Abstract
Objective
It is unknown if endothelial dysfunction precedes atrial fibrillation (AF) development. The objective of this study was to examine the association of brachial flow-mediated dilation (FMD) with incident AF.
Approach and Results
A total of 2,936 participants (mean age 61 ± 9.9; 50% women; 66% non-whites) from the Multi-Ethnic Study of Atherosclerosis with available ultrasound brachial FMD measurements who were free of baseline AF were included in this analysis. Baseline (2000-2002) FMD was computed from the percent difference (%FMD) in brachial artery diameter and maximum diameter during measured vasodilator response. AF was ascertained from hospitalization data including Medicare claims during a median follow-up of 8.5 years. Probability-weighted Cox proportional-hazards regression was used to compute hazard ratios (HR) and 95% confidence intervals (95%CI) for the association between FMD as a continuous variable (%FMD values per 1-SD increase) and incident AF. Incident AF was detected in 137 (4.7%) participants. Those with %FMD values below the sex-specific median value (median %FMD; males=3.6%, females=4.2%) (Incidence rate per 1000 person-years=7.3, 95%CI=5.9, 9.0) were more likely to develop AF than persons whose %FMD values were above the median value (Incidence rate per 1000 person-years=4.5, 95%CI=3.4, 5.8) (log-rank p=0.0043). In a multivariable Cox regression analysis, 1-SD increase in %FMD values (SD=2.8%) was associated with less incident AF (HR=0.84, 95%CI=0.70, 0.99). These results were consistent across subgroups stratified by age, sex, and race/ethnicity.
Conclusions
Smaller brachial FMD values are associated with higher rates of AF, suggesting a role for endothelial dysfunction in AF pathogenesis.
Keywords: atrial fibrillation, endothelial dysfunction, epidemiology
INTRODUCTION
Arterial flow-mediated dilation (FMD) is an indirect measurement of endothelial nitric oxide (NO) release.1 Impaired FMD represents systemic vascular endothelial dysfunction that is commonly associated with cardiovascular disease (CVD).2, 3 Additionally, the association of FMD with CVD is evidenced by its ability to predict future CVD events in population-based studies.4, 5
Endothelial dysfunction, defined by impaired FMD, has been reported in patients with atrial fibrillation (AF).6, 7 Also, abnormalities in NO signaling have been implicated in atrial ectopy near the pulmonary veins.8, 9 These findings suggest a potential role for endothelial dysfunction in the development of AF. However, data from population-based studies to support this claim are lacking. The purpose of this study was to examine the association of FMD with incident AF in the Multi-Ethnic Study of Atherosclerosis (MESA).
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement.
RESULTS
Of the 3,026 participants from the FMD ancillary study with available FMD measurements, 28 participants had a diagnosis of AF before enrolment in MESA. These cases were detected by Centers for Medicare & Medicaid Services linkage and were not detected in the baseline study electrocardiogram. Of those that remained, 3 participants with missing follow-up data and 59 participants missing either baseline characteristics or medication data were excluded. A total of 2,936 study participants (mean age 61 ± 9.9; 50% women; 66% non-whites) were included in the final analysis.
FMD was computed from the percent difference (%FMD) in brachial artery diameter and maximum diameter during measured vasodilator response. Baseline characteristics stratified by sex-specific median %FMD are shown in Table 1. Participants with %FMD values below the median were more likely to be older, diabetic, and non-white, and to have lower educational attainment and income compared with higher %FMD values. Persons with %FMD values below the median value were more likely to have increased values for systolic blood pressure and high-density lipoprotein cholesterol, and lower values for total cholesterol than those with %FMD values above the median value. Higher rates of antihypertensive medications, aspirin, lipid-lowering therapies, and left ventricular hypertrophy also were observed in persons with %FMD values below the median value.
Table 1. Baseline Characteristics (N=2,936)*.
| Variable | <Median %FMD (n=1,470) |
≥Median %FMD (n=1,466) |
P-value† |
|---|---|---|---|
| Age, mean (SD), years | 64 (10) | 58 (9.1) | <0.0001 |
| Male Sex (%) | 738 (50) | 722 (49) | 0.61 |
| Race/ethnicity | |||
| White (%) | 440 (30) | 561 (38) | |
| Black (%) | 396 (27) | 205 (14) | |
| Chinese-American (%) | 263 (18) | 326 (22) | |
| Hispanic (%) | 371 (25) | 374 (26) | <0.0001 |
| High school or less (%) | 569 (39) | 496 (34) | 0.0060 |
| Income <$20,000 (%) | 407 (28) | 351 (24) | 0.020 |
| Body mass index, mean (SD) kg/m2 | 28 (5.1) | 28 (5.4) | 0.44 |
| Current or former smoker (%) | 697 (47) | 679 (46) | 0.55 |
| Diabetes (%) | 229 (16) | 146 (10) | <0.0001 |
| Systolic blood pressure, mean (SD), mm Hg | 128 (20) | 121 (19) | <0.0001 |
| Total cholesterol, mean (SD), mg/dL | 193 (36) | 196 (34) | 0.048 |
| HDL-cholesterol, mean (SD), mg/dL | 51 (14) | 50 (14) | 0.014 |
| Antihypertensive medications (%) | 570 (39) | 427 (29) | <0.0001 |
| Statins (%) | 222 (15) | 185 (13) | 0.052 |
| Aspirin (%) | 357 (24) | 311 (21) | 0.047 |
| Lipid-lowering medications (%) | 245 (17) | 205 (14) | 0.044 |
| hs-CRP, mean (SD), mg/L | 3.5 (5.3) | 3.4 (5.7) | 0.13 |
| Left ventricular hypertrophy (%) | 70 (4.8) | 34 (2.3) | 0.0003 |
Median %FMD values for male and female participants were 3.6% and 4.2%, respectively.
Statistical significance was tested for categorical variables using the chi-square method and for continuous variables the Wilcoxon-rank sum method was used.
AF=atrial fibrillation; FMD=flow-mediated dilation; HDL=high-density lipoprotein; hs-CRP=high-sensitivity C-reactive protein; SD=standard deviation.
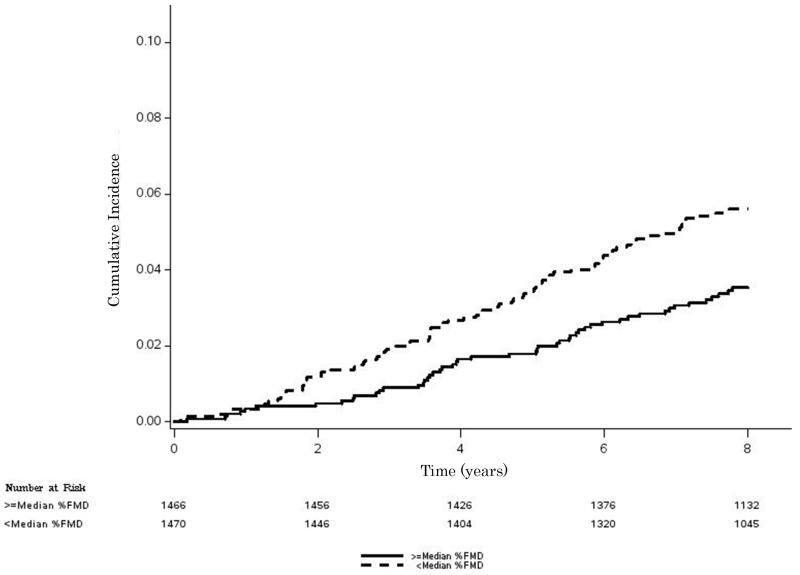
A total of 137 (4.7%) participants developed AF during the study period. Median follow-up for study participants was 8.5 years (interquartile range=7.9, 8.7). Unadjusted cumulative incidence curves stratified by median %FMD are shown in Figure 1. Participants with %FMD less than the median value (incidence rate per 1000 person-years=7.3, 95%CI=5.9, 9.0) were more likely to develop AF compared with participants who had %FMD values greater than the median value (incidence rate per 1000 person-years=4.5, 95%CI=3.4, 5.8) (log-rank p=0.0043).
Figure 1. Cumulative Incidence of AF by Median %FMD*.
*Median %FMD values for male and female participants were 3.6% and 4.2%, respectively. Kaplan-Meier estimates were significantly different (log-rank p=0.0043).
AF=atrial fibrillation; FMD=flow-mediated dilation.
In a multivariable Cox proportional hazards analysis, 1-SD increase in %FMD values (SD=2.8%) was associated with less incident AF (Table 2). The association between FMD and AF remained significant after further adjustment of Model 2 with amino-terminal-pro-brain natriuretic peptide (HR=0.83, 95%CI=0.69, 0.99). These results were consistent across subgroups of MESA participants stratified by age, sex, and race/ethnicity (Table 2).
Table 2. Association of FMD with AF by Age, Sex, and Race/Ethnicity*†.
| Events/No. at risk |
Model 1‡ HR (95%CI) |
P-value | Model 2§ HR (95%CI) |
P-value | P-interaction|| | |
|---|---|---|---|---|---|---|
| All | 137/2,936 | 0.82 (0.69, 0.98) | 0.029 | 0.84 (0.70, 0.99) | 0.048 | - |
| Age | ||||||
| ≤65 years | 47/1,898 | 0.68 (0.52, 0.89) | 0.0048 | 0.73 (0.56, 0.95) | 0.022 | 0.55 |
| >65 years | 90/1,038 | 0.79 (0.63, 0.99) | 0.037 | 0.81 (0.64, 1.01) | 0.065 | |
| Race | ||||||
| White | 70/1,001 | 0.88 (0.70, 1.1) | 0.27 | 0.86 (0.68, 1.1) | 0.22 | 0.97 |
| Non-White | 67/1,935 | 0.77 (0.59, 1.0) | 0.054 | 0.83 (0.64, 1.1) | 0.19 | |
| Sex | ||||||
| Male | 85/1,460 | 0.87 (0.69, 1.1) | 0.26 | 0.89 (0.70, 1.1) | 0.33 | 0.31 |
| Female | 52/1,476 | 0.77 (0.59, 0.99) | 0.048 | 0.77 (0.59, 1.01) | 0.062 |
HR presented are for %FMD per 1-SD increase (SD=2.8%).
Subgroups were adjusted according to Models 1 and 2 excluding the covariate of interest.
Adjusted for age, sex, race/ethnicity, income, and education.
Adjusted for Model 1 covariates plus smoking status, systolic blood pressure, diabetes, body mass index, total cholesterol, HDL-cholesterol, aspirin, antihypertensive and lipid-lowering medications, hs-CRP, and left ventricular hypertrophy.
Interactions tested using Model 2.
AF=atrial fibrillation; CI=confidence interval; FMD=flow-mediated dilation; HDL=high-density lipoprotein; hs-CRP=high-sensitivity C-reactive protein; HR=hazard ratio; SD=standard deviation.
DISCUSSION
In this analysis from MESA, lower brachial FMD values were associated with increased rates of AF. These findings suggest that endothelial dysfunction, as measured by brachial FMD, plays a role in the pathogenesis of AF.
To our knowledge, only 2 studies have examined the association of brachial FMD with AF. A study of chronic AF participants showed that FMD measurements are significantly impaired compared with sinus rhythm controls.6 Another case-control study showed that participants with persistent AF have impaired FMD and that FMD improves after the restoration of sinus rhythm.7 However, both studies examined FMD among participants who already had AF. The current study examined FMD among participants without diagnosed AF and showed that abnormal FMD values are associated with an increased risk of AF development. Additionally, participants with lower %FMD values were observed to have a higher incidence of AF than those in the general population.10 Therefore, we provide evidence that impaired FMD precedes the development of AF, suggesting a role for endothelial dysfunction in the pathogenesis of AF.
Endothelial cells regulate oxidative stress, vascular permeability, platelet aggregation, thrombosis, and vascular tone by controlling the release of several vasoactive substances, including NO.11 The dysfunctional endothelium results in the down-regulation of NO and the up-regulation of adhesion molecules that promote increased levels of inflammation and oxidative stress. Recent evidence suggests that increased oxidant generation by endothelial NADPH oxidase promotes the uncoupling of NO synthase and subsequent generation of reactive oxygen species and oxidative injury that leads to the electrophysiological remodeling observed in AF.8 Additionally, exogenous NO has been shown to reduce spontaneous electrical activity in cardiomyocytes isolated from the pulmonary vein, implicating NO as a regulator of AF arrhythmiogenesis.9 Endothelial dysfunction also is associated increased levels of inflammation that result in atrial ectopy in discharging cells near the pulmonary veins.12, 13 Therefore, it is plausible that persons with endothelial dysfunction, as evidenced by abnormal FMD, are more likely to have dysfunctional regulation of the aforementioned processes that increase their risk for AF. FMD potentially is able to identify persons with abnormal vascular biological profiles that precede this arrhythmia. However, further studies are needed to validate our findings before screening programs that use FMD are introduced.
Alternatively, a number of shared risk factors for AF, such as increasing age, diabetes, hypertension, and smoking have been associated with endothelial dysfunction.14-18 Potentially, these conditions increase the level of vascular endothelial dysfunction and predispose individuals to AF. However, our results remained significant after adjustment for these risk factors, suggesting that the association between endothelial dysfunction and AF is not completely explained by shared risk factors.
Brachial FMD has been shown to independently predict incident CVD events among persons who are free of CVD at baseline.5 Abnormal FMD values possibly identify individuals with subclinical atherosclerosis who are at-risk for CVD. Additionally, as evidenced by our results, FMD potentially is able to identify at-risk persons for AF. However, further research is needed to examine the predictive ability of FMD for AF among at-risk populations.
Our results should be interpreted in the context of certain limitations. Paroxysmal cases of AF possibly were missed due to the time-dependent nature of the condition. Incident AF cases were ascertained from hospitalization discharge records and inpatient Medicare claims data using International Classification of Disease codes which possibly resulted in misclassification. However, this method has been reported to have adequate positive predictive value for AF case identification.19-21 Brachial FMD measurements were obtained during the initial MESA visit and the association of FMD with AF may vary with repeat FMD measurements. The clinical significance of the FMD values (e.g., increase per 1-SD, median) used in this study is unknown and those used were designed to demonstrate exploratory associations (e.g., lower FMD values are associated with increased AF risk). Future studies are needed to define clinically relevant values. Non-significant interactions were observed by age, sex, and race/ethnicity but the current analysis potentially was underpowered to detect such differences. Furthermore, our results are limited regarding generalizability to other populations due to the older age of participants with FMD values lower than the median value for study participants.
In conclusion, we have shown that brachial FMD values are inversely associated with incident AF in MESA. Our results suggest that endothelial dysfunction precedes the development of AF and may play an important role in the pathogenesis of this common arrhythmia. Further research is needed to confirm our findings and also to explore the clinical utility of FMD to identify those who are at-risk for developing AF.
Supplementary Material
SIGNIFICANCE.
Endothelial dysfunction has been reported in patients with atrial fibrillation. However, the temporal association between impaired endothelial function and atrial fibrillation has not been examined. In this analysis from the Multi-Ethnic Study of Atherosclerosis, we have shown that impaired brachial artery flow-mediated dilation, an indirect marker of endothelial dysfunction, precedes the development of atrial fibrillation. These findings support the hypothesis that alterations in vascular biology predispose to the arrhythmiogenesis of atrial fibrillation.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of Funding
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-RR-024156 and UL1-RR-025005 from NCRR.
Nonstandard Abbreviations and Acronyms
- AF
Atrial fibrillation
- CVD
Cardiovascular disease
- FMD
Flow-mediated dilation
- hs-CRP
High-sensitivity C-reactive protein
- MESA
Multi-Ethnic Study of Atherosclerosis
- NO
Nitric oxide
Footnotes
Disclosures
Dr. Nazarian is a consultant and principal investigator for research funding awarded to Johns Hopkins University from Biosence Webster Inc.
REFERENCES
- 1.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 2.Neunteufl T, Katzenschlager R, Hassan A, Klaar U, Schwarzacher S, Glogar D, Bauer P, Weidinger F. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129:111–118. doi: 10.1016/s0021-9150(96)06018-2. [DOI] [PubMed] [Google Scholar]
- 3.Perrone-Filardi P, Cuocolo A, Brevetti G, et al. Relation of brachial artery flow-mediated vasodilation to significant coronary artery disease in patients with peripheral arterial disease. Am J Cardiol. 2005;96:1337–1341. doi: 10.1016/j.amjcard.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 4.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The cardiovascular health study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 5.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freestone B, Chong AY, Nuttall S, Lip GY. Impaired flow mediated dilatation as evidence of endothelial dysfunction in chronic atrial fibrillation: Relationship to plasma von willebrand factor and soluble e-selectin levels. Thromb Res. 2008;122:85–90. doi: 10.1016/j.thromres.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Skalidis EI, Zacharis EA, Tsetis DK, Pagonidis K, Chlouverakis G, Yarmenitis S, Hamilos M, Manios EG, Vardas PE. Endothelial cell function during atrial fibrillation and after restoration of sinus rhythm. Am J Cardiol. 2007;99:1258–1262. doi: 10.1016/j.amjcard.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 8.Kim YM, Guzik TJ, Zhang YH, Zhang MH, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. A myocardial nox2 containing nad(p)h oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res. 2005;97:629–636. doi: 10.1161/01.RES.0000183735.09871.61. [DOI] [PubMed] [Google Scholar]
- 9.Lin YK, Lu YY, Chen YC, Chen YJ, Chen SA. Nitroprusside modulates pulmonary vein arrhythmogenic activity. J Biomed Sci. 2010;17:20. doi: 10.1186/1423-0127-17-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in olmsted county, minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 11.Endemann DH, Schiffrin EL. Endothelial dysfunction. JASN. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 12.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 13.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 14.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 15.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 16.Paniagua OA, Bryant MB, Panza JA. Role of endothelial nitric oxide in shear stress-induced vasodilation of human microvasculature: Diminished activity in hypertensive and hypercholesterolemic patients. Circulation. 2001;103:1752–1758. doi: 10.1161/01.cir.103.13.1752. [DOI] [PubMed] [Google Scholar]
- 17.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 18.Weiner SD, Ahmed HN, Jin Z, Cushman M, Herrington DM, Nelson JC, Di Tullio MR, Homma S. Systemic inflammation and brachial artery endothelial function in the multi-ethnic study of atherosclerosis (mesa) Heart. 2014;100:862–866. doi: 10.1136/heartjnl-2013-304893. [DOI] [PubMed] [Google Scholar]
- 19.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and african-americans: The atherosclerosis risk in communities (aric) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21:141–147. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bengtson LG, Kucharska-Newton A, Wruck LM, Loehr LR, Folsom AR, Chen LY, Rosamond WD, Duval S, Lutsey PL, Stearns SC, Sueta C, Yeh HC, Fox E, Alonso A. Comparable ascertainment of newly-diagnosed atrial fibrillation using active cohort follow-up versus surveillance of centers for medicare and medicaid services in the atherosclerosis risk in communities study. PLoS One. 2014;9:e94321. doi: 10.1371/journal.pone.0094321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.