Abstract
Objective
Current antiplatelet strategies to prevent myocardial infarction and stroke are limited by bleeding risk. A better understanding of the roles of distinct platelet-activating pathways is needed. We determined whether platelet activation by two key primary activators, thrombin and collagen, plays distinct, redundant, or interacting roles in tail bleeding and carotid thrombosis in mice.
Approach and Results
Platelets from mice deficient for the thrombin receptor Par4 and the collagen receptor GPVI lack responses to thrombin and collagen, respectively. We examined tail bleeding and FeCl3-induced carotid artery occlusion in mice lacking Par4, GPVI or both. We also examined a series of Par mutants with increasing impairment of thrombin signaling in platelets. Ablation of thrombin signaling alone by Par4 deficiency increased blood loss in the tail bleeding assay and impaired occlusive thrombus formation in the carotid occlusion assay. GPVI deficiency alone had no effect. Superimposing GPVI deficiency upon Par4 deficiency markedly increased effect size in both assays. In contrast to complete ablation of thrombin signaling, 9- and 19-fold increases in EC50 for thrombin-induced platelet activation had only modest effects.
Conclusion
The observation that loss of Par4 uncovered large effects of GPVI deficiency implies that Par4 and GPVI made independent, partially redundant contributions to occlusive thrombus formation in the carotid and to hemostatic clot formation in the tail under the experimental conditions examined. At face value, these results suggest that thrombin- and collagen-induced platelet activation can play partially redundant roles despite important differences in the how these agonists are made available to platelets.
Keywords: Protease-activated receptor-3, Protease-activated receptor-4, Glycoprotein VI, Platelets, thrombin, collagen, mouse assays, hemostasis, thrombosis
Introduction
Platelet-dependent thrombi are a major cause of myocardial infarction and stroke1–9. The efficacy and use of established antiplatelet agents for preventing these events is limited by bleeding risk2–9. Improved understanding of the relative importance and interactions of different pathways of platelet activation in hemostasis and thrombosis is needed to determine whether and how these processes can be better separated.
Thrombin and collagen are potent activators of platelets ex vivo10–12. Platelet activation by thrombin generated at sites of vascular injury and by vessel wall collagen exposed at such sites contributes to hemostasis and thrombosis in vivo. The extent to which these primary platelet activators serve independent, redundant or interacting roles in vivo has not been directly examined.
Thrombin is arguably the most potent platelet activator12,13. Mouse platelets utilize Protease-Activated Receptor-3 (Par3) and Par4 (gene names F2rl2 and F2rl3, a.k.a. Par3 and Par4, respectively) to sense and respond to thrombin11,14. Mouse Par3 appears incapable of signaling by itself and instead serves as a thrombin-binding cofactor that promotes cleavage and activation of Par4 at low concentrations of thrombin15. In accord, platelets from Par3-deficient mice require more thrombin than wild-type platelets for normal activation, and platelets from Par4-deficient mice fail to respond to even micromolar thrombin11,14,16. In the FeCl3-induced carotid artery thrombosis assay, Par4-deficient mice are protected against thrombotic occlusion triggered by application of 250 mM (4%) FeCl3 compared to wild type, but application of 1.25 M (20%) FeCl3 reliably causes thrombotic occlusion of carotid arteries in Par4-deficient mice (albeit more slowly than in wild type)16. Par4-deficient mice also show increased blood loss in a tail bleeding assay compared to wild type, but hemostasis is still reliably achieved and blood loss is modest compared to the maximal that can occur in this assay16,17. Thus, mechanisms independent of thrombin-induced platelet activation are sufficient to drive formation of occlusive thrombi in the carotid and hemostatic clots in the tail.
Like thrombin, collagen is an effective primary activator of platelets10. Collagen activates platelets initially by binding to platelet glycoprotein VI (GPVI, gene name Gp6), and platelets from GPVI-deficient mice fail to respond to collagen10,18,19. Loss of GPVI function caused by null mutations in Gp6 or FcRγ (which is necessary for GPVI biogenesis and signaling20) or treatment with a GPVI-depleting antibody has been associated with variable effects on tail bleeding and FeCl3-induced carotid occlusion in mice18,21–29. However, isolated GPVI deficiency can be associated with modest or no effects in these assays in some conditions27,28 (see Discussion). Thus, mechanisms independent of collagen-induced platelet activation can be sufficient to drive thrombus formation in these assays.
Ex vivo, collagen and thrombin can each activate platelets in the absence of the other. Whether redundant functions of these activators in vivo might account for the "missing" mechanisms that support tail hemostasis and carotid thrombosis in the absence of collagen or thrombin signaling alone is unknown. The mechanisms by which these activators become available to platelets are distinct. Collagen is an insoluble polymer present in the vessel wall and can directly activate only those platelets that touch it at the immediate site of damage10. Thrombin is soluble and hence can act at a distance from the surface on which it is generated, and it can be generated on activated platelets30. Thus, in contrast to collagen, thrombin may directly activate platelets at a distance from the immediate site of damage. In a laser injury-induced arteriole thrombosis assay, thrombin signaling in platelets is required for growth of platelet thrombi away from the vessel wall but not for formation of small juxtamural platelet thrombi at the site of injury31. These observations raised the possibility that collagen and thrombin might have distinct rather than redundant roles, e.g., collagen signaling serving primarily to activate platelets adherent to the vessel wall and thrombin signaling driving growth of larger platelet thrombi necessary for vessel occlusion.
We utilized mice with different Par3 and Par4 genotypes to probe the effects of different levels of thrombin signaling in platelets as well as mice that lack thrombin signaling in platelets (Par4−/−), collagen signaling in platelets (Gp6−/−) or both to determine whether thrombin- and collagen-induced platelet activation serve independent, redundant or interacting roles in the tail bleeding and FeCl3-induced carotid occlusion assays in mice. Under the moderate to high injury conditions explored, partial inhibition of thrombin signaling alone had no or modest effects, but complete ablation of thrombin signaling decreased thrombus formation in both assays even when GPVI was intact. By contrast, ablation of collagen signaling had no effect on tail bleeding or carotid occlusion when thrombin signaling was intact but had large effects when thrombin signaling was absent. These results suggest that thrombin- and collagen-induced platelet activation can independently support thrombus formation in these assays and that their contributions are partially redundant such that removal of both pathways produces outsized effects. Such redundancy is perhaps surprising given the very different mechanisms by which these ligands become available to platelets. Implications for hypotheses regarding therapeutic strategies are discussed.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
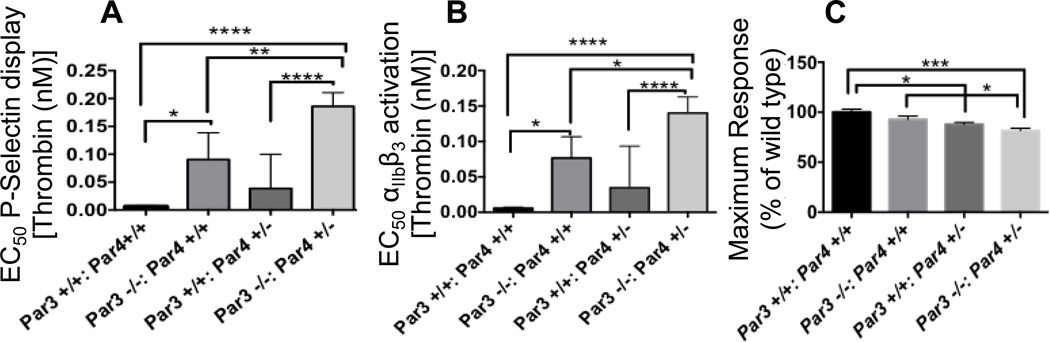
Our previous studies revealed that subtle impairment of thrombin signaling in Par3−/− platelets protects against carotid artery thrombosis triggered by low (250 mM; 4%) FeCL3 without significantly increasing blood loss in the tail bleeding assay used here17. To further explore the effect of different levels of impairment of thrombin-induced platelet activation and toward establishing conditions in which interactions with GPVI deficiency might be detected, we first characterized the concentration response to thrombin in platelets from mice homozygous or heterozygous for Par3 and Par4 alone and in combination as assessed by thrombin-induced P-selectin display measured by flow cytometry (Figure 1). EC50s for thrombin-induced P-selectin display in platelets from wild-type, Par3+/+, Par4+/−, Par3−/−:Par4+/+, and Par3−/−:Par4+/− mice were 0.1, 0.3, 0.9, and 1.9 nM, respectively (Figure 1A). Similar results were found when αIIbβ3 activation was used as an endpoint (Figure 1B), and maximal αIIbβ3 activation to thrombin was ~20% decreased in Par3−/−:Par4+/− platelets (Figure 1C). Par4−/− platelets showed no responses to even 1000 nM thrombin as previously described16,32. Par3−/−:Par4+/− mice were of special interest because, in addition to the 20% decrease in maximal response, platelets from these mice showed a 19-fold increase in the EC50 for thrombin over wild type, about twice the effect of isolated Par3 deficiency and in a range of partial impairment of thrombin signaling not previously explored. Accordingly, we next assessed the effect of these varying levels of loss of platelet responsiveness to thrombin in vivo.
Figure 1. Effect of combined partial or complete Par3 and Par4 deficiency on mouse platelet responses to thrombin.
Washed platelets from mice of the indicated genotypes were activated with different concentrations of thrombin (1 pM-100 nM) and platelet P-selectin exposure or αIIbβ3 activation were measured by flow cytometry. Maximal responses and the concentration of thrombin required to elicit half-maximal responses (EC50) were determined from the resulting concentration-response curves. Results (mean +/− SEM for 3 curves per genotype) are shown for A) EC50 for P-selectin display, B) EC50 for αIIbβ3 activation, C) maximal responses for αIIbβ3 activation. Groups were different at P<0.05 (*), <0.001 (***) or <0.0001 (****).
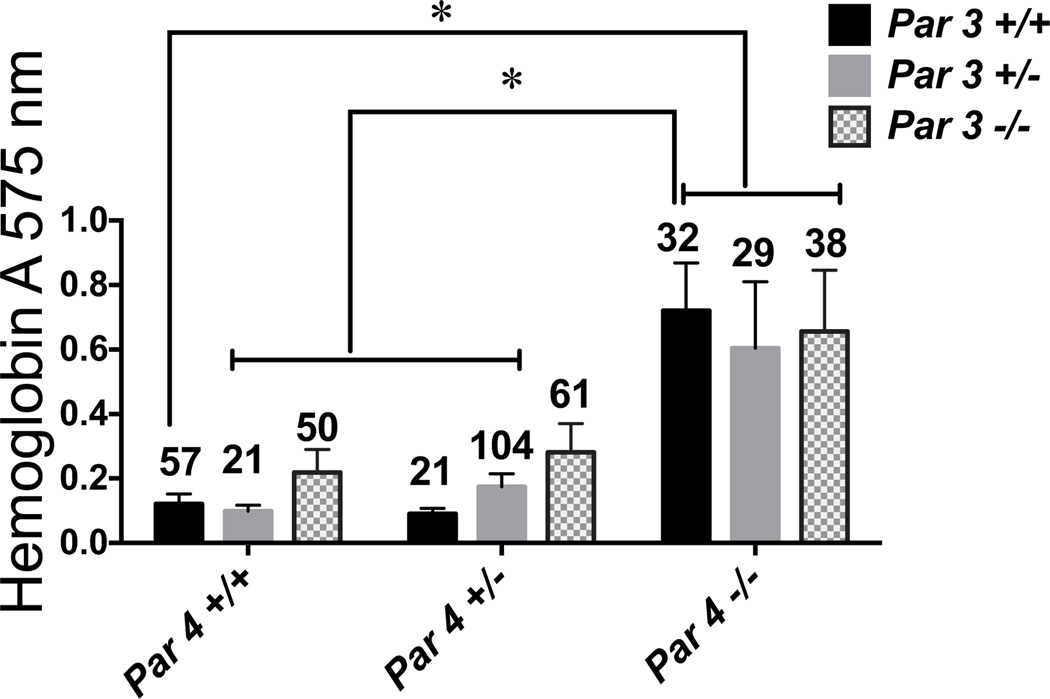
In the tail bleeding assay (Figure 2), blood loss was increased about 6-fold in Par4−/− mice compared to wild type (~0.1 AU to ~0.6 AU) and this increase was independent of Par3 genotype as expected (Par4 deficiency alone is sufficient to ablate thrombin signaling16,17). By contrast, neither Par3−/−:Par4+/+ nor Par3−/−:Par4+/− mice showed a statistically significant increase in tail bleeding compared to wild type (Figure 2); the point estimate for mean hemoglobin loss was 0.2 AU in Par3−/−:Par4+/+ and 0.26 AU in Par3−/−:Par4+/− mice compared to 0.1 AU in wild type.
Figure 2. Effect of combined partial or complete Par3 and Par4 deficiency on tail bleeding.
Blood loss after tail transection was measured in anesthetized 28 ± 3 day-old mice with the indicated genotypes. Mean ± SEM (n=21–104) is shown. Par4 but not Par3 genotype affected blood loss by two-way ANOVA. By Tukey's test, blood loss was different between all Par4−/− genotypes and other genotypes except Par3−/−:Par4+/− at P<0.05. Sample size is indicated above each bar.
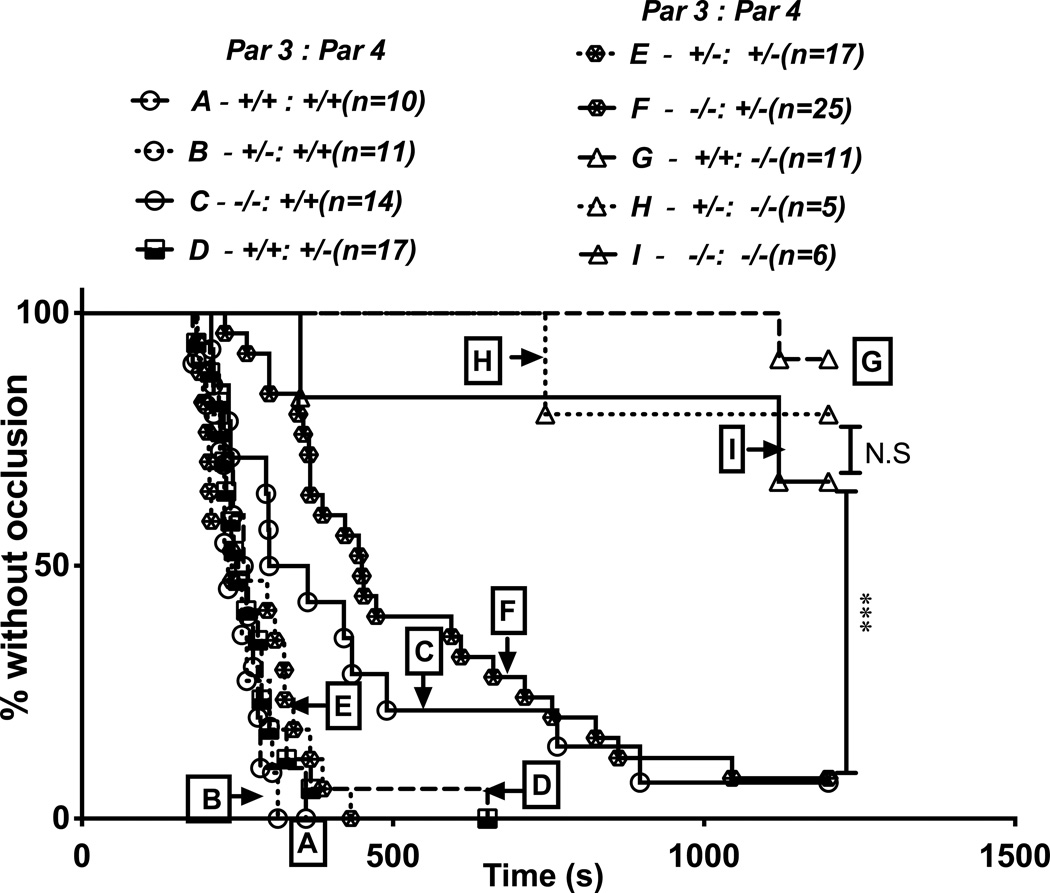
In the carotid artery occlusion assay (Figure 3), Par4−/− mice were protected against occlusive thrombus formation under the conditions used, and this effect was again independent of Par3 genotype. In wild-type mice, carotids occluded at a median time of ~250 seconds after injury, while in Par4−/− mice, ~80% of carotids never occluded (median >1200 seconds). Unlike in the tail bleeding assay, Par3−/−:Par4+/+ did show statistically significant albeit modest difference compared to wild-type mice with an increase in median time to occlusion to ~300 seconds, consistent with previous reports16,17. Median time to occlusion in Par3−/−:Par4+/− mice was also different from wild-type at ~400 seconds, but the difference between Par3−/−:Par4+/+ and Par3−/−:Par4+/− did not reach statistical significance.
Figure 3. Effect of combined partial or complete Par3 and Par4 deficiency on carotid artery occlusion.
Carotid arteries of anesthetized 8–14 week-old mice were injured by exposure to filter paper saturated with 0.5 M (8%) FeCL3, flow after injury was measured, and time to stable occlusion determined. Data are shown as percent of arteries without occlusion as a function of time after injury for each genotype. Genotype affected time to occlusion by log-rank analysis (P<0.0001). In multiple comparison-corrected follow-on comparisons, all Par4−/− genotypes (G,H,I) were different from all others, and Par3−/−:Par4+/+ (C) and Par3−/−:Par4+/− (F) mice were different from wild-type (A) (P<0.001) but not from each other.
The results described above reveal that even in an otherwise wild-type background in which other platelet-activating pathways are intact, increasing the EC50 for thrombin-induced platelet activation from 0.1 nM for wild-type to 0.9 nM for Par3−/− or to 1.9 nM for Par3−/−:Par4+/− platelets produced detectable protection in the carotid occlusion assay. These changes in signaling did not produce statistically significant increases in tail bleeding, but point estimates suggested a positive trend. Ablation of thrombin signaling in Par4−/− mice in an otherwise wild-type background conferred more substantial protection in the carotid occlusion assay and increased blood loss in the tail bleeding assay.
We next examined the relative importance of GPVI in the same assays and its interactions of thrombin-induced platelet activation using mice homozygous and heterozygous for Gp6 and Par4 null alleles, alone and in combination. In contrast to the case with alterations in thrombin signaling via PARs, even ablation of collagen signaling via Gp6 produced no detectable effect in an otherwise wild-type background.
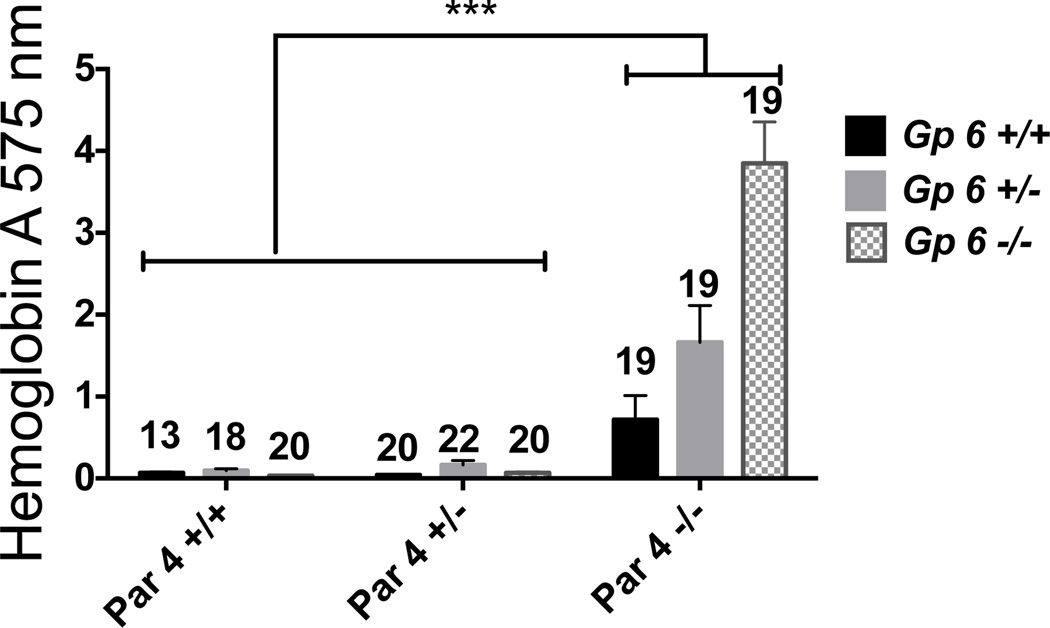
In the tail bleeding assay (Figure 4), isolated Par4 deficiency caused an increase in hemoglobin loss from 0.1 AU in Gp6+/+:Par4+/+ mice to 0.7 AU in Gp6+/+:Par4−/− mice, consistent with the previous experiment (Figure 2). In contrast to Par4 deficiency, GPVI deficiency in a Par4+/+ or Par4+/− background did not cause increased blood loss. Strikingly, however, superimposing GPVI deficiency upon Par4 deficiency resulted in a marked increase in blood loss over and above that associated with Par4 deficiency alone. Mean hemoglobin loss in Gp6−/−:Par4−/− mice was 3.8 AU, corresponding to about 15% of total blood volume and more than 5-fold the hemoglobin loss in Gp6+/+:Par4 −/−. Gp6 heterozygosity also had a significant effect in a Par4−/− background, with mean hemoglobin loss increasing from 0.7 AU in Gp6+/+:Par4−/− mice to 1.8 AU in Gp6+/−:Par4−/− mice.
Figure 4. Effect of combined partial or complete Par4 and GPVI deficiency on tail bleeding.
Blood loss after tail transection was measured in anesthetized 28 ± 3 day-old mice with the indicated Gp6 and Par4 genotypes. Mean ± SEM (n=13–22) hemoglobin loss is shown. Par4 and Gp6 genotypes each had statistically significant effects and interactions by 2-way ANOVA (all P<0.0001). By Tukey's test, blood loss in all Par4−/− genotypes was different from that in all Par4+/+ and Par+/− genotypes regardless of Gp6 genotype (P<0.001). By contrast, blood loss in Gp6+/− and Gp6−/− mice was not different from that in Gp6+/+ mice in Par4+/+ or Par4+/− backgrounds, but blood loss in Gp6+/−:Par4−/− mice and Gp6−/−:Par4−/− mice was significantly different from that in Gp6+/+:Par4−/− mice (P<0.001).
In the carotid artery thrombosis assay, we showed that even at a low level of injury (0.25M (4%) FeCl3), GPVI deficiency alone provided no protection against thrombosis while under the same conditions Par4 deficiency alone provided complete protection (not shown). These results are consistent with at least some published studies (see Discussion) in which Par4 and GPVI mutants were studied separately16–18,26,29. Thus, in contrast to thrombin-signaling, GPVI signaling was unnecessary for normal thrombus formation when other platelet signaling pathways were left intact.
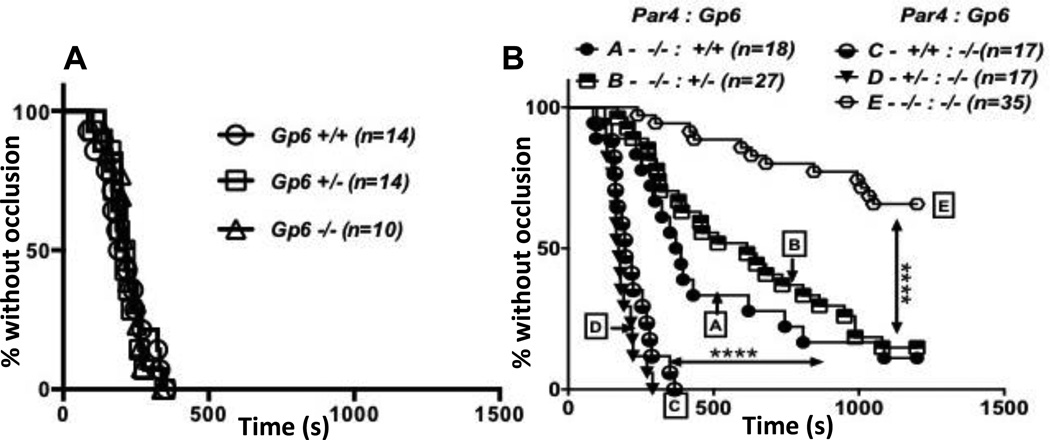
To provide a system in which interactions of the kind found in the tail bleeding assay could be detected, we increased the concentration of FeCl3 to a level at which Par4−/− mice were only partially protected (Figure 5). As expected, GPVI deficiency alone had no effect on the rate or frequency of occlusive thrombus formation in carotid arteries after injury with 1.25 M (20%) FeCl3; median time to occlusion was about 200 seconds for wild-type, Gp6+/− and Gp6−/− mice, and all arteries were occluded by about 300 seconds (Figure 5A). Par4 deficiency alone caused a statistically significant delay in occlusion even at this level of injury (Figure 5B; curve A); median time to occlusion was about 350 seconds in Gp6+/+:Par4−/− mice compared to about 200 seconds in wild-type and in Gp6−/−:Par4+/+ and Gp6−/−:Par4+/− mice. Also in contrast to these genotypes, about 10% of vessels in Par4−/− mice remained open to 1200 seconds, the end of the protocol (Figure 5B; curve A).
Figure 5. Effect of combined partial or complete Par4 and GPVI deficiency on carotid artery occlusion.
Carotid arteries of anesthetized 8 to 14 week-old mice were injured by exposure to filter paper saturated with 1.25 M (20%) FeCL3, flow after injury was measured, and time to stable occlusion determined. Data are shown as percent of arteries without occlusion as a function of time after injury for each genotype. A) Littermate offspring of Gp6+/− crosses were examined. B) Littermate offspring of Par4+/−:Gp6+/− crosses and Par4−/−:Gp6+/−×Par4−/−:Gp6−/− crosses were examined. Note that GPVI deficiency alone had no effect. In (B), genotype affected time to occlusion by log-rank analysis (P<0.0001). In multiple comparison-corrected follow-on comparisons, Par4 deficiency alone prolonged time to occlusion (curve A vs. C,D; P<0.0001), and combined Par4 and GPVI deficiency provided a substantial further increase in time to occlusion (curve E vs. A; P<0.0001).
While GPVI deficiency alone had no effect in the thrombosis assay, superimposing GPVI deficiency upon Par4 deficiency had a major effect. Compared to 10% in Gp6+/+:Par4−/− mice, ~70% of vessels in Gp6−/−:Par4−/− remained open through the end of the protocol such that median time to occlusion was indeterminate (>1200 seconds) (Figure 5B; curve E). Median time to occlusion in Gp6+/−: Par4−/− mice was intermediate between Gp6+/+:Par4−/− and Gp6−/−: Par4−/− mice (Figure 5B; curve B).
The results described above suggest that collagen-induced platelet activation via GPVI is not necessary for normal clot formation in the tail bleeding assay nor for occlusive thrombus formation in the carotid occlusion assay when thrombin signaling is intact. By contrast, thrombin-induced platelet activation is necessary for wild-type levels of hemostatic clot and arterial thrombus formation in these assays even when collagen signaling is intact. The fact that combined GPVI and Par4 deficiency had large effects compared to either deficiency alone suggests that collagen- and thrombin-induced platelet activation each make independent contributions that serve partially redundant functions in these assays.
Discussion
We report a comparison of the effects of ablating or reducing thrombin-induced platelet activation and ablating collagen-induced platelet activation, alone and in combination, on tail bleeding and thrombotic carotid occlusion in mice. A lack of an effect of isolated GPVI deficiency in these assays under conditions in which even modest changes in thrombin signaling (e.g., Par3 deficiency) produced detectable effects suggests that, under the conditions examined, collagen-induced platelet activation via GPVI is not necessary for normal hemostasis in the tail bleeding assay nor for normal formation of occlusive thrombi in the carotid occlusion assay when thrombin signaling is intact. By contrast, the clear (albeit less than maximal) effect of isolated Par4 deficiency in the same assays suggests that thrombin-induced platelet activation is necessary for normal thrombus formation in these assays even when collagen signaling is intact. The large increase in bleeding and the marked decrease in carotid occlusion associated with combined GPVI and Par4 deficiency compared to either single deficiency suggests that collagen- and thrombin-induced platelet activation make substantially redundant contributions to thrombus formation in these assays such that either pathway becomes critical in the absence of the other.
Previous studies have reported variable effects of loss of GPVI function in tail bleeding and carotid occlusion assays10,18–29,33. Some variability might be due to different means of achieving GPVI loss of function, e.g., FcRγ mutation and antibody-induced GPVI shedding might have effects beyond loss of GPVI signaling. Variability across studies of Gp6 nulls may also be due in part to different genetic backgrounds26. The Gp6 and Par mutant mice examined in this study had been backcrossed >7 times into a C57BL6 background and littermates were compared. Different experimental conditions may also contribute to variability across studies in the literature. Indeed, the sensitivity of the tail bleeding and carotid occlusion assays can be "tuned" by varying the site of transection and concentration of FeCl3 used, respectively17,25,34. In the present study, assay conditions in which isolated GPVI or Par4 deficiency had a modest effect were chosen to enable detection of additive or synergistic effects of combined deficiencies. Under conditions in which isolated Par4 deficiency had a modest effect, isolated GPVI deficiency had no detectable effect. It is certainly possible that different results would have been observed under different experimental conditions or in a different genetic background.
Large effects of superimposing anticoagulation upon defective collagen-induced platelet activation in tail bleeding, FeCl3-induced carotid occlusion and other thrombosis assays have been described previously25,28. Anticoagulants inhibit both fibrin formation and thrombin-induced platelet activation, and the effects of anticoagulation in these studies might be due to either or both. Indeed, combined loss of fibrin formation and thrombin-induced platelet activation in mice is incompatible with hemostasis and survival, while loss of either function alone has no or modest effects by comparison35. The current study focused specifically on platelet activation, utilizing deficiencies of the receptors required for initial activation of mouse platelets by collagen and thrombin to directly probe the roles and interactions of platelet activation by these mechanisms. Expression of these receptors is relatively restricted to platelets, and effects of their deficiency in hemostasis and thrombosis assays can almost certainly be attributed to altered platelet function36,37.
Finding that collagen- and thrombin-induced platelet activation can have substantially redundant roles in thrombus formation is perhaps surprising given that collagen is a preformed insoluble polymer that presumably can activate only those platelets that touch it at the damaged vessel wall while thrombin is locally generated soluble mediator that can act on platelets in the growing thrombus31. However, collagen signaling via GPVI does trigger release of soluble mediators like adenosine diphosphate (ADP) and thromboxane that can sustain and extend platelet activation in time and space10, perhaps allowing collagen-induced platelet activation to partially mimic the ability of thrombin-induced platelet activation to drive growth of platelet thrombi at a distance from the vessel wall. Given the known dependence of collagen-induced platelet aggregation on ADP10, the past observation that combined ablation of thrombin signaling via Par4 and ADP signaling via P2Y12 had generally additive effects in the assays used herein17 is consistent with this view.
Although combined loss of thrombin- and collagen-induced platelet activation was associated with remarkable protection against FeCl3-induced carotid artery occlusion, 30% of vessels still clotted. Thrombus formation in these vessels might be driven by fibrin formation and/or by platelet-activating pathways independent of thrombin and collagen signaling.
At face value, the observation that superimposition of GPVI deficiency upon Par4 deficiency has large effects in the FeCl3-induced carotid occlusion assay suggests that ligation of platelet GPVI by collagen occurs in this assay. A recent reported that relatively little subendothelial collagen is exposed by FeCl3-induced carotid injury as assessed by scanning electron microscopy38. Whether collagen exposure sufficient to trigger an important level of GPVI-dependent platelet activation went undetected by SEM or whether GPVI can contribute to thrombus formation by collagen-independent mechanisms is not known. However, there is no need to invoke the latter possibility to explain the observation that superimposition of GPVI deficiency upon Par4 deficiency has large effects in the tail bleeding assay, in which mechanical disruption of vascular integrity likely provides both substantial collagen exposure and thrombin generation.
In the studies outlined above, we failed to separate effects on tail bleeding vs. carotid occlusion by ablating collagen signaling, thrombin signaling or both. Our past studies suggested that Par3 deficiency, which produces a relatively subtle decrease in platelet responsiveness to thrombin, had no effect on tail bleeding under the conditions employed here but protected against carotid occlusion at a low level of FeCl3 injury, while Par4 deficiency, which ablates thrombin signaling in platelets, increased both bleeding and protection17,39. By combining Par3 deficiency with Par4 heterozygosity, we obtained Par3−/−:Par4+/− mice that provided a unique opportunity to probe the effect of a stable, approximately two-fold further increase in the EC50 for thrombin-induced platelet activation compared to Par3−/− mice. Like Par3−/− mice, Par3−/−:Par4+/− mice showed protection against thrombosis without a statistically significant increase in bleeding compared to wild-type mice. This result is interesting as a point of contrast in that even a partial decrease in thrombin signaling in platelets is associated with decreased carotid occlusion under conditions in which complete loss of collagen-induced responses has no detectable effect. Doubling the EC50 for thrombin-induced platelet activation from 0.9 in Par3−/− mice to 1.9 nM in Par3−/−:Par4+/− did not produce a statistically significant increase in protection against carotid occlusion nor in tail bleeding, but point estimates suggested a trend in both assays. This result is perhaps not surprising, and limited power and small effect size may have prevented detection of real but modest effects of doubling the EC50 for thrombin-induced platelet activation.
Important caveats must be weighed in considering whether hypotheses generated from these mouse studies might be relevant to human hemostasis and thrombosis. First, there are important species differences in PAR utilization between human and mouse platelets. As noted in the introduction, mouse platelets utilize Par3 and Par4, and rather than serving as a independent transmembrane signaling molecule, mouse Par3 functions as a cofactor that binds thrombin and promotes Par4 activation at low thrombin concentrations. By contrast, human platelets utilize PAR1 and PAR4 and these receptors signal independently; PAR1 is necessary for human platelet activation by low concentrations of thrombin and PAR4 contributes at higher concentrations40. These and other species differences prevent direct extrapolation of studies of Par mutant mice to humans. Second, FeCl3-induced carotid occlusion and tail bleeding assays in mice do not recapitulate the complexity of human thrombosis and hemostasis. While FeCl3-induced carotid occlusion in mice provides a tractable assay of thrombotic occlusion in an artery, it lacks important aspects of human pathophysiology, e.g., exposure of atheromatous plaque contents after erosion or rupture, stenosis. Similarly, while mouse tail bleeding provides an assay of hemostatic clot formation after transection of blood vessels under one set of conditions, it does not represent the different tissues, blood vessel and injury types, flow rates, pressures, and varying levels of tamponade involved in human hemostasis. Importantly, the tail bleeding and most other bleeding assays measure procedure-induced bleeding from normal vessels; bleeding that causes morbidity and mortality in humans is often spontaneous and may occur at sites of abnormal vasculature (e.g., neovessels, inflammation, vascular malformations, etc.) in which the relative importance of different contributors to clot formation may differ from normal vessels.
Notwithstanding the important caveats listed above, it is worth noting that in an absolute sense, the ability of inhibition of a pathway to increase tail bleeding or decrease carotid occlusion in mice has correlated with an ability to impair hemostasis and protect against thrombosis in humans7,8,14,16,17,28,35,39,41–43. PARs mediate platelet activation by thrombin in mice and humans and, at least in some settings, can contribute to hemostasis and thrombosis in both species11,15–17,35,40,44. GPVI mediates initial platelet activation by collagen in mice and humans and, at least in some settings, can contribute to hemostasis in both species18–29,45. The role for GPVI in human thrombosis remains to be tested. New antiplatelet agents are often tested as an addition to standard of care, and use of antiplatelet agents in combination is growing2,4–7,44. The findings of substantially redundant functions for protease-activated receptor- and GPVI-dependent platelet activation in tail bleeding and carotid occlusion assays in mice and of strong interactive effects of removing both functions might be considered in the design of future studies exploring these targets for antithrombotic therapy.
Supplementary Material
Significance.
We demonstrated that thrombin- and collagen-dependent platelet activation via protease-activated receptors (PARs) and GPVI have substantially redundant roles in mouse assays of hemostasis and thrombosis, such that removing both pathways had much larger effects than removing either alone. This redundancy is somewhat surprising given spatial and other differences in the way that thrombin and collagen become available to platelets in a growing thrombus growth. Testing of antiplatelet agents is often accomplished in combination with established ones. The strong interactive effects seen with loss of PAR and GPVI function in the in vivo hemostasis and thrombosis assays used here might be considered in further exploration of these receptors as targets for antithrombotic therapy.
Acknowledgements
Funding: This work was supported in part by NIH HL44907 to S.R.C.
Abbreviations
- Par
Protease-activated receptor
- GPVI
Glycoprotein VI protein
- Gp6
Glycoprotein VI gene
Footnotes
Disclosures: None.
References
- 1.Abrams C. Platelet Signal Transduction. In: Coleman RW, MV CA, George JN, Goldhaber SZ, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Philadelphia: J.B. Lippincott; 2006. [Google Scholar]
- 2.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial I. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 3.Mehta SR, Yusuf S. Clopidogrel in Unstable angina to prevent Recurrent Events Study I. The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J. 2000;21:2033–2041. doi: 10.1053/euhj.2000.2474. [DOI] [PubMed] [Google Scholar]
- 4.Bakhru MR, Bhatt DL. Interpreting the CHARISMA study. What is the role of dual antiplatelet therapy with clopidogrel and aspirin? Cleve Clin J Med. 2008;75:289–295. doi: 10.3949/ccjm.75.4.289. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ, Investigators C. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Topol EJ. Clopidogrel for High Atherothrombotic R, Ischemic Stabilization M, Avoidance Executive C. Clopidogrel added to aspirin versus aspirin alone in secondary prevention and high-risk primary prevention: rationale and design of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Am Heart J. 2004;148:263–268. doi: 10.1016/j.ahj.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Scirica BM, Bonaca MP, Braunwald E, De Ferrari GM, Isaza D, Lewis BS, Mehrhof F, Merlini PA, Murphy SA, Sabatine MS, Tendera M, Van de Werf F, Wilcox R, Morrow DA. Investigators TRAdP-TSC. Vorapaxar for secondary prevention of thrombotic events for patients with previous myocardial infarction: a prespecified subgroup analysis of the TRA2oP-TIMI 50 trial. Lancet. 2012;380:1317–1324. doi: 10.1016/S0140-6736(12)61269-0. [DOI] [PubMed] [Google Scholar]
- 8.Morrow DA, Scirica BM, Fox KA, Berman G, Strony J, Veltri E, Bonaca MP, Fish P, McCabe CH, Braunwald E. Investigators TPT. Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: design and rationale for the Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA2oP)-TIMI 50 trial. Am Heart J. 2009;158:335–341. e333. doi: 10.1016/j.ahj.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 9.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Investigators T-T. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 10.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 11.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 12.Davey MG, Luscher EF. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967;216:857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- 13.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. Journal of thrombosis and haemostasis : JTH. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 14.Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- 16.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 17.Cornelissen I, Palmer D, David T, Wilsbacher L, Concengco C, Conley P, Pandey A, Coughlin SR. Roles and interactions among protease-activated receptors and P2ry12 in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 2010;107:18605–18610. doi: 10.1073/pnas.1013309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, Marchese P, Reininger A, Ruggeri ZM, Ware J. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 2003;102:1701–1707. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- 19.Nieswandt B, Brakebusch C, Bergmeier W, Schulte V, Bouvard D, Mokhtari-Nejad R, Lindhout T, Heemskerk JW, Zirngibl H, Fassler R. Glycoprotein VI but not alpha2beta1 integrin is essential for platelet interaction with collagen. EMBO J. 2001;20:2120–2130. doi: 10.1093/emboj/20.9.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieswandt B, Bergmeier W, Schulte V, Rackebrandt K, Gessner JE, Zirngibl H. Expression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRgamma chain. J Biol Chem. 2000;275:23998–24002. doi: 10.1074/jbc.M003803200. [DOI] [PubMed] [Google Scholar]
- 21.Nieswandt B, Schulte V, Bergmeier W, Mokhtari-Nejad R, Rackebrandt K, Cazenave JP, Ohlmann P, Gachet C, Zirngibl H. Long-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in mice. J Exp Med. 2001;193:459–469. doi: 10.1084/jem.193.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruner S, Prostredna M, Aktas B, Moers A, Schulte V, Krieg T, Offermanns S, Eckes B, Nieswandt B. Anti-glycoprotein VI treatment severely compromises hemostasis in mice with reduced alpha2beta1 levels or concomitant aspirin therapy. Circulation. 2004;110:2946–2951. doi: 10.1161/01.CIR.0000146341.63677.3C. [DOI] [PubMed] [Google Scholar]
- 23.Massberg S, Gawaz M, Gruner S, Schulte V, Konrad I, Zohlnhofer D, Heinzmann U, Nieswandt B. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J Exp Med. 2003;197:41–49. doi: 10.1084/jem.20020945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruner S, Prostredna M, Koch M, Miura Y, Schulte V, Jung SM, Moroi M, Nieswandt B. Relative antithrombotic effect of soluble GPVI dimer compared with anti-GPVI antibodies in mice. Blood. 2005;105:1492–1499. doi: 10.1182/blood-2004-06-2391. [DOI] [PubMed] [Google Scholar]
- 25.Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo. Blood. 2006;107:3902–3906. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheli Y, Jensen D, Marchese P, Habart D, Wiltshire T, Cooke M, Fernandez JA, Ware J, Ruggeri ZM, Kunicki TJ. The Modifier of hemostasis (Mh) locus on chromosome 4 controls in vivo hemostasis of Gp6−/− mice. Blood. 2008;111:1266–1273. doi: 10.1182/blood-2007-09-111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bender M, May F, Lorenz V, Thielmann I, Hagedorn I, Finney BA, Vogtle T, Remer K, Braun A, Bosl M, Watson SP, Nieswandt B. Combined in vivo depletion of glycoprotein VI and C-type lectin-like receptor 2 severely compromises hemostasis and abrogates arterial thrombosis in mice. Arterioscler Thromb Vasc Biol. 2013;33:926–934. doi: 10.1161/ATVBAHA.112.300672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangin P, Yap CL, Nonne C, Sturgeon SA, Goncalves I, Yuan Y, Schoenwaelder SM, Wright CE, Lanza F, Jackson SP. Thrombin overcomes the thrombosis defect associated with platelet GPVI/FcRgamma deficiency. Blood. 2006;107:4346–4353. doi: 10.1182/blood-2005-10-4244. [DOI] [PubMed] [Google Scholar]
- 29.Konstantinides S, Ware J, Marchese P, Almus-Jacobs F, Loskutoff DJ, Ruggeri ZM. Distinct antithrombotic consequences of platelet glycoprotein Ibalpha and VI deficiency in a mouse model of arterial thrombosis. J Thromb Haemost. 2006;4:2014–2021. doi: 10.1111/j.1538-7836.2006.02086.x. [DOI] [PubMed] [Google Scholar]
- 30.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 31.Vandendries ER, Hamilton JR, Coughlin SR, Furie B, Furie BC. Par4 is required for platelet thrombus propagation but not fibrin generation in a mouse model of thrombosis. Proc Natl Acad Sci U S A. 2007;104:288–292. doi: 10.1073/pnas.0610188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornelissen I, Palmer D, David T, Wilsbacher L, Concengco C, Conley P, Pandey A, Coughlin SR. Roles and interactions among protease-activated receptors and P2ry12 in hemostasis and thrombosis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18605–18610. doi: 10.1073/pnas.1013309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bender M, Hagedorn I, Nieswandt B. Genetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl(3)-induced thrombosis. J Thromb Haemost. 2011;9:1423–1426. doi: 10.1111/j.1538-7836.2011.04328.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Xu L. An optimized murine model of ferric chloride-induced arterial thrombosis for thrombosis research. Thromb Res. 2005;115:95–100. doi: 10.1016/j.thromres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Camerer E, Duong DN, Hamilton JR, Coughlin SR. Combined deficiency of protease-activated receptor-4 and fibrinogen recapitulates the hemostatic defect but not the embryonic lethality of prothrombin deficiency. Blood. 2004;103:152–154. doi: 10.1182/blood-2003-08-2707. [DOI] [PubMed] [Google Scholar]
- 36.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 37.Jung SM, Moroi M. Platelet glycoprotein VI. Adv Exp Med Biol. 2008;640:53–63. doi: 10.1007/978-0-387-09789-3_5. [DOI] [PubMed] [Google Scholar]
- 38.Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood. 2013;121:3733–3741. doi: 10.1182/blood-2012-11-468983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss EJ, Hamilton JR, Lease KE, Coughlin SR. Protection against thrombosis in mice lacking PAR3. Blood. 2002;100:3240–3244. doi: 10.1182/blood-2002-05-1470. [DOI] [PubMed] [Google Scholar]
- 40.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andre P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, Jurek M, Koller B, Phillips DR, Conley PB. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uzunova A, Ramey E, Ramwell PW. Effect of testosterone, sex and age on experimentally induced arterial thrombosis. Nature. 1976;261:712–713. doi: 10.1038/261712a0. [DOI] [PubMed] [Google Scholar]
- 43.Lewis HD, Jr, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE, 3rd, Schnaper HW, LeWinter MM, Linares E, Pouget JM, Sabharwal SC, Chesler E, DeMots H. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med. 1983;309:396–403. doi: 10.1056/NEJM198308183090703. [DOI] [PubMed] [Google Scholar]
- 44.Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Fish MP, Fox KA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJ, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA. Committee TPTS, Investigators. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–1413. doi: 10.1056/NEJMoa1200933. [DOI] [PubMed] [Google Scholar]
- 45.Moroi M, Jung SM, Okuma M, Shinmyozu K. A patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesion. J Clin Invest. 1989;84:1440–1445. doi: 10.1172/JCI114318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.