Abstract
Objective
To estimate atherosclerosis progression and identify influencing factors in rheumatoid arthritis (RA).
Methods
We used carotid ultrasound to measure intima-media thickness (IMT) in RA patients, and ascertained cardiovascular (CV) risk factors, inflammation markers and medications. A second ultrasound was performed approximately 3 years later. We calculated the progression rate by subtracting the baseline from the follow-up IMT, divided by the time between the two scans. We used logistic regression to identify baseline factors predictive of rapid progression. We tested for interactions of erythrocyte sedimentation rate (ESR) with CV risk factors and medication use.
Results
Results were available for 487 RA patients. The mean (SD) common carotid IMT at baseline was 0.571 mm (0.151). After a mean of 2.8 years, the IMT increased by 0.050 mm (0.055), p≤0.001, a progression rate of 0.018 mm/year (95% CI 0.016 to 0.020). Baseline factors associated with rapid progression included the number of CV risk factors (OR 1.27 per risk factor, 95% CI 1.01 to 1.61), and the ESR (OR 1.12 per 10 mm/h, 95% CI 1.02 to 1.23). The ESR×CV risk factor and ESR×medication product terms were significant, suggesting these variables modify the association between the ESR and IMT progression.
Conclusions
Systemic inflammation and CV risk factors were associated with rapid IMT progression. CV risk factors may modify the role of systemic inflammation in determining IMT progression over time. Methotrexate and antitumour necrosis factor agents may influence IMT progression by reducing the effect of the systemic inflammation on the IMT.
INTRODUCTION
Patients with rheumatoid arthritis (RA) have increased cardiovascular (CV) risk,1–3 which may be due to a predisposition to atherosclerosis, as assessed using ultrasound measurement of the carotid intima-media thickness (IMT).4,5 Cross-sectional studies suggest IMT in RA is associated with systemic inflammation,6–10 CV risk factors and glucocorticoid exposure,11 and may predict CV events in RA patients.12 Longitudinal studies of IMT change in RA have also suggested a role for inflammation and glucocorticoids in atherosclerosis progression,6,13–20 and have hinted at a potential therapeutic effect for certain drugs.14,16,21 However, longitudinal studies to date have been somewhat limited by small sample sizes. Here, we studied a large RA cohort whose members underwent measurement of the common carotid IMTat two points in time.
PATIENTS AND METHODS
Patients
A high resolution carotid ultrasound was performed on consecutive patients meeting RA criteria.22 Approximately 3 years later, a follow-up scan was done. The following variables were assessed at baseline.
RA manifestations
A physician assessed patients for tenderness, swelling or deformity in 48 joints, and for subcutaneous nodules. Reliability coefficients were 0.94 for tenderness, 0.90 for swelling and 0.98 for deformity.23 We used the 28-joint swollen and tender joint counts and the erythrocyte sedimentation rate (ESR) to calculate the disease activity score in 28 joints (DAS28) score.24
CV risk factor assessment
We defined hypertension by the use of antihypertensive medications, diastolic blood pressure ≥90 mm Hg or systolic pressure ≥140 mm Hg; diabetes mellitus by the use of antidiabetic medications or fasting blood sugar ≥126 mg/dL; and hypercholesterolaemia by use of lipid-lowering medications or fasting plasma cholesterol ≥200 mg/dL. Hypertension, diabetes and hypercholesterolaemia were also considered present if diagnosed previously by a physician. Patients were considered current smokers if they smoked at baseline, and former smokers if they had quit. We defined obesity as a body mass index of 30 kg/m2 or greater.
Antirheumatic medication use
We examined patients’ medication bottles and recorded all their prescription and non-prescription drugs. We also reviewed medical and pharmacy records, resolving discrepancies by discussion with patients. We noted if patients were taking methotrexate, antitumour necrosis factor (anti-TNF) agents (infliximab, etanercept, adalimumab, certolizumab or golimumab) or both, including the date when the drug was started.
Laboratory studies
The ESR was measured using the Westergren technique. Rheumatoid factor was measured by latex agglutination. Anti-cyclic citrullinated peptides (CCP) was measured by enzyme-linked immune assay. Total plasma cholesterol and glucose were measured by a Synchron LX automated system. HLA-DRB1 genotyping was performed by PCR sequence-specific primer amplification with Fastype kits, as described previously.25 The HLA-DRB1 shared epitope was classified as described previously.25
Carotid ultrasound
Carotid ultrasound was performed as previously described:9 Briefly, a duplex scan of the carotid arteries was performed following a standardised vascular protocol developed for the Multi-Ethnic Study of Atherosclerosis (MESA).26 We used an ATL HDI-3000 High Resolution Imaging machine with a L7-4 Transducer (Philips Medical Systems North America Company, Bothell, Washington, USA). A single longitudinal lateral view of the distal 10 mm of the right and left common carotid arteries (CCAs) and three longitudinal views in different imaging planes of each internal carotid artery (ICA) were obtained, recorded on Super VHS tapes and mailed to a central facility (Ultrasound Reading Center, Tufts Medical Center, Boston, Massachusetts, USA) for reading of the carotid artery IMT. At the reading centre, the images were digitised at end diastole (smallest CCA diameter). We focused on CCA IMT measurements because progression is more consistently measured for CCAs than for the ICA.27,28 Scan images obtained at baseline and follow-up for each participant were reanalysed concurrently by a single reader to guard against reader drift in measurements.29 The reader was masked to clinical characteristics of subjects. The maximum IMT (millimetre) of the CCA was defined as the mean of the maximum IMT for near and far walls on both right and left sides.
Our study involved a single ultrasonographer and a single reader. Nevertheless, to assess the technique’s reliability, our reader reread 50 images, and a different reader reread a separate set of 50 images. The intrareader intraclass correlation coefficient for CCA IMT was 0.99, and the inter-reader coefficient was 0.94.
Analysis
We calculated IMT change by subtracting the baseline from the follow-up IMT. The yearly rate of change in IMT was calculated by dividing IMT change by the time between the two measurements. We used IMT progression rate as the dependent variable in linear regression models that examined its association with demographic variables (age at RA onset, sex and ethnic group): CV risk factors (diabetes, smoking, hypertension, hypercholesterolaemia and obesity), RA manifestations (RA duration, tender, swollen and deformed joint counts) and laboratory markers (rheumatoid factor, ESR and shared epitope). We calculated a CV risk factor score by summing the number of CV risk factors present (diabetes, hypertension, hypercholesterolaemia, obesity and smoking). We examined the association of the IMT progression rate with anti-TNF agents or methotrexate. We used a stepwise variable selection technique, dependent on the strength of association with IMT progression rate, and in all subsequent models, we used the variables identified in the stepwise model together with age (age at RA onset+disease duration) and sex as covariates. To assess the possibility of non-linear behaviour of continuous variables such as the ESR, we stratified the variable into quintiles, using the lowest quintile as the referent. We also defined a subgroup of patients with ‘rapid progression’, defined as the upper quartile in the IMT change. We used logistic regression to examine independent variables associated with rapid IMT progression, and from these models generated predicted probabilities of rapid progression, adjusted for covariates. For both the linear and logistic regression models, we tested for interaction between the CV risk factors score and inflammation markers (ie, the ESR), and between the use of anti-TNF agents or methotrexate and the ESR, by including CV risk factor×ESR or medication×ESR product terms in the regression models. All analyses were performed using Stata V.9.0 (College Station, Texas, USA).
RESULTS
A baseline carotid ultrasound was performed on 631 RA patients. After a mean of 2.8 years (range 1–4.3 years), 56 of the patients had died, eight were lost to follow-up and one refused further participation, leaving 566 patients eligible for a follow-up scan. Of these, we scanned 487 (86%). The mean (SD) common carotid IMT at baseline was 0.571 mm (0.151). The same measure at follow-up increased significantly to a mean of 0.621 mm (0.171), an increase of 0.050 mm (0.055), p≤0.001, 95% CI 0.045 to 0.055. Dividing the IMT change by the time between the baseline and follow-up scans, we obtained an IMT progression rate of 0.018 mm/year, 95% CI 0.016 to 0.020. The Pearson correlation between the baseline and the follow-up IMT was 0.93. The IMT increased in 425 patients (87%), and actually decreased in 62 of them (13%).
The stepwise variable selection procedure to identify baseline factors associated with the IMT progression began with the variables listed in table 1. Variables associated with the IMT progression rate with a p value higher than 0.05 were removed, re-entering only if the p value became 0.045 or less. We tested models with unstratified and stratified ESR. The stepwise process selected the following variables, with their corresponding regression coefficients, expressed in millimetres (mm)/year: the baseline common carotid IMT, 0.0201 mm/year (95% CI 0.0844 to 0.3187), p=0.001; an ESR ≥55 mm/h, 0.0464 mm/year (95% CI 0.0028 to 0.0900), p=0.03; and the presence of four CV risk factors, 0.0119 mm/year (95% CI 0.0305 to 0.2080), p=0.009. We also tested models with individual CV risk factors of diabetes, hypertension, hypercholesterolaemia, smoking or obesity, but none of them was individually associated with IMT progression.
Table 1.
Characteristics of 487 RA patients at baseline carotid ultrasound scan, according to observed progression in carotid intima-media thickness (IMT)
| Common carotid IMT progression |
||
|---|---|---|
| Upper quartile (rapid progressors) |
Lower three quartiles | |
| N | 121 | 366 |
| Progression | ||
| Baseline IMT, mean mm (SD) | 0.601 (0.221)* | 0.561 (0.117) |
| Follow-up IMT, mean mm (SD) | 0.721 (0.251)*** | 0.581 (0.118) |
| Absolute progression, mean mm (SD) | 0.119 (0.063) | 0.0278 (0.027) |
| Progression rate, mean mm/yr (CI) | 0.042 (0.038 to 0.046) | 0.010 (0.009 to 0.011)*** |
| Demographics | ||
| Age, mean yrs (SD) | 58.2 (11.2) | 56.0 (11.7) |
| Disease duration mean yrs (SD) | 11.9 (9.6) | 12.5 (9.2) |
| Women, n (%) | 82 (67.8) | 273 (74.6) |
| Hispanic, n (%) | 70 (57.8) | 223 (60.9) |
| Non-Hispanic white, n (%) | 39 (32.2) | 112 (30.6) |
| CV risk factors | ||
| No CV risk factors, n (%) | 3 (2.4)* | 33 (9.0) |
| Diabetes, n (%) | 25 (20.7) | 60 (16.4) |
| Hypertension, n (%) | 79 (65.3)* | 201 (54.9) |
| Hypercholesterolaemia, n (%) | 45 (37.2) | 116 (31.7) |
| Smoking, n (%) | 75 (62.0) | 206 (56.3) |
| Obesity (BMI ≥30), n (%) | 56 (46.3) | 160 (43.7) |
| Four or more CV risk factors, n (%) | 210 (17.3)* | 38 (10.3) |
| Clinical manifestations of RA | ||
| Tender joint count, mean (SD) | 14.3 (12.2) | 14.9 (13.6) |
| Swollen joint count, mean (SD) | 4.3 (5.0) | 4.1 (4.6) |
| Deformed joint count, mean (SD) | 12.5 (11.7) | 11.3 (10.7) |
| Subcutaneous nodules, n (%) | 30 (24.8) | 90 (24.6) |
| DAS28, mean (SD) | 5.25 (1.52) | 5.05 (1.45) |
| Laboratory | ||
| ESR, mean mm/h (SD) | 41.1 (24.8)** | 34.2 (23.3) |
| Rheumatoid factor, n (%) | 93 (76.9) | 260 (71.1) |
| Anti-CCP, n (%) | 97 (80.8) | 281 (76.9) |
| HLA-DRB1 shared epitope, n (%) | 89 (74) | 248 (67) |
| Medication use | ||
| Methotrexate, n (%) | 100 (82) | 323 (88) |
| Anti-TNF agents, n (%) | 26 (21) | 82 (22) |
| Methotrexate and anti-TNF, n (%) | 25 (21) | 80 (22) |
| Glucocorticoids, n (%) | 51 (42) | 179 (49) |
p≤0.05;
p≤0.01;
p≤0.001.
BMI, Body Mass Index; CV, cardiovascular; ESR, erythrocyte sedimentation rate; RA, rheumatoid arthritis; TNF, tumour necrosis factor.
Among patients in the top IMT progression rate quartile, the IMT increased at a rate of 0.4280 mm/year (95% CI 0.3880 to 0.4690). In the lowest quartile, the change rate was −0.0110 mm/year (95% CI −0.0213 to −0.0009). Table 1 shows characteristics of rapid progressors at baseline, compared with the patients in lower three IMT progression quartiles. The rapid progressors had more frequent hypertension and higher mean ESR (table 1). There were also fewer patients with no CV risk factors, and more patients with four or more CV risk factors among the rapid progressors.
The stepwise logistic regression to identify factors associated with rapid IMT progression selected the same variables that were associated with IMT progression rate: baseline carotid IMT, OR 4.13 (95% CI 1.10 to 1.54), p=0.03; four CV risk factors, OR 3.11 (95% CI 1.21 to 8.00), p=0.01; and an ESR ≥55 mm/h, OR 1.93 (95% CI 1.18 to 3.15), p=0.008.
We then tested models with an ESR×CV risk factor product term. Table 2 shows the results of linear regression models with IMT progression rate as dependent variable, and the ESR and the number of CV risk factors as independent variables, in un-interacted and interacted forms. The interaction term was significant with a p value of 0.04 (table 2). To interpret this interaction, we stratified the association of the ESR with IMT progression by the number of CV risk factors. The results of these models are shown in table 3; in the absence of CV risk factors, or with one single CV risk factor, the ESR was not significantly associated with the IMT progression rate. However, with two or more CV risk factors, the ESR-IMT progression rate association grew progressively stronger.
Table 2.
Association of the intima-media thickness progression rate with CV risk factors and ESR
| Un-interacted model |
Interacted model |
|||||
|---|---|---|---|---|---|---|
| Rate difference versus referent | 95% CIs | p Value | Rate difference versus referent | 95% CIs | p Value | |
| Number of CV risk factors (0 referent) | ||||||
| 1 | 0.0023 | −0.0035 to 0.0082 | 0.4 | −0.0021 | −0.0094 to 0.0051 | 0.5 |
| 2 | 0.0012 | −0.0048 to 0.0073 | 0.4 | −0.0068 | −0.0167 to 0.0031 | 0.1 |
| 3 | −0.0017 | −0.0087 to 0.0051 | 0.6 | −0.0137 | −0.0272 to −0.0002 | 0.04 |
| ≥4 | 0.0162 | 0.0060 to 0.0265 | 0.002 | −0.0000 | −0.0188 to 0.0188 | 0.9 |
| ESR strata, in mm/h (< 17 referents) | ||||||
| 17–26 | 0.0018 | −0.0036 to 0.0072 | 0.5 | −0.0002 | −0.0060 to 0.0055 | 0.9 |
| 27–37 | 0.0050 | −0.0005 to 0.0106 | 0.07 | 0.0008 | −0.0060 to 0.0077 | 0.8 |
| 38–54 | 0.0018 | −0.0037 to 0.0074 | 0.5 | −0.0042 | −0.0123 to 0.0038 | 0.3 |
| > 54 | 0.0076 | 0.0021 to 0.0131 | 0.006 | −0.0011 | −0.0112 to 0.0090 | 0.8 |
| Interaction terms | ||||||
| No. CV risk factors×ESR | – | – | – | 0.0012 | 0.0000 to 0.0024 | 0.04 |
CV, cardiovascular; ESR, erythrocyte sedimentation rate.
Table 3.
Association of ESR with the IMT progression rate, stratified by the number of CV risk factors present
| Strata | Rate difference per ESR 10 mm/h |
95% CIs | p Value |
|---|---|---|---|
| Unstratified | |||
| 0.0008 | 0.0001 to 0.0016 | 0.02 | |
| No. of CV risk factors | |||
| 0 | −0.0006 | −0.0019 to 0.0007 | 0.3 |
| 1 | 0.0002 | −0.0006 to 0.0011 | 0.5 |
| 2 | 0.0011 | 0.0003 to 0.0019 | 0.004 |
| 3 | 0.0020 | 0.0008 to 0.0031 | 0.001 |
| ≥ 4 | 0.0029 | 0.0011 to 0.0045 | 0.001 |
Models are adjusted for age at RA onset, RA duration and sex.
Rate difference represents the difference in progression rates, in millimetres per year, associated an ESR increase of 10 mm/h.
CV, cardiovascular; ESR, erythrocyte sedimentation rate; IMT, intima-media thickness; RA, rheumatoid arthritis.
Logistic regression models of rapid progression also showed a significant CV risk factor×ESR interaction term. Figure 1 shows the probability of being a rapid progressor, according to the number of CV risk factors versus ESR quintiles. This analysis suggested that in the absence of CV risk factors, ESR was not associated with rapid progression. However, with increasing CV risk factors, the association between ESR and rapid IMT progression strengthened.
Figure 1.
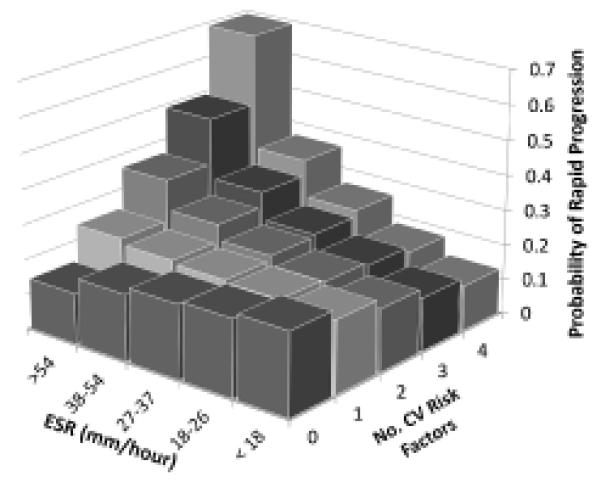
Predicted probability of rapid progression, according to the number of cardiovascular (CV) risk factors and the erythrocyte sedimentation rate (ESR). Adjustment model from a logistic regression that included age at rheumatoid arthritis (RA) onset, RA duration, sex, baseline common carotid intima-media thickness, ESR, number of CV risk factors and a CV risk factor×ESR interaction term.
None of the medications displayed a significant ‘main effect’ association with IMT change rate. However, methotrexate×ESR and anti-TNF×ESR product terms were significantly associated with rapid progression. Table 4 shows these results. To interpret these interactions, we graphed the predicted probability of being a rapid progressor, according to ESR quintiles, stratified by drug, as shown in figure 2. This suggests that association of the ESR with rapid IMT progression was abrogated by these two medications.
Table 4.
Association of ESR with rapid progression, according to the use of medication
| Medication | OR of rapid progression |
95% CIs | p Value |
|---|---|---|---|
| Methotrexate | |||
| Absent | 2.29 | 1.45 to 3.61 | ≤ 0.001 |
| Present | 1.17 | 1.001 to 1.37 | 0.04 |
| Interaction p value | 0.006 | – | – |
| Anti-TNF | |||
| Absent | 1.36 | 1.15 to 1.61 | ≤ 0.001 |
| Present | 1.003 | 0.73 to 1.37 | 0.9 |
| Interaction p value | 0.08 | – | – |
Odds ratio are per ESR difference of 10 mm/h, from logistic regression models that adjusted for age at RA onset, RA duration, sex and CV risk factors.
CV, cardiovascular; ESR, erythrocyte sedimentation rate; RA, rheumatoid arthritis; TNF, tumour necrosis factor.
Figure 2.

Predicted probability of rapid intima-media thickness progression, according to the erythrocyte sedimentation rate (ESR), stratified by: (A) use of methotrexate; (B) use of anti-TNF agents. Adjustment model from a logistic regression that included age at rheumatoid arthritis (RA) onset, RA duration and sex. Error bars represent 95% CI. TNF, tumour necrosis factor.
DISCUSSION
We observed an increase in the common carotid IMT in most, though not all, members of this RA cohort. The mean rate of increase, 0.018 mm/year, was similar to that reported by Giles and colleagues, who found a rate of 0.016 mm/year.18 Other authors have reported higher progression rates: Nagata-Sakurai et al6 found 0.027 mm/year, while Gonzalez-Juanatey and colleagues report the pooled progression of the two groups they studied was 0.036 mm/year.13
The lack of a non-RA control group limits our ability to infer whether IMT progressed any differently in our patients than in individuals without RA. There are however a substantial number of randomised trials in the non-RA population where IMT has served as outcome for various interventions. Among 2693 normal control subjects in 17 randomised trials, the IMT progression rate was estimated to be 0.0147 mm/year (95% CI 0.0122 to 0.0173),27 a rate appreciably lower than in our patients. In the MESA, a population-based observational study that used IMT measurement techniques similar to ours, and which employed our ultrasound reading centre as a reader,30 the progression rate was estimated at a mean of 0.01 mm/year, SD of 0.05, a rate even lower than in the controls who participated in the randomised trials cited above.27 Although comparisons of the results of these control studies with our own require caution given potential methodological and patient population differences, it is of note that the few IMT progression studies in RA that have included a non-RA control group have also found more rapid progression in the RA patients.6,17,19 These observations suggest that IMT may progress more rapidly in RA than in individuals without RA.
The key factors that we found associated with mean IMT progression and with rapid progression were the ESR and the number of CV risk factors. We also noted an interaction between these two variables, similar to the cross-sectional interactions we reported from the baseline data of this same cohort.9 The interaction suggests that CV risk factors modify the association between ESR and IMT progression: the ESR is most strongly associated with progression in patients with four CV risk factors, and weakest among patients who are free of CV risk factors. These findings suggest that interventions aimed at reducing systemic inflammation are more likely to succeed in reducing the rate of IMT progression if combined with interventions to reduce CV risk factors. Tight control of RA disease activity combined with strict CV risk factor management deserves further study as a strategy to reduce atherosclerosis in RA.
We did not find association of methotrexate or anti-TNF with the mean IMT progression rate. However, we did note a separate statistical interaction of ESR with methotrexate and anti-TNF drugs. Both drugs modified the association of ESR with rapid progression: a high ESR was not associated with rapid progression in patients receiving either of these drugs. This finding is consistent with reports that anti-TNF therapy may slow progression or lead to regression of IMT in RA.13,14,16 However, our findings and those of others reporting on the effect of medications on the carotid IMT in RA should be interpreted with caution given that the drugs were not allocated at random in any of those studies. In the randomised trial reported by Tam and colleagues,21 methotrexate alone was compared with methotrexate plus infliximab in their ability to prevent IMT progression over 12 months. IMT did not progress significantly in either group, which could represent a beneficial effect of both treatments. However, with only 20 RA patients in each group, an alternative explanation was insufficient statistical power to detect change.21 In view of the above findings, the medication effect on IMT progression in RA patients requires clarification in adequately-powered randomised trials.
We conclude that the common carotid IMT progressed significantly in this RA cohort over an average of 2.8 years. IMT progressed more rapidly in patients with high degrees of systemic inflammation and with multiple CV risk factors. Our findings suggest higher levels of systemic inflammation are associated with rapid IMT progression when traditional CV risk factors are present. Methotrexate and the anti-TNF drugs seem to diminish the likelihood of rapid IMT progression among patients with high degrees of systemic inflammation. Confirmation of this effect of medications on the progression of atherosclerosis requires a randomised trial. Although we did not examine the role of carotid ultrasound in the identification of CV outcomes, evidence suggests that carotid ultrasound may improve CV risk stratification in RA,31,32 lending clinical relevance to the findings we presented here.
Acknowledgments
Funding Supported by grants RO1-HL085742, RO1-HD37151, and UL1-RR-025767 from the National Institutes of Health.
Footnotes
Contributors IdR conceived, designed and obtained funding for the study, designed data collection tools and monitored data collection for the whole study; JFP and DHO codeveloped the ultrasound reading protocols; DFB assisted with patient recruitment and follow-up and contributed to study conception and design; JMK, JFR and EM assisted with data collection; AE conceived, designed and obtained funding for the study and conducted the statistical analysis.
Competing interests None.
Ethics approval UTHSCSA Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.del Rincón I, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 2.Avina-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–9. doi: 10.1136/annrheumdis-2011-200726. [DOI] [PubMed] [Google Scholar]
- 3.Avina-Zubieta JA, Choi HK, Sadatsafavi M, et al. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–7. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 4.Tyrrell PN, Beyene J, Feldman BM, et al. Rheumatic disease and carotid intima-media thickness: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2010;30:1014–26. doi: 10.1161/ATVBAHA.109.198424. [DOI] [PubMed] [Google Scholar]
- 5.van Sijl AM, Peters MJ, Knol DK, et al. Carotid intima media thickness in rheumatoid arthritis as compared to control subjects: a meta-analysis. Semin Arthritis Rheum. 2011;40:389–97. doi: 10.1016/j.semarthrit.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Nagata-Sakurai M, Inaba M, Goto H, et al. Inflammation and bone resorption as independent factors of accelerated arterial wall thickening in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48:3061–7. doi: 10.1002/art.11327. [DOI] [PubMed] [Google Scholar]
- 7.del Rincón I, Williams K, Stern MP, et al. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–40. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- 8.Gerli R, Schillaci G, Giordano A, et al. CD4+CD28− T lymphocytes contribute to early atherosclerotic damage in rheumatoid arthritis patients. Circulation. 2004;109:2744–8. doi: 10.1161/01.CIR.0000131450.66017.B3. [DOI] [PubMed] [Google Scholar]
- 9.del Rincón I, Freeman GL, Haas RW, et al. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52:3413–23. doi: 10.1002/art.21397. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Gay MA, Gonzalez-Juanatey C, Pineiro A, et al. High-grade C-reactive protein elevation correlates with accelerated atherogenesis in patients with rheumatoid arthritis. J Rheumatol. Jul. 2005;32:1219–23. [PubMed] [Google Scholar]
- 11.del Rincón I, O’Leary DH, Haas RW, et al. Effect of glucocorticoids on the arteries in rheumatoid arthritis. Arthritis Rheum. 2004;50:3813–22. doi: 10.1002/art.20661. [DOI] [PubMed] [Google Scholar]; Arthritis Rheum. 2005;52:678. Erratum in: [Google Scholar]
- 12.Gonzalez-Juanatey C, Llorca J, Martin J, et al. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2009;38:366–71. doi: 10.1016/j.semarthrit.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Juanatey C, Llorca J, Garcia-Porrua C, et al. Effect of anti-tumor necrosis factor alpha therapy on the progression of subclinical atherosclerosis in severe rheumatoid arthritis. Arthritis Rheum. 2006;55:150–3. doi: 10.1002/art.21707. [DOI] [PubMed] [Google Scholar]
- 14.Del Porto F, Laganà B, Lai S, et al. Response to anti-tumour necrosis factor alpha blockade is associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1111–15. doi: 10.1093/rheumatology/kem089. [DOI] [PubMed] [Google Scholar]
- 15.Raterman HG, Voskuyl AE, Simsek S, et al. Increased progression of carotid intima media thickness in thyroid peroxidase antibodies-positive rheumatoid arthritis patients. Eur J Endocrinol. 2013;169:751–7. doi: 10.1530/EJE-13-0394. [DOI] [PubMed] [Google Scholar]
- 16.Ferrante A, Giardina AR, Ciccia F, et al. Long-term anti-tumour necrosis factor therapy reverses the progression of carotid intima-media thickness in female patients with active rheumatoid arthritis. Rheumatol Int. 2009;30:193–8. doi: 10.1007/s00296-009-0935-2. [DOI] [PubMed] [Google Scholar]
- 17.Södergren A, Karp K, Boman K, et al. Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res Ther. 2010;12:R158. doi: 10.1186/ar3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giles JT, Post WS, Blumenthal RS, et al. Longitudinal predictors of progression of carotid atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2011;63:3216–25. doi: 10.1002/art.30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veselinovic M, Jakovljevic V, Jurisic-Skevin A, et al. Carotid enlargement and serum levels of von Willebrand factor in rheumatoid arthritisa follow-up study. Clin Rheumatol. 2012;31:1727–32. doi: 10.1007/s10067-012-2079-0. [DOI] [PubMed] [Google Scholar]
- 20.Guin A, Chatterjee Adhikari M, Chakraborty S, et al. Effects of disease modifying anti-rheumatic drugs on subclinical atherosclerosis and endothelial dysfunction which has been detected in early rheumatoid arthritis: 1-year follow-up study. Semin Arthritis Rheum. 2013;43:48–54. doi: 10.1016/j.semarthrit.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Tam LS, Shang Q, Li EK, et al. Infliximab is associated with improvement in arterial stiffness in patients with early rheumatoid arthritis—a randomized trial. J Rheumatol. 2012;39:2267–75. doi: 10.3899/jrheum.120541. [DOI] [PubMed] [Google Scholar]
- 22.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 23.Orces CH, del Rincón I, Abel MP, et al. The number of deformed joints as a surrogate measure of damage in rheumatoid arthritis. Arthritis Rheum. 2002;47:67–72. doi: 10.1002/art1.10160. [DOI] [PubMed] [Google Scholar]
- 24.Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 25.del Rincón I, Escalante A. HLA-DRB1 alleles associated with susceptibility or resistance to rheumatoid arthritis, articular deformities, and disability in Mexican Americans. Arthritis Rheum. 1999;42:1329–38. doi: 10.1002/1529-0131(199907)42:7<1329::AID-ANR5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 27.Bots ML, Evans GW, Riley WA, et al. Carotid intima-media thickness measurements in intervention studies: design options, progression rates, and sample size considerations: a point of view. Stroke. 2003;34:2985–94. doi: 10.1161/01.STR.0000102044.27905.B5. [DOI] [PubMed] [Google Scholar]
- 28.Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell CK, Aeschlimann SE, Korcarz CE. Carotid intima-media thickness testing: technical considerations. J Am Soc Echocardiogr. 2004;17:690–2. doi: 10.1016/j.echo.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Polak JF, Pencina MJ, O’Leary DH, et al. Common carotid artery intima-media thickness progression as a predictor of stroke in multi-ethnic study of atherosclerosis. Stroke. 2011;42:3017–21. doi: 10.1161/STROKEAHA.111.625186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corrales A, Parra JA, González-Juanatey C, et al. Cardiovascular risk stratification in rheumatic diseases: carotid ultrasound is more sensitive than Coronary Artery Calcification Score to detect subclinical atherosclerosis in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1764–70. doi: 10.1136/annrheumdis-2013-203688. [DOI] [PubMed] [Google Scholar]
- 32.Corrales A, González-Juanatey C, Peiró ME, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: results of a population-based study. Ann Rheum Dis. 2014;73:722–7. doi: 10.1136/annrheumdis-2012-203101. [DOI] [PubMed] [Google Scholar]


