Abstract
Background
Endocrine measures of ovarian reserve prior to breast cancer treatment may predict post-chemotherapy ovarian function, providing prognostic information at cancer diagnosis. The study tested if pre-chemotherapy anti-mullerian hormone (AMH), follicle stimulating hormone (FSH), and inhibin B (inhB) levels were associated with return of ovarian function post-chemotherapy and generated a prognostic score for ovarian recovery in young breast cancer patients.
Methods
A prospective cohort study recruited 109 participants, median age 39 (range 23–45), prior to chemotherapy from two breast clinics and followed them longitudinally. Using time-to-event analysis, we tested the association between pre-chemotherapy AMH, FSH and inhB levels and time to return of ovarian function, measured by menstrual pattern.
Results
After median follow up of 163 days (range 4–1009) after chemotherapy, 62 participants (57%) experienced return of ovarian function. In adjusted analyses, AMH levels >0.7ng/mL (HR 2.9, 95%CI 1.5–5.6) and FSH levels ≤10IU/L (HR 4.7, 95%CI 1.3–16.8) were associated with shorter time to ovarian recovery. InhB levels were not related. A prognostic score based on age<40, AMH >0.7ng/mL and BMI≥25 estimated timing of recovery.
Conclusions
In reproductive-aged women newly diagnosed with breast cancer, pre-chemotherapy AMH and FSH levels were associated with return of ovarian function, independent of age. A novel prognostic score incorporating AMH, age, and body size was able to estimate ovarian recovery. Pending validation, these data support using pre-chemotherapy ovarian reserve measures, particularly AMH, to prospectively counsel young patients on future ovarian function. As ovarian function does not equate fertility, follow up studies on predicting fertility are needed.
Keywords: AMH, breast cancer, chemotherapy, ovarian reserve
INTRODUCTION
Breast cancer is the most common cancer in reproductive-aged women with approximately 15,000 new cases in the U.S. each year.[1] Most young women diagnosed with breast cancer will undergo chemotherapy. While chemotherapy improves cancer outcomes, it also induces ovarian damage by direct toxicity to the finite pool of ovarian follicles.[2] Consequently, young breast cancer survivors face higher risks of infertility, primary ovarian insufficiency and early menopause.[3, 4] Post-chemotherapy ovarian function is also important to adjuvant endocrine therapy decisions, cancer recurrence and survival.[5] Hence, prognostic information on post-chemotherapy ovarian function that is available at cancer diagnosis can help inform decisions from fertility preservation to selection of appropriate anti-estrogen therapy. Despite clinical relevance, there are limited data on predicting post-chemotherapy ovarian function.[6–9]
Post-chemotherapy ovarian function is most commonly measured by menstrual bleeding pattern. In prospectively collected bleeding data, the majority of young women experience some duration of amenorrhea after chemotherapy because of direct ablation of ovarian follicles.[10, 11] After chemotherapy, women who have residual ovarian follicles will menstruate when follicular growth and ovulation resume, whereas women with depleted ovarian reserve will not resume menstrual bleeding. Although return of menses does not equate fertility, this event does identify the resumption of ovulatory function that is necessary for fertility and important to adjuvant endocrine therapy decisions. However, measuring ovarian function by bleeding pattern alone requires watchful waiting, diagnosing recovery of ovarian function only in retrospect.
Age and cancer treatment regimen are consistently associated with resumption of menstrual bleeding in cancer survivors, supporting the concept that post-treatment ovarian function is determined by both intrinsic factors, i.e. ovarian reserve, and extrinsic exposures, i.e. toxicity of treatment.[11, 12] In addition to age, endocrine measures of ovarian reserve prior to cancer treatment may aid in predicting ovarian function after treatment. Expressed by granulosa cells in small growing follicles, anti-mullerian hormone (AMH) levels decrease with declining size of the follicle pool and are relatively stable across the menstrual cycle.[13, 14] Follicle stimulating hormone (FSH) from the pituitary increases with declining ovarian reserve, but varies within and across menstrual cycles.[15, 16] Inhibin B (inhB) from granulosa cells decreases with declining ovarian reserve and fluctuates across the menstrual cycle.[15, 16] These endocrine measures are impacted by gonadotoxic cancer treatments, and several small studies suggest an association between pre-chemotherapy AMH and FSH and post-chemotherapy ovarian function.[7, 17–22] However, data are lacking on individualizing risk based on these biomarkers. Moreover, no study to date has been designed to predict the timing of ovarian function after chemotherapy in order to inform patients and providers on the expected course of ovarian recovery and contribute to clinical decisions that depend on ovarian function status.
The objectives of the study were to examine the association of pre-chemotherapy AMH, FSH, and inhB with the timing of post-chemotherapy ovarian function in young breast cancer patients and generate a prognostic score for ovarian recovery. We hypothesized that lower FSH, higher AMH and higher inhB prior to chemotherapy would predict shorter time to ovarian recovery after chemotherapy, as measured by menstrual pattern.
MATERIALS AND METHODS
Study population
A prospective cohort study was performed to determine predictors of ovarian function in young breast cancer survivors. Participants were identified by systematic medical record screening of all new breast cancer patients at breast clinics at the University of California San Diego (UCSD) and University of Pennsylvania (Penn) between 2009 and 2012. Patients were eligible if they were ages 18–45, diagnosed with early stage breast cancer (American Joint Committee on Cancer Stages I–III), had a uterus and at least one ovary, and reported at least one menses over the prior 12 months, representative of stages of reproductive aging prior to menopause.[16] Pregnancy, breastfeeding, use of psychotropic drugs known to impact ovulation, and history of prior cancer, chemotherapy or pelvic radiation were exclusion criteria. To test whether endocrine measures predict the return of ovarian function after chemotherapy, this analysis included participants who 1) underwent chemotherapy and 2) subsequently experienced secondary amenorrhea, defined as ≥ 3 months of amenorrhea with chemotherapy.[23] All participants provided written informed consent, and the study was approved by the Institutional Review Boards at UCSD and Penn.
Study procedures
Enrolled prior to chemotherapy, participants completed daily bleeding calendars and underwent in-person follow up visits in the breast clinic every 6 months, during which they completed a study questionnaire and blood draw. Due to urgency in starting chemotherapy, enrollment blood specimens were drawn across the menstrual cycle. Specimens were processed for serum and frozen at −80°C. Study questionnaires obtained demographics, breast cancer and medical history, and interval menstrual history.
Sera were assayed for AMH, FSH, inhB and estradiol (E2) at the University of Southern California Reproductive Endocrine Research Laboratory. AMH was measured by the AMH enzyme-linked immunosorbent assay kits with limit of detectability of 0.17 ng/mL (Beckman Coulter, AMH Gen II assay, Brea, CA, USA). FSH was measured by a direct immunochemiluminometric assay using the automated Immulite system (Siemens Medical Solutions, Los Angeles, CA). E2 was measured by radioimmunoassay after an organic solvent extraction step, with limit of detectability of 3 pg/mL. InhB assays used a monoclonal two site enzyme-linked immunosorbent assay, with limit of detectability of 9.4 pg/mL (Diagnostic Systems Laboratories, Webster, TX). Inter-assay coefficients of variation were <10% for all assays. Values below detection thresholds were given half of the threshold value in analyses.
Statistical analysis
Analyses were performed using STATA (Release 12, Stata Corporation, College Station, TX) and SPSS, v.21.0 (SPSS Inc., Chicago, IL). The primary exposures of interest were pre-chemotherapy AMH, FSH, and inhB. The primary outcome was return of ovarian function after chemotherapy, defined as time from the end of chemotherapy (to minimize impact of variable durations of chemotherapy) to the first episode of vaginal bleeding. Time to return of ovarian function was censored at the time of last follow up or for any of the following events which occurred prior to return of menses: start of GnRH agonist therapy, bilateral oophorectomy, hysterectomy, cancer recurrence requiring further treatment, death, or study withdrawal. Bleeding was determined using daily bleeding calendars and verified by study questionnaires and primary medical records. Bleeding episodes related to procedures such as IUD insertion were excluded.
Baseline characteristics were summarized by frequencies and proportions, or means, medians, standard deviations and range, as appropriate. AMH, FSH, inhB, and age were dichotomized by median levels, as well as FSH 10 IU/L and AMH 1.0 ng/mL to indicate decreased ovarian reserve.[24–26] Time-to-event methods were used. Kaplan-Meier survival curves were generated, and time to return of menses was compared by baseline characteristics using logrank tests. Cox proportion hazard regression model was developed to examine predictors of return of ovarian function and control for confounding. All variables with p<0.05 based on the Wald test from univariate analysis and known potential confounders (BMI, race, chemotherapy regimen and tamoxifen) were included in the multivariable model.[27, 28] P-values < 0.05 in the multivariable model were considered significant. A priori identified interactions of age by FSH and AMH were evaluated; neither achieved statistical significance and were not considered further. Sensitivity analysis was performed by 1) varying the definition of return of ovarian function to be two or more episodes of vaginal bleeding within 6 months after chemotherapy and 2) including only women who were followed for at least 3 months after chemotherapy.
To build a scoring system for return of ovarian function, model simplification was undertaken. Further categorization (quartiles, tertiles, medians and clinical cutpoints for age, AMH, FSH, and BMI) of risk factors from the final multivariable model was considered. The final multiviable model and proposed simplifications were compared using Harrel’s C to assure that the score had similar discrimination. We considered both weighted, using estimates from the Cox model, and unweighted scores. Resampling methods, bootstrap, were used to further assessed model performance.[29]
A priori, we set the sample size at 120 for feasibility and assumed the proportion with ovarian recovery to be 50%.[3] Using standard deviations for hormone levels from women of this age range [15, 20], the cohort would be powered to detect a difference of 0.15 ng/ml in AMH, 0.62 mIU/ml in FSH, and 3.70 pg/ml in inhibin B in pre-chemotherapy bloods between participants who recover ovarian function and those who do not (β=0.80, α=0.05).
RESULTS
A total of 124 participants underwent chemotherapy, of whom 109 (88%) experienced at least 3 months of amenorrhea with chemotherapy and were included in this analysis. Among participants excluded, 9 (8%) never experienced amenorrhea, and 5 (4%) did not have sufficient follow up to determine amenorrhea status. Participants who never experienced amenorrhea had a median age of 37.1 (interquartile range [IQR] 35.2–41.5); median AMH level of 1.0 ng/mL (IQR 0.6–1.5); median FSH level of 3.3 IU/L (IQR 2.3–4.1); and median inhB level of 20 pg/mL (IQR 10.9–30.5); they did not differ in these characteristics from participants included in this analysis.
Table 1 depicts baseline characteristics of the 109 women. Median age was 39.5 (IQR 36.4–42.9) years, and mean BMI (standard deviation [SD]) was 24.6 (5.1). All participants were premenopausal prior to the start of chemotherapy, with 85.3% of participants having greater than nine periods in the year prior to start of chemotherapy. Median cycle day of blood draw was 14 (IQR 4–21). Most participants were diagnosed with ductal type breast cancer (90%) and treated with cyclophosphamide-based chemotherapy (83%). The most common chemotherapy regimens included doxorubicin/cyclophosphamide/paclitaxel (55%) and docetaxel/ cyclophosphamide (22%). Approximately half of participants (51.4%) were treated with tamoxifen; 1 participant received an aromatase inhibitor. Pre-chemotherapy levels of AMH, FSH, inhB and E2 are depicted in Table 2. The median AMH level was 0.7 ng/mL (IQR 0.3–1.6), while median FSH and inhB levels were 5.3 IU/L (IQR 3.6–8.3) and 45.0 pg/mL (14.3–78.6), respectively.
Table 1.
Baseline characteristics of participants
| Characteristic | All (N=109) | Unadjusted Hazard Ratioa (95% CI) | p-value |
|---|---|---|---|
| Age at enrollment (years) | |||
| Median (interquartile range [IQR]) | 39.5 (36.4, 42.9) | 0.87 (0.83, 0.92) | <0.001 |
| <40, N(%) | 57 (52.2) | 4.03 (2.31, 7.01) | <0.001 |
| Body mass index (kg/m2), N(%) | |||
| <25 | 70 (64.2) | 1.0 | Ref |
| 25–30 | 22 (20.2) | 1.20 (0.64, 2.26) | 0.57 |
| >30 | 17 (15.6) | 1.41 (0.70, 2.84) | 0.34 |
| Race, N(%) | |||
| White | 80 (73.4) | 1.0 | Ref |
| African American | 14 (12.8) | 0.82 (0.40, 1.69) | 0.83 |
| Other | 15 (13.8) | 0.76 (0.34, 1.68) | 0.75 |
| Education > high school, N(%) | 89 (82.4) | 0.77 (0.39, 1.52) | 0.45 |
| Income > $60,000, N(%) | 72 (66.1) | 0.63 (0.35, 1.16) | 0.14 |
| Current smoking, N(%) | 7 (6.4) | 0.34 (0.09, 1.54) | 0.38 |
| Number of periods in past year, N(%) | |||
| 10–12 | 93 (85.3) | 1.0 | Ref |
| 7–9 | 2 (1.8) | 0.76 (0.30, 1.90) | 0.55 |
| 1–6 | 11 (10.1) | 1.52 (0.37, 6.26) | 0.57 |
| Age at menarche, median (IQR) | 13 (12, 14) | 0.98 (0.84, 1.14) | 0.98 |
| Previous pregnancy, N(%) | 92 (84.4) | 0.80 (0.35, 1.58) | 0.75 |
| Breast cancer type, N(%) | |||
| Ductal | 98 (89.9) | 1.0 | Ref |
| Lobular | 5 (4.6) | 1.55 (0.56, 4.31) | 0.4 |
| Mixed | 6 (5.5) | 0.28 (0.04, 2.01) | 0.2 |
| Breast cancer stage, N(%) | |||
| I | 26 (23.9) | 1.0 | Ref |
| II | 61 (56.0) | 1.36 (0.76, 2.45) | 0.3 |
| III | 22 (20.2) | 0.62 (0.26, 1.44) | 0.62 |
| Estrogen receptor +, N(%) | 70 (64.2) | 0.95 (0.56, 1.60) | 0.83 |
| Progesterone receptor +, N(%) | 63 (58.3) | 0.80 (0.47, 1.34) | 0.39 |
| Positive lymph node, N(%) | 54 (54) | 0.97 (0.88, 1.06) | 0.45 |
| Radiation therapy, N(%) | 74 (68.5) | 0.72 (0.43, 1.20) | 0.210 |
| Chemotherapy regimen | |||
| Cyclophosphamide-based | 91 (83.5) | 1 | Ref |
| Carboplatin-based | 16 (14.7) | 1.11 (0.52–2.33) | 0.26 |
| Dose dense treatment, N(%) | 57 (52.3) | 0.92 (0.56, 1.52) | 0.75 |
| Tamoxifen, N(%) | 56 (51.4) | 1.17 (0.70, 1.94) | 0.55 |
| GnRH agonist during chemotherapy, N (%) | 12 (11) | 0.89 (0.38–2.07) | 0.78 |
| Recruitment site, N(%) | |||
| UCSD | 34 (31.2) | 1.0 | Ref |
| Penn | 75 (68.8) | 1.05 (0.60, 1.83) | 0.87 |
Cox Regression
Table 2.
Pre-chemotherapy levels of anti-mullerian hormone (AMH), follicle stimulating hormone (FSH), inhibin B (inhB) and estradiol
| All (N=109) | Unadjusted Hazard Ratioa (95% CI) | p-value | |
|---|---|---|---|
| AMH, ng/mLb | |||
| Median (IQR) | 0.71 (0.29, 1.60) | 1.53 (1.31, 1.80) | <0.001 |
| >0.7 ng/mL, N(%) | 52 (47.7) | 3.93 (2.21, 7.00) | <0.001 |
| >0.17 ng/mL, N(%) | 20 (19.2) | 3.68 (1.65, 8.19) | 0.001 |
| FSH, IU/L | |||
| Median (IQR) | 5.31 (3.59, 8.32) | 0.92 (0.86, 0.98) | 0.006 |
| <5.3 IU/L, N(%) | 54 (50.0) | 2.17 (1.28, 3.69) | 0.004 |
| <10.0 IU/L, N(%) | 16 (15.0) | 4.29 (1.53, 12.0) | 0.003 |
| InhB, pg/mLc | |||
| Median (IQR) | 45.0 (14.3, 78.6) | 1.00 (1.00, 1.01) | 0.32 |
| <45.0 pg/mL, N(%) | 43 (39.9) | 1.46 (0.87, 2.46) | 0.15 |
| <9.4 pg/mL, N(%) | 18 (16.5) | 1.79 (0.88, 3.65) | 0.110 |
| Estradiol, pg/mL | |||
| Median (IQR) | 88.03 (52.50, 155.50) | 1.00 (1.00, 1.01) | 0.06 |
Cox Regression,
104 participants,
86 participants, interquartile range (IQR)
Sixty-two participants (57%) reported return of menses after median follow up of 163 days (range 4–1009). Seventeen participants were censored: 8 (7%) for bilateral oophorectomy; 6 (5%) for starting GnRH agonist therapy; 1 (1%) for recurrence; and 2 (2%) for death. On univariate analysis of baseline covariates, younger age, higher AMH and lower FSH levels were associated with return of ovarian function (Tables 1, 2). InhB, cancer or treatment characteristics, and GnRH agonist co-treatment for fertility preservation during chemotherapy were not associated with return of menses.
In a multivariable explanatory model adjusting for age, body size, race, chemotherapy regimen, and tamoxifen exposure, both AMH and FSH remained significantly associated with return of ovarian function (Table 3). Participants with pre-chemotherapy AMH levels >0.7 ng/mL experienced significantly shorter time to return of ovarian function compared to those with AMH levels ≤0.7 ng/mL (HR 2.9, 95% CI 1.5–5.6). FSH levels ≤10 IU/L were also associated with shorter time to return of ovarian function (HR 4.7, 95% 1.3, 16.8). In addition, participants who were younger, overweight or obese, and white were more likely to regain ovarian function. In sensitivity analysis, results were consistent whether varying the outcome as two episodes of vaginal bleeding within 6 months after chemotherapy or including only participants with at least 3 months of follow up post chemotherapy.
Table 3.
Multivariable model of time to return of ovarian function
| Risk factor | Comparison | Adjusted Hazard Ratio (95% CI)a | p-value |
|---|---|---|---|
| Age | <40 vs. ≥ 40 | 3.39 (1.74, 6.60) | < 0.001 |
| AMH | >0.7 vs. ≤0.7 ng/mL | 2.90 (1.51, 5.56) | 0.002 |
| FSH | <11.0 vs. ≥ 11 IU/L | 4.69 (1.31, 16.81) | 0.018 |
| BMI | ≥ 25 vs. <25 | 5.07 (2.44, 10.54) | < 0.001 |
| Race | White vs. non-white | 2.33 (1.20, 4.53) | 0.01 |
| Chemotherapy Regimen | Carboplatin to Cyclophosphamide | 1.33 (0.60, 2.93) | 0.49 |
| Tamoxifen | Exposed to Unexposed | 1.38 (0.78, 2.43) | 0.27 |
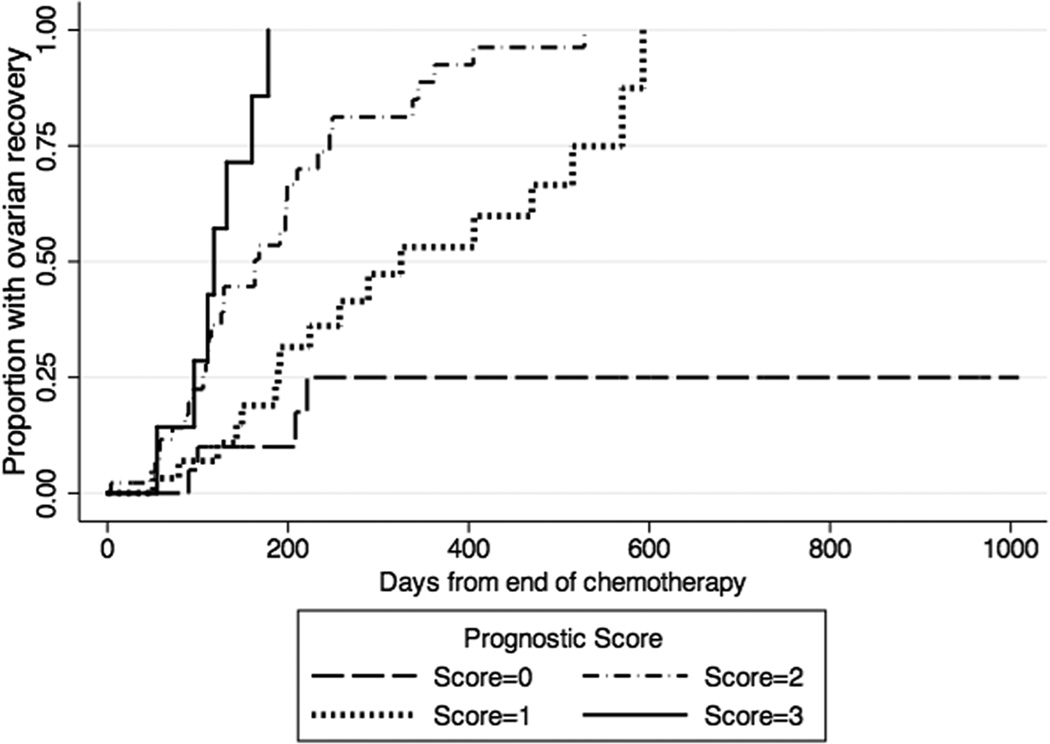
An internal validation of the multivariable explanatory model was conducted using 1000 bootstrap samples of size 109 to determine final variables for the return of ovarian function. Only age, AMH and BMI remained significantly associated with return of ovarian function from the bootstrap models. Two prognostic scores (weighted and unweighted) were created and compared, with one point given per variable present. Score A weighted the three variables equally (Score A=[age <40]+[AMH >0.7 ng/mL] + [BMI ≥25]), while Score B used coefficient weights from the Cox model (Score B= 3.3[age <40] + 2.9[AMH>0.7 ng/mL] + 2.7[BMI ≥25]). Bootstrap estimates of Harrel C were similar for the two prognostic scores (Score A 0.70 vs. Score B 0.71). Hence, the unweighted Score A was used in Table 4 and Figure 1 to demonstrate differential times to return of ovarian function as a function of prognostic score.
Table 4.
Prognostic score to estimate time to return of ovarian function after chemotherapy
| Scorea | Total N |
Return N |
Days to return of ovarian function |
||
|---|---|---|---|---|---|
| 25% | 50% | 75% | |||
| 0 | 20 | 4 | 221 | - | - |
| 1 | 31 | 17 | 189 | 325 | 570 |
| 2 | 45 | 33 | 106 | 163 | 246 |
| 3 | 8 | 7 | 96 | 118 | 160 |
1 point for each of the following: age <40, AMH>0.7 ng/mL, BMI ≥25
Figure 1.
Kaplan-Meier curves of time to return of ovarian function after chemotherapy by Prognostic Score, where 1 point is given for each of the following pre-chemotherapy measures: age <40, AMH>0.7 ng/mL, BMI ≥25. Hatch marks on the curves represent censored events.
DISCUSSION
In reproductive-aged women who are newly diagnosed with breast cancer, pre-chemotherapy AMH and FSH levels were associated with the return of ovarian function after chemotherapy, independent of age. A novel prognostic score incorporating AMH, age, and body size was able to estimate the timing of resumption of menstrual bleeding. These data support using pre-chemotherapy measures of ovarian reserve to prospectively counsel young breast cancer patients on future ovarian function.
In this cohort of breast cancer patients undergoing cyclophosphamide-based chemotherapy, the vast majority of participants (88%) experienced secondary amenorrhea once chemotherapy was initiated. This observation was expected and reflects acute loss of ovarian function from chemotherapy-induced ablation of ovarian follicles. To determine if and when ovarian function returns after chemotherapy exposure (via resumption of folliculogenesis, ovulation, and menstrual bleeding), the outcome was time to resuming menses, as menstrual pattern remains the gold standard measure of ovarian function.[16] Over the follow up period, half of participants (57%) showed evidence of ovarian recovery by resumption of menses. This rate is consistent with those reported in three smaller cohorts.[7, 19, 22] In estimating the timing of ovarian recovery and associated confidence bounds, this study generated novel data aimed to inform clinical decisions that depend on ovarian function status as well as future research on characterizing the pattern(s) of ovarian aging after cancer.
Pre-chemotherapy measures of ovarian reserve, specifically AMH and FSH, were associated with post-chemotherapy ovarian recovery. These data confirm prior observations in four small cohorts of breast cancer patients. Anderson, et al. reported that pre-treatment AMH levels were lower in 30 women who were amenorrheic at 2 years post-chemotherapy, compared to 9 women who continued to menstruate.[6, 9] In a second cohort, pre-chemotherapy AMH and inhibin B levels were lower in 16 women who remained amenorrheic for 12 months post-chemotherapy compared to 5 women who were menstruating.[7] Similarly, a third cohort study of 27 patients followed for a median of 13 months recently reported that detectable AMH ≥0.16 ng/mL, but not FSH or inhibin B, prior to chemotherapy was associated with return of menses.[22] The final cohort of 26 patients showed non-statistically significant lower AMH and higher FSH levels in women who did not resume menses 6 months following chemotherapy.[19] Among studies, AMH was most consistently associated with post-treatment menstrual pattern. The relationships between FSH or inhibin B and menstrual pattern were in expected directions, but did not reliably demonstrate significant associations. We posit that this is potentially due to sampling throughout the menstrual cycle in most studies, which increases overall variability and limits study power. In practice, early follicular phase blood draws are a challenge in newly diagnosed patients moving into chemotherapy. Therefore, pre-treatment ovarian reserve markers with significant intra-cycle variability may be of limited clinical utility for prediction.
The current study developed a novel prognostic score for ovarian recovery in order to shift the focus of studies on ovarian reserve in young cancer patients from association studies into clinical prediction. Without predictors, young women with breast cancer undergo watchful waiting for return of menses after chemotherapy, with the diagnoses of ovarian recovery versus ovarian failure made only in retrospect. Because of a relative large sample size and close follow up of menstrual bleeding pattern (by diary and frequent verification in study visits), we were able to define the outcome with more precision than prior studies. Moreover, the study was powered a priori to identify multiple independent predictors of post-chemotherapy ovarian function measured by bleeding pattern. In deriving cutpoints in hormone levels, we tested medians, quartiles and clinically used cutpoints for decreased ovarian reserve and found that median AMH (0.7 ng/mL) and FSH cutpoint for decreased ovarian reserve (11 IU/L) provided better discrimination. Interestingly, the cutpoint of 0.7 ng/mL in AMH levels was identified empirically by two small cohorts. In the Anderson cohort,[6] receiver-operating characteristics curve analysis identified 0.7 ng/mL as the AMH cutpoint for peak likelihood ratio of 6 months of amenorrhea by 4–5 years post-chemotherapy; this study used the Beckman Coulter active MIS/AMH kit to which the Gen II assay used in this study was standardized.[30] Anders et al also identified a median level of 0.7 ng/mL using the Diagnostic Systems Labs AMH assay, which uses the same antibody pair as Gen II assay.[7] Similar to the multivariable logistic model in Anderson study, FSH did not remain in the simplified prediction model after bootstrapping in our dataset.
Younger age and higher body mass index were predictors of shorter time to return of menses. Age was expected, and the cutpoint at 40 is clinically used to dichotomize participants in CRA literature. Quartiles of age did not improve prediction. Age was additive to AMH, a finding also observed by Anderson, et al. using a classification tree approach. To our knowledge, pre-treatment body size has not been associated with post-chemotherapy ovarian function. However, the Study of Women’s Health Across the Nation recently reported higher baseline weight to be associated with later age at the natural final menstrual period in a longitudinal study of 3,200 women in the U.S., a finding also reported by several other smaller studies on menopause in non-cancer populations.[28, 31, 32] While the mechanism by which larger body size would result in later ovarian senescence is not clear, if confirmed in future studies, body size may be an additional intrinsic factor related to ovarian function after gonadotoxic therapy.
Cancer and treatment risk factors including stage, exposure to cyclophosphamide, dose dense therapy, and tamoxifen exposure were not related to timing of ovarian recovery. This was expected and considered a strength of the study as we recruited a relatively homogeneous population to allow us to examine the primary exposures of interest: endocrine measures of ovarian reserve. The identification of AMH, in addition to age, provides candidate predictors for future investigations on predicting ovarian function in more diverse cancer populations, where efforts to classify the gonadotoxicity of various treatment approaches are much needed.
Several limitations are important to discuss. First, post-treatment ovarian function was measured by return of menses. We chose a menstrual bleeding outcome because menstrual pattern is associated with hormone biomarkers, menopausal symptoms, and age at final menses.[16, 33–36] Although presence of menses does not equate fertility, the return of menses does identify the resumption of ovulatory function that is necessary for fertility and important to adjuvant endocrine therapy decisions. By selecting the first bleeding episode as the outcome, it is possible that women who had bleeding for other reasons, e.g. atrophy, were misclassified. Hence, we performed sensitivity analysis varying the bleeding outcome definition and length of follow up post-chemotherapy, which did not change the observed associations. Moreover, misclassification would bias our results toward the null. With further follow up, the future goals of this cohort are to identify the end of the window of ovarian function and explore if these endocrine markers predict fertility. Post-chemotherapy ovarian reserve markers were intentionally not selected as the outcome, as there are no longitudinal studies to link these levels to clinical outcomes from pregnancy to menopause to date.
Second, prognostic models require validation.[37] External validation is ideal but challenging because of longitudinal studies in this population are few and small, outcome data have not been collected in a consistent manner, and multiple immunoassays for AMH have been used. We approached this limitation by internal validation with bootstrapping results that were robust.[29] For ongoing and future studies, we propose careful collection of menstrual bleeding and biospecimens post-chemotherapy to allow for future cross-validation and characterization of stages of menopausal transition in populations exposed to gonadotoxic treatments.
Although study withdrawal and loss to follow up was minimal in our cohort, twelve percent of the population was censored because of temporary or permanent ovarian ablation by GnRH agonist or oophorectomy. Similar loss of evaluable patients was observed in prior studies. While sensitivity analysis omitting these patients from the analysis did not change the overall findings, this observation informs the need to inflate sample size in future studies to account for attrition.
The ability to predict post-chemotherapy ovarian function is clinically important in young cancer survivors, because of implications on fertility, menopause, choice of endocrine therapy and cancer prognosis. Prognostic markers measured prior to cancer treatment and relate risk of post-treatment ovarian function would change clinical management. Building upon cross-sectional and early longitudinal studies on endocrine ovarian reserve markers in cancer survivors, this study identified a prediction rule for return of ovarian function after chemotherapy in breast cancer patients based on pre-treatment AMH, age and body size.
Acknowledgments
Support: American Cancer Society MRSG-08-110-01-CCE (HIS), NIH HD058799 (HIS)
We thank our participants and the study staff Leslie Barbier, Samantha Roberts, and Sally Dominick for their support. We also wish to thank Dr. Frank Stanczyk and University of Southern California Reproductive Endocrine Research Laboratory for endocrine biomarker measurements.
Footnotes
Disclosure: HIS and KC have served on the Advisory Board for Ferring Pharmaceuticals.
REFERENCES
- 1.Cancer Facts & Figures. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Nicosia SV, Matus-Ridley M, Meadows AT. Gonadal effects of cancer therapy in girls. Cancer. 1985;55:2364–2372. doi: 10.1002/1097-0142(19850515)55:10<2364::aid-cncr2820551011>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Su HI, Sammel MD, Green J, et al. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116:592–599. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Letourneau JM, Ebbel EE, Katz PP, et al. Acute ovarian failure underestimates age-specific reproductive impairment for young women undergoing chemotherapy for cancer. Cancer. 2012;118:1933–1939. doi: 10.1002/cncr.26403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Network NCC. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer [serial online] 2012 v2.2007. [Google Scholar]
- 6.Anderson RA, Cameron DA. Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–1343. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 7.Anders C, Marcom PK, Peterson B, et al. A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Invest. 2008;26:286–295. doi: 10.1080/07357900701829777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su HI, Sammel MD, Velders L, et al. Association of cyclophosphamide drug-metabolizing enzyme polymorphisms and chemotherapy-related ovarian failure in breast cancer survivors. Fertil Steril. 2010;94:645–654. doi: 10.1016/j.fertnstert.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson RA, Rosendahl M, Kelsey TW, Cameron DA. Pretreatment anti-Mullerian hormone predicts for loss of ovarian function after chemotherapy for early breast cancer. Eur J Cancer. 2013;49:3404–3411. doi: 10.1016/j.ejca.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 11.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 12.Byrne J, Fears TR, Gail MH, et al. Early menopause in long-term survivors of cancer during adolescence. Am J Obstet Gynecol. 1992;166:788–793. doi: 10.1016/0002-9378(92)91335-8. [DOI] [PubMed] [Google Scholar]
- 13.Weenen C, Laven JS, Von Bergh AR, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 14.van Disseldorp J, Lambalk CB, Kwee J, et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod. 2010;25:221–227. doi: 10.1093/humrep/dep366. [DOI] [PubMed] [Google Scholar]
- 15.Freeman EW, Sammel MD, Gracia CR, et al. Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril. 2005;83:383–392. doi: 10.1016/j.fertnstert.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 16.Harlow SD, Gass M, Hall JE, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutchman Singh K, Muttukrishna S, Stein RC, et al. Predictors of ovarian reserve in young women with breast cancer. Br J Cancer. 2007;96:1808–1816. doi: 10.1038/sj.bjc.6603814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillon KE, Sammel MD, Prewitt M, et al. Pretreatment antimullerian hormone levels determine rate of posttherapy ovarian reserve recovery: acute changes in ovarian reserve during and after chemotherapy. Fertil Steril. 2013;99:477–483. doi: 10.1016/j.fertnstert.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu B, Douglas N, Ferin MJ, et al. Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer. 2010;116:2099–2105. doi: 10.1002/cncr.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21:2583–2592. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 21.Brougham MF, Crofton PM, Johnson EJ, Evans N, Anderson RA, Wallace WH. Anti-Mullerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab. 2012;97:2059–2067. doi: 10.1210/jc.2011-3180. [DOI] [PubMed] [Google Scholar]
- 22.Henry NL, Xia R, Schott AF, McConnell D, Banerjee M, Hayes DF. Prediction of Post-Chemotherapy Ovarian Function Using Markers of Ovarian Reserve. Oncologist. 2013 doi: 10.1634/theoncologist.2013-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Current evaluation of amenorrhea. Fertil Steril. 2008;90:S219–S225. doi: 10.1016/j.fertnstert.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 24.La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 25.Nardo LG, Gelbaya TA, Wilkinson H, et al. Circulating basal anti-Mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92:1586–1593. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 26.Jayaprakasan K, Campbell B, Hopkisson J, Johnson I, Raine-Fenning N. A prospective, comparative analysis of anti-Mullerian hormone, inhibin-B, and three-dimensional ultrasound determinants of ovarian reserve in the prediction of poor response to controlled ovarian stimulation. Fertil Steril. 2008;93:855–864. doi: 10.1016/j.fertnstert.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 27.Randolph JF, Jr, Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516–1522. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 28.Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178:70–83. doi: 10.1093/aje/kws421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moons KG, Kengne AP, Woodward M, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012;98:683–690. doi: 10.1136/heartjnl-2011-301246. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Mullerian hormone (AMH) ELISA. J Immunol Methods. 2010;362:51–59. doi: 10.1016/j.jim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 32.Rodstrom K, Bengtsson C, Milsom I, Lissner L, Sundh V, Bjourkelund C. Evidence for a secular trend in menopausal age: a population study of women in Gothenburg. Menopause. 2003;10:538–543. doi: 10.1097/01.GME.0000094395.59028.0F. [DOI] [PubMed] [Google Scholar]
- 33.Broer SL, Eijkemans MJ, Scheffer GJ, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96:2532–2539. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 34.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–1680. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. Predicting age at menopause from serum antimullerian hormone concentration. Menopause. 2011;18:766–770. doi: 10.1097/gme.0b013e318205e2ac. [DOI] [PubMed] [Google Scholar]
- 36.Santoro N, Brockwell S, Johnston J, et al. Helping midlife women predict the onset of the final menses: SWAN, the Study of Women's Health Across the Nation. Menopause. 2007;14:415–424. doi: 10.1097/gme.0b013e31802cc289. [DOI] [PubMed] [Google Scholar]
- 37.Altman DG, De Stavola BL, Love SB, Stepniewska KA. Review of survival analyses published in cancer journals. Br J Cancer. 1995;72:511–518. doi: 10.1038/bjc.1995.364. [DOI] [PMC free article] [PubMed] [Google Scholar]