Abstract
Background
Histologic response to chemotherapy has been shown to be an independent prognostic factor in patients with osteosarcoma and Ewing’s sarcoma. However, in patients with soft tissue sarcoma (STS), the prognostic impact of histologic response to therapy is less clear. We sought to determine the prognostic significance of treatment-induced pathologic necrosis in patients receiving neoadjuvant chemoradiotherapy for STS.
Methods
Between 1989 and 2011, we identified 113 patients with grade 2 or 3 extremity or truncal STS who received protocol neoadjuvant interdigitated chemoradiotherapy followed by surgery. We quantified the extent of tumor necrosis in the resected specimens and correlated this with outcome.
Results
The median tumor necrosis was 90%, and 103 (91%) patients received all 3 cycles of planned neoadjuvant chemotherapy. The likelihood of achieving ≥ 95% necrosis was not related to the number of pre-operative cycles of chemotherapy received but was related to tumor histology (MFH 62% versus synovial sarcoma 0%, p<0.001; myxoid liposarcoma 56% versus synovial sarcoma 0%, p=0.002). At a median follow-up of 6 years, there were no statistically significant differences in the 5-year local control, disease-specific, and overall survival rates for patients with ≥ 95% necrosis (n = 50, 44%) and < 95% necrosis (n = 63, 56%), even when stratifying by histology.
Conclusions
In a homogeneous population of patients with high-grade extremity and truncal STS treated with neoadjuvant chemoradiotherapy, the extent of pathologic tumor necrosis did not correlate with outcome.
Keywords: Pathologic necrosis, soft tissue sarcoma, neoadjuvant therapy, outcome, prognosis
Introduction
There are several potential advantages to the preoperative, or neoadjuvant, administration of chemoradiation therapy (CRT) for soft tissue sarcomas (STS). These include: (1) tumor shrinkage that may facilitate a less radical resection and, in some cases, enable a limb-salvage approach in lieu of amputation; (2) lower morbidity owing to lower treatment volumes and doses in the preoperative setting; (3) immediate treatment of micrometastatic disease, thus potentially forestalling the development of overt metastases; and (4) an in vivo test of the sensitivity of the tumor to CRT, thus providing an early indication of the potential effectiveness of the neoadjuvant regimen. The most objective, reliable measure of this in vivo sensitivity to neoadjuvant therapy is the extent of pathologic tumor necrosis. Treatment-induced pathologic necrosis has been shown to be an independent prognostic factor in patients with osteosarcoma and Ewing’s sarcoma,1–5 though not all studies have conclusively demonstrated the correlation of histologic response with treatment and survival in osteosarcoma.6–7 However, in patients with extremity or truncal STS, the prognostic impact of histologic response to therapy is much less clear, with only a few published studies offering conflicting results.8–13 These studies are limited by either their small study populations or by the heterogeneity of their treatment regimens. We sought to determine the prognostic significance of treatment-induced pathologic necrosis in STS in a large, homogeneous group of patients receiving a uniform regimen of neoadjuvant CRT.
Patients and Methods
Study Cohort
After approval from the Massachusetts General Hospital (MGH) Institutional Review Board was obtained, the MGH Department of Radiation Oncology sarcoma database was searched for patients age 18 or older who were treated between 1989 and 2011 with preoperative chemoradiotherapy for localized extremity and superficial trunk STS. We excluded patients with tumors located primarily in the bone, cartilage, head, neck, retroperitoneum, and brain. We also excluded patients with the following histologies: desmoid tumor, dermatofibrosarcoma protuberans, chondrosarcoma, osteosarcoma, rhabdomyosarcoma, Ewing’s sarcoma, and peripheral neuroectodermal tumors.
The study design and patient evaluation have been previously described in detail.14 In brief, patients ≥ 18 years of age with high-grade (grade 2 or 3 in a three-tier grading system) extremity STS ≥ 8 cm who were judged to be medically fit were offered treatment after granting informed consent. Diagnostic core needle biopsies or incisional biopsies of the tumors were obtained in all patients. The protocol therapy is outlined in Figure 1. Patients were to receive a total of 6 cycles of MAID chemotherapy and 44 Gy of preoperative radiation therapy. Three cycles of chemotherapy were given preoperatively, interdigitated with 44 Gy in split courses of 22 Gy in 11 fractions of 2 Gy per day between the first and second cycles and between the second and third cycles. The MAID chemotherapy regimen was as follows: mesna 2500 mg/m2/d by continuos i.v. infusion on Days 1 – 4; adriamycin (doxorubicin) 20 mg/m2/d continuous i.v. infusion on Days 1 – 3; ifosfamide 2000 mg/m2/d continuous i.v. infusion on Days 1 – 3; and dacarbazine 250 mg/m2/d continuous i.v. infusion on Days 1 – 4 with or without granulocyte colony-stimulating factor (G-CSF) 5 μg/kg/d starting on Day 5. Surgery was planned for 80 days after the initiation of the first cycle of chemotherapy.
Figure 1.
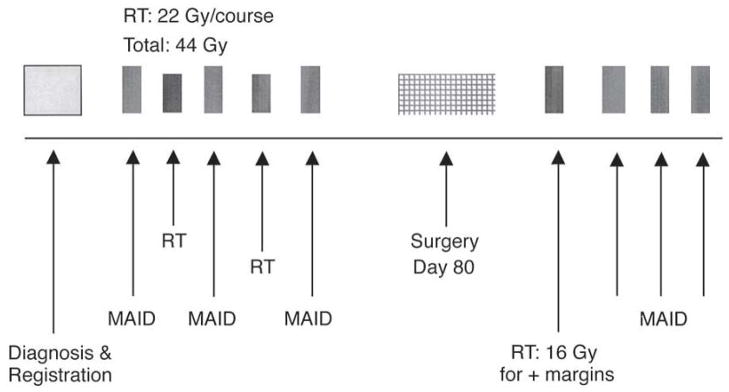
Neoadjuvant MAID chemoradiation treatment protocol.
Surgical resection was planned 3 weeks after the completion of the preoperative chemoradiation therapy. Tumors were resected with the intent of limb salvage with negative margins (R0 resection). The biopsy site was excised en bloc with the definitive surgical specimen. The wounds were either closed primarily or reconstructed with rotational flaps, with or without a skin graft. If the surgical margins were deemed positive, another 16 Gy in 8 fractions was delivered postoperatively to the tumor bed with a 1-cm margin. A boost was not given to patients with 100% tumor necrosis. Postoperative MAID chemotherapy, planned for an additional 3 cycles, was resumed after completion of the postoperative RT or 21 – 35 days postoperatively in patients with negative margins not receiving postoperative RT.
All patients had pre-treatment imaging of their primary tumors with either magnetic resonance imaging (MRI) or computed tomography (CT), and the majority of patients had follow-up imaging after three cycles of chemotherapy and radiotherapy, prior to surgery. The resected tumors were graded according to the National Cancer Institute (NCI) grading system using three-tiers, and tumors with overlapping grades were classified at the higher tier. According to an institutional protocol, the resection specimens were bivalved along the long axis of the tumor, and a slab of the long axis was submitted for microscopic examination. Additionally, one section per centimeter was taken of the remaining two halves and submitted for microscopic examination, and the extent of necrosis was assessed relative to the percentage of residual viable tumor based on these representative tumor sections. Pathology reports of surgical specimens were evaluated in conjunction with operative reports to determine the type of resection and margin status. Resections were classified as being R0 (macroscopically complete with negative microscopic margins), R1 (macroscopically complete with positive microscopic margins), or R2 (macroscopically incomplete). A positive microscopic margin was defined as tumor present at the inked surface of the specimen.
Patients were routinely seen in follow-up in our multidisciplinary Connective Tissue Oncology Clinic. Patients were seen at 1 month and 3 months after the end of treatment, then every 3 months for the next two years, every 4 months during year 3, every 6 months for years 4 and 5, and then yearly thereafter indefinitely. Radiographic imaging of the chest (typically chest computed tomography scans) was performed at least every 6 months for the first 5 years of follow-up, and magnetic resonance imaging of the primary site was obtained as clinically indicated.
Data Collection and Analysis
The MGH Department of Radiation Oncology sarcoma database contains prospectively entered data obtained from patient notes and medical records. Data entered and retrieved for this study included tumor histology and grade, location, and stage at diagnosis; dates of operations; pathologic information, such as tumor size, margin status, percent tumor necrosis, and vascular invasion; radiation treatment dates, doses, and modalities; patients’ dates of diagnosis, treatment, and follow-up; and status at each follow-up (no evidence of disease, alive with unknown status, alive with disease, dead of disease, dead of unknown cause).
Data were analyzed for associations between percent pathologic necrosis and outcomes, including rates of local control (LC), disease-specific survival (DSS), and overall survival (OS). Dates of death for patients with social security numbers were obtained from the Social Security Death Index (SSDI). LC was calculated from the date of treatment initiation to the date of first local recurrence. DSS was calculated from the date of treatment initiation to the date of documented death due to sarcoma. OS was calculated from the date of treatment initiation to the date of documented death by SSDI. Censoring occurred at the earlier of date of death or date of last contact. Estimates for LC, DSS, and OS rates were calculated using the method of Kaplan and Meier (KM). Unadjusted intergroup comparisons based on each outcome were made using the log-rank test. Necrosis percentage comparisons by histology and by number of pre-operative cycles of chemotherapy were made using Fisher’s exact test. All reported p-values are two-sided using a significance threshold of 0.05. Statistical analyses were performed using SAS version 9.2.
Results
Patient and tumor characteristics
Clinicopathologic features of the 113 patients in this series are shown in Table 1. The majority of the patients was male, approximately 50 years old, presented with a primary tumor, and had grade 2 or 3 tumors of the lower extremity. The most prevalent histologic types were malignant fibrous histiocytoma (now referred to as undifferentiated pleomorphic sarcoma) and myxoid liposarcoma, and the vast majority of patients underwent a R0 resection. A total of 103 (91%) patients received all 3 planned cycles of preoperative chemotherapy, whereas 4 (4%) and 6 (5%) patients received only 2 and 1 cycle of preoperative chemotherapy, respectively. All 113 patients received 44 Gray of preoperative XRT. The median pathologic tumor necrosis rate was 90%, and 50 (44%) patients had ≥ 95% necrosis. The likelihood of achieving ≥ 95% necrosis was not related to the number of pre-operative cycles of chemotherapy received but was related to tumor histology (MFH 62% versus synovial sarcoma 0%, p<0.001; myxoid liposarcoma 56% versus synovial sarcoma 0%, p=0.002).
Table 1.
Patient and tumor characteristics (n = 113).
| N (%) | |
|---|---|
| Gender: | |
| Male | 74 (65) |
| Female | 39 (35) |
| Age at diagnosis: | |
| ≤ 50 years | 56 (49) |
| > 50 years | 57 (51) |
| Primary Site | |
| Upper Extremity | 12 (11) |
| Lower Extremity | 90 (80) |
| Trunk | 11 (9) |
| Presentation | |
| Primary | 110 (97) |
| Recurrent | 3 (3) |
| Size | |
| < 5 cm | 0 |
| 5 – 10 cm | 39 (34) |
| > 10 cm | 74 (66) |
| Histology | |
| Malignant fibrous histiocytoma | 29 (26) |
| Myxoid liposarcoma | 28 (25) |
| Synovial Sarcoma | 14 (12) |
| Myxofibrosarcoma | 5 (4) |
| Malignant Peripheral Nerve Sheath Tumor | 4 (3) |
| Leiomyosarcoma | 3 (3) |
| Other | 30 (27) |
| Grade | |
| 2 | 58 (51) |
| 3 | 55 (49) |
| Margin | |
| Negative | 99 (88) |
| Positive | 14 (12) |
| Pathologic Necrosis | |
| Median | 90% |
| < 95% | 63 (56) |
| 0 – 25% | 13 (12) |
| 26 – 50% | 8 (7) |
| 51 – 75% | 20 (18) |
| 76 – 94% | 22 (19) |
| ≥ 95% | 50 (44) |
| 100% | 9 (8) |
Recurrence and survival outcomes
At a median follow-up of 6 years (range, 0.8 – 17.4 years), the overall 5-year local recurrence rate for all 113 patients was 7.0%. The time to local failure ranged from 6.6 to 139 months. The 5-year local control (LC) rates for patients who received CRT with < 95% and ≥ 95% pathologic necrosis were not statistically different at 92.3% and 93.9% (p = 0.65), respectively (Table 2 and Figure 2). Furthermore, the 5-year disease-specific survival (DSS) rates for patients with < 95% and ≥ 95% pathologic necrosis were not statistically different at 89.3% and 85.0% (p = 0.25), respectively (Table 2 and Figure 2). Lastly, the 5-year overall survival (OS) rates for patients in the CRT cohort with < 95% and ≥ 95% pathologic necrosis were also not statistically different at 86.7% and 85.0% (p = 0.34), respectively (Table 2 and Figure 2). When stratifying by histology, there was also no difference in any outcome measure (LC, DSS, or OS) according to the extent of pathologic necrosis.
Table 2.
Patient outcomes based on the extent of treatment-induced pathologic necrosis.
| Outcome (95% CI) | ≥ 95% tumor necrosis | < 95% tumor necrosis | P-value |
|---|---|---|---|
| 5-year local control | 93.9% (77.7%, 98.5%) | 92.3% (80.6%, 97.1%) | 0.65 |
| 5-year disease-specific survival | 85.0% (69.1%, 93.1%) | 89.3% (77.7%, 95.1%) | 0.25 |
| 5-year overall survival | 85.0% (69.1%, 93.1%) | 86.7% (73.8%, 93.5%) | 0.34 |
Figure 2.
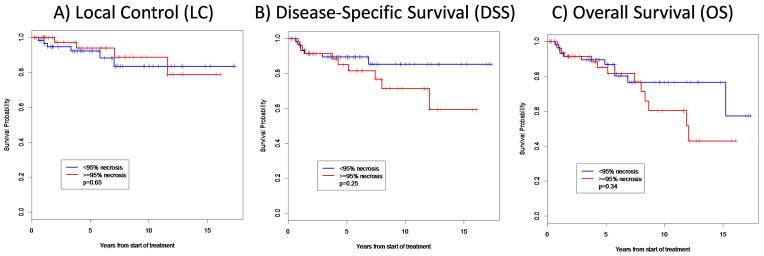
Overall survival (OS), disease-specific survival (DSS), and local control (LC) based on the extent of treatment-induced pathologic necrosis.
Discussion
In this study of a relatively large number of patients with high-grade extremity or truncal STS who were treated in a homogeneous fashion with a protocol of preoperative CRT at a single institution, we demonstrate that the extent of pathologic tumor necrosis does not correlate with outcome. In fact, of 9 patients who enjoyed complete, 100% treatment-induced pathologic necrosis, 3 died of metastatic disease. By contrast, all 7 patients whose tumors showed the lowest rates of histologic response (≤ 10% necrosis) are alive and well.
The percentage of patients in our series achieving at least 95% pathologic necrosis after neoadjuvant CRT (44%) is on par with the rates reported in other series.8–11,13 The few published studies reporting on histologic response after preoperative XRT alone show relatively low rates of treatment-induced necrosis. For example, Choong et al.15 demonstrated that only 15 of 38 tumors (39%) exhibited > 80% necrosis after 50.4 Gy of preoperative XRT. Among studies that employed only preoperative systemic (not intra-arterial) chemotherapy without XRT, high pathologic necrosis rates of 85% to ≥ 95% were reported in 13% to 48% of patients.8,11,16,17 However, not surprisingly, the highest pathologic necrosis rates have been reported in series in which intra-arterial chemotherapy is at least one component of the neoadjuvant regimen.9,10,18
Though treatment-induced pathologic necrosis has generally been accepted as an important prognostic variable in bone sarcomas,1–5 few studies have investigated the correlation between pathologic necrosis and outcome in patients with STS. Several studies have shown that higher rates of treatment-induced pathologic necrosis do in fact correlate with lower metastatic potential and improved survival. In the largest study by Eilber et al., including 496 patients receiving a variety of different neoadjuvant treatment regimens over a nearly 25-year period, patients whose tumors exhibited ≥ 95% pathologic necrosis had a 2.5-fold lower risk of local recurrence and a 1.9-fold lower risk of death than those patients whose tumors exhibited < 95% necrosis.9 However, only the final 125 patients included in this study received ifosfamide, which is perhaps the most active chemotherapy in the treatment of STS, and it was this group that had the highest rates (48%, or 39 of 81 assessable tumors) of ≥ 95% pathologic necrosis. Thus, it is no surprise that this group survived longer and that this improved survival correlated with higher rates of pathologic necrosis. In comparison, only 13% (30 of 228 assessable tumors) of the patients who were treated on protocols including intra-arterial doxorubicin and XRT achieved a pathologic necrosis rate of ≥ 95%. Intuitively, one would expect that this group would have exhibited the highest rates of pathologic necrosis, since the tumors are receiving such aggressive, locally cytotoxic, therapy. However, the tumors treated on these protocols in fact had low rates of pathologic necrosis, and not surprisingly, the patients treated on these protocols had poorer survival rates, as these patients received no systemic chemotherapy; the intra-arterial administration of doxorubicin does nothing to treat potential sites of metastatic disease. These important facts may help explain why Eilber et al. found a correlation between the extent of pathologic necrosis and outcome in their large study.
In a much smaller study of 33 patients by Henshaw et al., 76% of the patients had > 95% tumor necrosis, and although none of the patients received preoperative XRT, all of them were given intra-arterial cisplatin.10 They demonstrated that patients with > 95% tumor necrosis had a longer survival time than those who did not, though among the 4 patients who developed metastatic disease, 3 had > 95% necrosis. Similarly, Pezzi et al., in a study of 45 patients receiving preoperative adriamycin-based chemotherapy alone, showed that histologic response to chemotherapy (graded on a scale of 0 to 4) correlated with a significantly longer survival time.8 Lastly, MacDermed et al. found that in 34 patients with locally advanced STS of the extremity treated with neoadjuvant ifosfamide and XRT, necrosis rates were predictive of subsequent metastatic risk.13
On the other hand, several other studies, including our own, show that the extent of pathologic tumor necrosis does not correlate with outcome. Menendez et al. in a study of 82 patients treated with neoadjuvant chemotherapy alone did not find a correlation between histologic response and outcome, concluding that tumor necrosis following chemotherapy has no prognostic value in STS.11 Similarly, Lucas et al., in a study of 31 patients homogeneously treated with the same neoadjuvant chemotherapy regimen, showed no correlation between percentage of post-treatment viable tumor and overall or event-free survival.12 Importantly, these two studies included only neoadjuvant chemotherapy in the treatment protocol, and thus other therapies such as XRT and intra-arterial chemotherapy, which certainly affect the histology of the tumor, are not present to mask the effects of the systemic chemotherapy alone.
Similar to Eilber et al.,9 we were able to achieve high rates of ≥ 95% pathologic necrosis in patients who received an ifosfamide-containing chemotherapy regimen. But unlike Eilber et al., we found no correlation between percent pathologic necrosis and any outcome measure, including local control, disease-specific, or overall survival, and this was true even when stratifying by histology, including the particularly chemosensitive histologies of MFH and myxoid liposarcoma. In fact, similar to the findings of Lucas et al.,12 we found that a high percentage of patients with high necrosis rates went on to die of metastatic disease, and conversely, that several patients with virtually no tumor necrosis at all are alive and well. Thus, histologic response in and of itself may not be a reliable predictor of biologic behavior. Furthermore, using pathologic tumor necrosis as a parameter to decide who may have enjoyed a beneficial response to a given preoperative chemotherapy regimen, and thus who may continue to benefit from more of the same chemotherapy after surgery, may not be wise. Similarly, we urge caution in the use of the treatment-induced pathologic necrosis rate as an endpoint to judge the effectiveness of therapies in clinical trials. Ultimately, until we have a more reliable predictor of outcome, it may be most prudent to use the outcome of survival itself as the true measure of the effectiveness of a given therapy; after all, prolonging survival is what we all want to achieve at the end of the day.
Another potential measure of tumor response to neoadjuvant therapy is the radiographic response, most commonly measured by the RECIST criteria.19 We have previously reported that in a subset of 58 patients treated on this CRT protocol, that the majority (36 of 58, or 62%) of patients have stable disease (SD), and that half (n=18) of these patients with SD showed < 95% pathologic necrosis and the other half showed ≥ 95% necrosis.20 Another 29% (17 of 58) of the patients had a partial response, and 13 (76%) of these patients had ≥ 95% necrosis compared to 4 that did not. The remaining 9% (5 of 58) of patients exhibited progressive disease, and 2 of these patients had ≥ 95% pathologic necrosis in the resected tumors. Though a greater percentage of patients who had a PR by RECIST criteria also showed ≥ 95% pathologic necrosis, this was not statistically significant. Furthermore, radiographic response as measured by RECIST did not correlate with any outcome measure, making this measurement a similarly poor indicator of the effectiveness of a given neoadjuvant therapy. A tumor may dramatically shrink in response to neoadjuvant therapy (uncommon) but show mostly viable tumor, whereas on the other hand, a tumor may stay the same size or even grow but then show dramatic cell death. Indeed, we have noted that radiographic “progression” by RECIST criteria in patients undergoing neoadjuvant chemoradiation for soft tissue sarcomas can reflect significant necrosis of tumor with enlargement of the mass from osmotic effects.14 Recent studies have shown that the Choi criteria,21 which are based on changes not only in tumor size but also in attenuation at CT or tumor contrast enhancement at MR imaging, are a better predictor of both pathologic response22 and survival23 in patients with STS who receive preoperative chemotherapy alone. As we move forward, perhaps we should adopt the Choi criteria for radiographic tumor response assessment in STS treated with neoadjuvant chemotherapy and (possibly) CRT.
This study has some important limitations that deserve mention. First, as with many of the previous studies examining the impact of pathologic tumor necrosis on outcome, our study is limited by small patient numbers, though it is the second largest published study on this subject. A much larger, multi-institutional study would be necessary to render a more conclusive result. Second, our approach to the measurement of the percentage of pathologic necrosis may differ from that of others, as there is no acceptable, standardized grading system employed by all soft tissue pathologists around the world. Furthermore, our particular neoadjuvant regimen is unique to our institution, and thus the results of this study may not be generalizable to other institutions. Lastly, the use of preoperative XRT in addition to systemic chemotherapy in our protocol potentially masks the effect of the chemotherapy by enhancing the treatment effect on the primary tumor while leaving systemic disease unaffected. Unfortunately, we do not have a cohort of patients treated with chemotherapy alone for comparison, and given the undeniable salutory effect of XRT on local tumor control in STS and the many advantages to its administration before surgery, it is unlikely that we will ever have such a cohort for comparison in the future.
In conclusion, patients with high-grade extremity and truncal STS who undergo an aggressive regimen of preoperative chemoradiotherapy achieve a high percentage of pathologic tumor necrosis. Importantly, however, the extent of pathologic tumor necrosis does not correlate with any outcome measure. Furthermore, radiographic tumor response as measured by RECIST criteria does not correlate with the extent of pathologic tumor necrosis nor outcome. Further research is needed to identify better predictors of outcome based on the clinical, radiographic, and pathologic tumor response to neoadjuvant therapy in STS.
Acknowledgments
Funding: Dana Farber/Harvard Cancer Center Biostatistics Core is supported in part by the National Cancer Institute Cancer Center Support Grant #NIH 5 P30 CA06516.
Footnotes
Financial Disclosures: None.
References
- 1.Picci P, Rougraff BT, Bacci G, et al. Prognostic significance of histopathologic response to chemotherapy in nonmetastatic Ewing’s sarcoma of the extremities. J Clin Oncol. 1993;11:1763–1769. doi: 10.1200/JCO.1993.11.9.1763. [DOI] [PubMed] [Google Scholar]
- 2.Picci P, Sangiorgi L, Rougraff BT, et al. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994;12:2699–2705. doi: 10.1200/JCO.1994.12.12.2699. [DOI] [PubMed] [Google Scholar]
- 3.Picci P, Bohling T, Bacci G, et al. Chemotherapy-induced tumor necrosis as a prognostic factor in localized Ewing’s sarcoma of the extremities. J Clin Oncol. 1997;15:1553–1559. doi: 10.1200/JCO.1997.15.4.1553. [DOI] [PubMed] [Google Scholar]
- 4.Zunino JH, Johnston JO. Prognostic value of histologic tumor necrosis assessment in osteogenic sarcoma of bone. Am J Orthop. 2000;29:369–372. [PubMed] [Google Scholar]
- 5.Bacci G, Mercuri M, Longhi A, et al. Grade of chemotherapy-induced necrosis as a predictor of local and systemic control in 881 patients with non-metastatic osteosarcoma of the extremities treated with neoadjuvant chemotherapy in a single institution. Eur J Cancer. 2005;41:2079–2085. doi: 10.1016/j.ejca.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Lewis IJ, Nooij MA, Whelan J, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: A randomized phase III trial of the European Osteosarcoma Intergroup. J Natl Cancer Inst. 2007;99:112–128. doi: 10.1093/jnci/djk015. [DOI] [PubMed] [Google Scholar]
- 7.Zalupski MM, Rankin C, Ryan JR, et al. Adjuvant therapy of osteosarcoma – a phase II trial: Southwest Oncology Group study 9139. Cancer. 2004;100:818–825. doi: 10.1002/cncr.20021. [DOI] [PubMed] [Google Scholar]
- 8.Pezzi CM, Pollock RE, Evans HL, et al. Preoperative chemotherapy for soft tissue sarcomas of the extremities. Ann Surg. 1990;211:476–481. doi: 10.1097/00000658-199004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: A predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203–3209. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]
- 10.Henshaw RM, Priebat DA, Perry DJ, et al. Survival after induction chemotherapy and surgical resection for high-grade soft tissue sarcoma. Is radiation necessary? Ann Surg Oncol. 2001;8:484–495. doi: 10.1007/s10434-001-0484-8. [DOI] [PubMed] [Google Scholar]
- 11.Menedez LR, Ahlmann ER, Savage K, et al. Tumor necrosis has no prognostic value in neoadjuvant chemotherapy for soft tissue sarcoma. Clin Orthop Relat Res. 2007;455:219–224. doi: 10.1097/01.blo.0000238864.69486.59. [DOI] [PubMed] [Google Scholar]
- 12.Lucas DR, Kshirsagar MP, Biermann JS, et al. Histologic alterations from neoadjuvant chemotherapy in high-grade extremity soft tissue sarcoma: clinicopathological correlation. Oncologist. 2008;13:451–458. doi: 10.1634/theoncologist.2007-0220. [DOI] [PubMed] [Google Scholar]
- 13.Macdermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in locally advanced soft-tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147–1153. doi: 10.1016/j.ijrobp.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–1127. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 15.Choong PF, Nizam I, Ngan SY, et al. Thallium-201 scintigraphy – a predictor of tumor necrosis in soft tissue sarcoma following preoperative radiotherapy? Eur J Surg Oncol. 2003;29:908–915. doi: 10.1016/j.ejso.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Casper ES, Gaynor JJ, Harrison LB, et al. Preoperative and postoperative adjuvant combination chemotherapy for adults with high grade soft tissue sarcoma. Cancer. 1994;73:1644–1651. doi: 10.1002/1097-0142(19940315)73:6<1644::aid-cncr2820730616>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez RE, Zalupski MM, Frank JJ, et al. Multidrug resistance phenotype in high grade soft tissue sarcoma: correlation of P-glycoprotein immunohistochemistry with pathologic response to chemotherapy. Cancer. 1999;86:976–981. doi: 10.1002/(sici)1097-0142(19990915)86:6<976::aid-cncr12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt RA, Conrad EU, 3rd, Collins C, et al. Measurement and prediction of the short-term response of soft tissue sarcomas to chemotherapy. Cancer. 1993;72:2593–2601. doi: 10.1002/1097-0142(19931101)72:9<2593::aid-cncr2820720914>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Look Hong NJ, Hornicek FJ, Harmon DC, et al. Neoadjuvant chemoradiotherapy for patients with high-risk extremity and truncal sarcomas: a 10-year single institution retrospective study. Eur J Cancer. 2013;49:875–883. doi: 10.1016/j.ejca.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. [DOI] [PubMed] [Google Scholar]
- 22.Brisse H, Ollivier L, Edeline V, et al. Imaging of malignant tumors of the long bone in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol. 2004;34:595–605. doi: 10.1007/s00247-004-1192-x. [DOI] [PubMed] [Google Scholar]
- 23.Stacchiotti S, Verderio P, Messina A, et al. Tumor response assessment by modified Choi criteria in localized high-risk soft tissue sarcoma treated with chemotherapy. Cancer. 118:5857–5866. doi: 10.1002/cncr.27624. [DOI] [PubMed] [Google Scholar]


