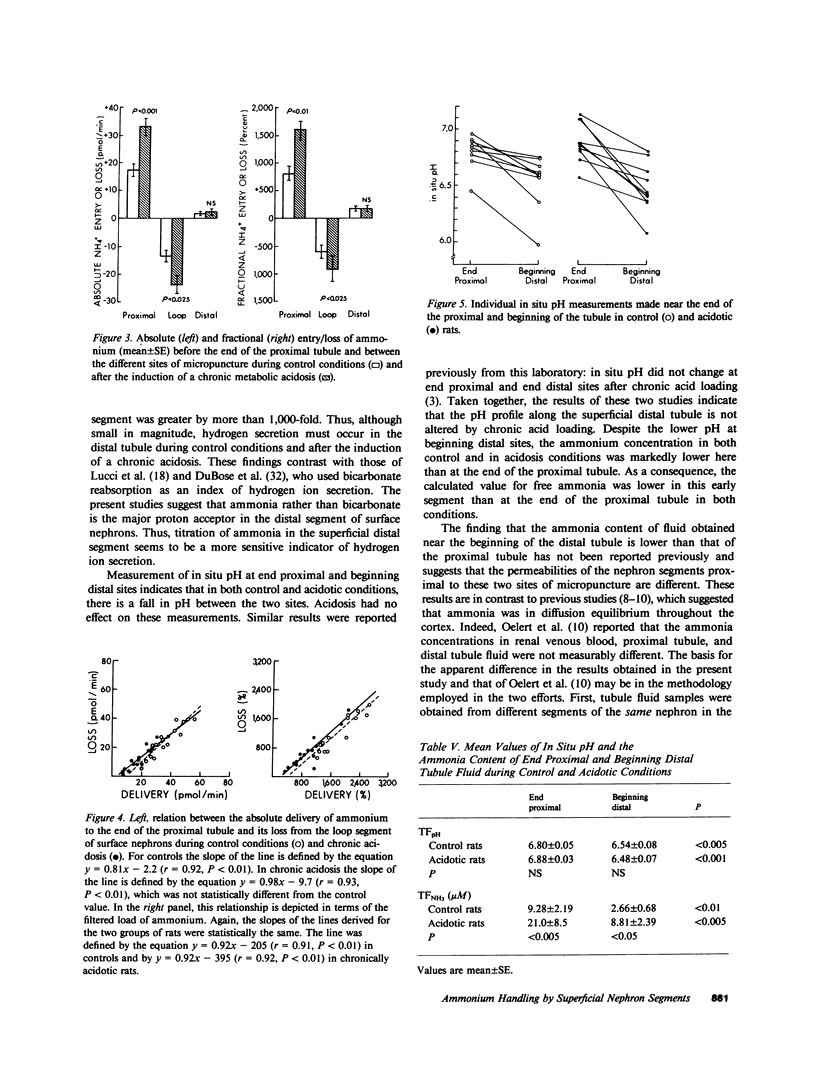
Abstract
Ammonia entry along surface nephron segments of rats was studied with micropuncture techniques under control and chronic metabolic acidosis conditions. Tubule fluid was collected successively from sites at the end and beginning of the distal tubule and at the end of the proximal tubule of the same nephron. During chronic metabolic acidosis, ammonium excretion doubled. As anticipated, the ammonium concentration (TFNH+4) was significantly higher in proximal tubule fluid during acidosis, and ammonium delivery to end proximal sites increased from 19.4 +/- 2.3 to 34.0 +/- 3.2 pmol/min (P less than 0.001). Although chronic acidosis did not affect TFNH+4 at the beginning of the distal tubule, ammonium delivery to the end of the distal tubule increased from 5.72 +/- 0.97 to 9.88 +/- 0.97 pmol/min. In both control and acidotic groups ammonium delivery was lower (P less than 0.001) to end distal sites than to end proximal sites, indicating net loss in the intervening segment. This loss was greater during chronic metabolic acidosis (23.9 +/- 3.3 vs. 13.6 +/- 2.0 pmol/min in controls, P less than 0.025). In both groups net entry of ammonia, in similar amounts, occurred along the distal tubule (P less than 0.05). In situ pH averaged 6.80 +/- 0.05 at end proximal tubule sites and fell to 6.54 +/- 0.08 at the beginning of the distal tubule (P less than 0.005). Chronic metabolic acidosis did not affect these measurements. The calculated free ammonia at the end of the proximal tubule rose from 9.3 +/- 2.2 to 21 +/- 9 microM (P less than 0.005) during chronic metabolic acidosis, and was also higher at beginning distal sites during acidosis (8.8 +/- 2.4 vs. 2.7 +/- 0.7 microM in controls, P less than 0.05). In both groups ammonia values for the beginning distal tubule fluid were lower than for end proximal tubule fluid. Thus, loss of ammonium in the loop segment is enhanced by chronic metabolic acidosis. Distal entry of ammonia is markedly less than along the proximal tubule and does not change in chronic metabolic acidosis, and ammonia permeabilities for the proximal and distal segments of surface nephrons seem different.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANK N., SCHWARTZ W. B. Influence of certain urinary solutes on acidic dissociation constant of ammonium at 37 degrees C. J Appl Physiol. 1960 Jan;15:125–127. doi: 10.1152/jappl.1960.15.1.125. [DOI] [PubMed] [Google Scholar]
- Buerkert J., Head M., Klahr S. Effects of acute bilateral ureteral obstruction on deep nephron and terminal collecting duct function in the young rat. J Clin Invest. 1977 Jun;59(6):1055–1065. doi: 10.1172/JCI108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D. Ammonium handling by superficial and juxtamedullary nephrons in the rat. Evidence for an ammonia shunt between the loop of Henle and the collecting duct. J Clin Invest. 1982 Jul;70(1):1–12. doi: 10.1172/JCI110581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Trigg D. Segmental analysis of the renal tubule in buffer production and net acid formation. Am J Physiol. 1983 Apr;244(4):F442–F454. doi: 10.1152/ajprenal.1983.244.4.F442. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Lowry O. H. The distribution of glutaminase isoenzymes in the various structures of the nephron in normal, acidotic, and alkalotic rat kidney. J Biol Chem. 1973 Jan 10;248(1):162–168. [PubMed] [Google Scholar]
- DENIS G., PREUSS H., PITTS R. THE PNH3 OF RENAL TUBULAR CELLS. J Clin Invest. 1964 Apr;43:571–582. doi: 10.1172/JCI104942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Carter N. W. Determination of disequilibrium pH in the rat kidney in vivo: evidence of hydrogen secretion. Am J Physiol. 1981 Feb;240(2):F138–F146. doi: 10.1152/ajprenal.1981.240.2.F138. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Seldin D. W., Carter N. W. Direct determination of PCO2 in the rat renal cortex. J Clin Invest. 1978 Aug;62(2):338–348. doi: 10.1172/JCI109134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbach G. M., Weise M., Stolte H. Amino acid reabsorption in the rat nephron. Free flow micropuncture study. Pflugers Arch. 1975;357(1-2):63–76. doi: 10.1007/BF00584545. [DOI] [PubMed] [Google Scholar]
- GLABMAN S., KOSE R. M., GIEBISCH G. Micropuncture study of ammonia excretion in the rat. Am J Physiol. 1963 Jul;205:127–132. doi: 10.1152/ajplegacy.1963.205.1.127. [DOI] [PubMed] [Google Scholar]
- Good D. W., Burg M. B. Ammonia production by individual segments of the rat nephron. J Clin Invest. 1984 Mar;73(3):602–610. doi: 10.1172/JCI111250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good D. W., Knepper M. A., Burg M. B. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Physiol. 1984 Jul;247(1 Pt 2):F35–F44. doi: 10.1152/ajprenal.1984.247.1.F35. [DOI] [PubMed] [Google Scholar]
- Graber M. L., Bengele H. H., Mroz E., Lechene C., Alexander E. A. Acute metabolic acidosis augments collecting duct acidification rate in the rat. Am J Physiol. 1981 Dec;241(6):F669–F676. doi: 10.1152/ajprenal.1981.241.6.F669. [DOI] [PubMed] [Google Scholar]
- HAYES C. P., Jr, MAYSON J. S., OWEN E. E., ROBINSON R. R. A MICROPUNCTURE EVALUATION OF RENAL AMMONIA EXCRETION IN THE RAT. Am J Physiol. 1964 Jul;207:77–83. doi: 10.1152/ajplegacy.1964.207.1.77. [DOI] [PubMed] [Google Scholar]
- Hamm L. L., Trigg D., Martin D., Gillespie C., Buerkert J. Transport of ammonia in the rabbit cortical collecting tubule. J Clin Invest. 1985 Feb;75(2):478–485. doi: 10.1172/JCI111723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills A. G., Reid E. L. PCO2 and PNH3 in mammalian kidney and urinary tract related to urine pH and flow. Am J Physiol. 1970 Aug;219(2):423–434. doi: 10.1152/ajplegacy.1970.219.2.423. [DOI] [PubMed] [Google Scholar]
- Jaeger P., Karlmark B., Giebisch G. Ammonium transport in rat cortical tubule: relationship to potassium metabolism. Am J Physiol. 1983 Nov;245(5 Pt 1):F593–F600. doi: 10.1152/ajprenal.1983.245.5.F593. [DOI] [PubMed] [Google Scholar]
- Karlmark B. The determination of titratable acid and ammonium ions in picomole amounts. Anal Biochem. 1973 Mar;52(1):69–82. doi: 10.1016/0003-2697(73)90332-1. [DOI] [PubMed] [Google Scholar]
- Lucci M. S., Pucacco L. R., Carter N. W., DuBose T. D., Jr Evaluation of bicarbonate transport in rat distal tubule: effects of acid-base status. Am J Physiol. 1982 Oct;243(4):F335–F341. doi: 10.1152/ajprenal.1982.243.4.F335. [DOI] [PubMed] [Google Scholar]
- Nagami G. T., Kurokawa K. Regulation of ammonia production by mouse proximal tubules perfused in vitro. Effect of luminal perfusion. J Clin Invest. 1985 Mar;75(3):844–849. doi: 10.1172/JCI111781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelert H., Uhlich E., Hills A. G. Messungen des Ammoniakdruckes in den corticalen Tubuli der Rattenniere. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;300(1):35–48. [PubMed] [Google Scholar]
- Pucacco L. R., Carter N. W. A glass-membrane pH microelectrode. Anal Biochem. 1976 Jun;73(2):501–512. doi: 10.1016/0003-2697(76)90200-1. [DOI] [PubMed] [Google Scholar]
- ROBINSON R. R., OWEN E. E. INTRARENAL DISTRIBUTION OF AMMONIA DURING DIURESIS AND ANTIDIURESIS. Am J Physiol. 1965 Jun;208:1129–1134. doi: 10.1152/ajplegacy.1965.208.6.1129. [DOI] [PubMed] [Google Scholar]
- Roy D. R., Blouch K. L., Jamison R. L. Effects of acute acid-base disturbances on K+ delivery to the juxtamedullary end-descending limb. Am J Physiol. 1982 Aug;243(2):F188–F196. doi: 10.1152/ajprenal.1982.243.2.F188. [DOI] [PubMed] [Google Scholar]
- SULLIVAN L. P. AMMONIUM EXCRETION DURING STOPPED FLOW: A HYPOTHETICAL AMMONIUM COUNTERCURRENT SYSTEM. Am J Physiol. 1965 Aug;209:273–282. doi: 10.1152/ajplegacy.1965.209.2.273. [DOI] [PubMed] [Google Scholar]
- Sajo I. M., Goldstein M. B., Sonnenberg H., Stinebaugh B. J., Wilson D. R., Halperin M. L. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int. 1981 Sep;20(3):353–358. doi: 10.1038/ki.1981.146. [DOI] [PubMed] [Google Scholar]
- Simon E., Martin D., Buerkert J. Handling of ammonium by the renal proximal tubule during acute metabolic acidosis. Am J Physiol. 1983 Dec;245(6):F680–F686. doi: 10.1152/ajprenal.1983.245.6.F680. [DOI] [PubMed] [Google Scholar]
- Stanton B. A., Biemesderfer D., Wade J. B., Giebisch G. Structural and functional study of the rat distal nephron: effects of potassium adaptation and depletion. Kidney Int. 1981 Jan;19(1):36–48. doi: 10.1038/ki.1981.5. [DOI] [PubMed] [Google Scholar]
- Stone W. J., Balagura S., Pitts R. F. Diffusion equilibrium for ammonia in the kidney of the acidotic dog. J Clin Invest. 1967 Oct;46(10):1603–1608. doi: 10.1172/JCI105652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C. S., Granges F., Kirk G., Gordon D., Giebisch G. Effects of saline infusion on titratable acid generation and ammonia secretion. Am J Physiol. 1984 Sep;247(3 Pt 2):F506–F519. doi: 10.1152/ajprenal.1984.247.3.F506. [DOI] [PubMed] [Google Scholar]


