Abstract
CMV remains an important opportunistic pathogen in solid organ transplantation, particularly in lung transplant recipients (LTRs). LTRs mismatched for CMV (donor+/recipient−; D+R−) are at high-risk for active CMV infection and increased mortality, however the immune correlates of viral control remain incompletely understood. We prospectively studied 23 D+R− LTRs during primary CMV infection to determine whether acute CD8+ T-cell parameters differentiated the capacity for viral control in early chronic infection. T-box transcription factors expression patterns of T-bet > Eomes differentiated LTR controllers from viremic relapsers, and reciprocally correlated with granzyme B loading, and CMV phosphoprotein 65 (pp65)-specific CD8+IFN-γ+ and CD107a+ frequencies. LTR relapsers demonstrated reduced CD8+Ki67+ cells ex vivo and substantially impaired CD8+pp65-specific in vitro proliferative responses at 6 days, with concomitantly lower pp65-specific CD4+IL-2+ frequencies, as compared to LTR controllers. However, CMV-specific in vitro proliferative responses could be significantly rescued, most effectively with pp65 antigen and exogenous IL-2, resulting in an increased T-bet:Eomes balance, and enhanced effector function. Using class I CMV tetramers, we observed similar frequencies between relapsers and controllers, though reduced T-bet:Eomes balance in tetramer+ cells from relapsers, along with impaired CD8+ effector responses to tetramer-peptide restimulation. Together, these data show impaired CMV-specific CD8+ effector responses is not for complete lack of CMV-specific cells, but rather, underscores the importance of the T-bet:Eomes balance, with CMV-specific proliferation a key factor driving early T-bet expression and effector function in CD8+ T cells during primary infection, and differentiating the capacity of high-risk LTRs to establish immune control during early chronic infection.
INTRODUCTION
Cytomegalovirus (CMV), a member of the β-herpesvirus family, remains a significant opportunistic infection and cause of morbidity/mortality in solid organ transplant recipients and hematopoietic cell transplant recipients(1-3). In particular, LTRs have increased susceptibility to CMV infection, perhaps due to the lung being a major reservoir for latent virus(4). LTRs mismatched for CMV (donor+/recipient−; D+R−), comprise 25% of all LTRs and have increased incidence of active CMV infection and end-organ disease, yet despite longer duration of antiviral prophylaxis in many programs, D+R− LTRs continue to have increased 5-year mortality(5). Additionally, several studies have implicated active CMV infection as a risk factor for the development of chronic allograft rejection or the bronchiolitis obliterans syndrome (BOS), the major limiting factor for long-term survival in LTRs(6, 7). Recent studies have shown that CMV viremia, including multiple episodes of viremia, are associated with an increased risk of BOS and decreased survival in LTRs(8, 9). However, an unanswered question in the field is whether all D+R− LTRs are at increased risk for mortality and/or BOS or whether there is heterogeneity among the group, with a subset of patients being at higher risk for poor clinical outcomes.
We recently have shown that D+R− LTRs differ in their capacity to establish immune control of CMV following discontinuation of antiviral therapy after primary infection, with approximately one-third of patients demonstrating relapsing viremia(10). We found that LTR ‘relapsers’ failed to induce high levels of the type-1 T-box transcription factor, T-bet, in the peripheral CD8+ T-cell pool during primary infection and had poor CD8+IFN-γ+ effector responses to the major CMV antigen phosphoprotein 65 (pp65) compared to LTR ‘controllers’. However in addition to T-bet, another T-box transcription factor family member, Eomesodermin (Eomes), has been shown to cooperate with T-bet to regulate CD8+ effector T-cell function in a Runx3-dependent manner(11, 12). While T-bet and Eomes mRNA have previously been shown to be detectable during primary CMV in renal transplant recipients(13), an assessment of Eomes protein expression, relative to T-bet and its relationship to CD8+ T-cell effector function has not been elucidated in human acute primary viral infection. We hypothesized the balance of T-bet/Eomes expression in CD8+ T cells would differ in relapser versus controller LTRs and impact acute primary effector function in CD8+ T-cells.
Herein, we report that the T-bet: Eomes balance in total CD8+ T-cells and CMV-specific CD8+tetramer+ cells differentiates D+R− LTR relapsers versus controllers, with T-bet and Eomes reciprocally correlating to CD8+ effector function and proliferation. Importantly, LTR relapsers with reduced T-bet expression demonstrated impaired CD8+ CMV pp65-specific in vitro proliferative responses, along with diminished CD4+ pp65-specific IL-2 secretion. Unexpectedly, exogenous IL-2 treatment in the presence of CMV antigen significantly rescued impaired CMV-specific proliferation in PBMCs from relapsers and enhanced the T-bet:Eomes balance, granzyme B expression, and functional CMV-specific IFNγ/CD107a responses in the CD8+ T-cells. Together, these findings show the T-bet:Eomes balance and in vitro CMV-specific effector and proliferative responses in CD8+ T-cells during primary CMV infection differentiate the capacity of high-risk LTRs to establish durable immune control during early chronic infection.
MATERIALS AND METHODS
Study subjects
D+R− LTRs from the Johns Hopkins Lung Transplant Program were identified and provided informed written consent for participation in a Johns Hopkins Medicine Institutional Review Board-approved protocol. All patients were treated with standard three-drug immunosuppression. Antiviral prophylaxis with ganciclovir and/or valganciclovir was used for the initial three months after transplant. Patients were prospectively monitored at least weekly for the development of primary CMV infection (defined as de novo detection of viral replication by quantitative PCR). CMV viral loads were determined using quantitative PCR of plasma by the Johns Hopkins Hospital Clinical Virology Laboratory. Patients developing primary CMV infection were treated with antiviral therapy (ganciclovir and/or valganciclovir) until two consecutive weekly quantitative CMV PCR measurements revealed undetectable viremia. Following completion of antiviral therapy for primary infection, patients continued to be prospectively monitored with quantitative CMV PCR measured at least biweekly, as well as during any symptomatic or clinically indicated time points, for the development of relapsing viremia (defined as the detection of >300 copies/mL of CMV by quantitative PCR on two consecutive samples after the completion of antiviral therapy for primary infection). Patients with relapsing viremia received antiviral therapy (ganciclovir and/or valganciclovir) if clinically indicated.
Tissue samples
Blood samples from LTRs were obtained prior to the discontinuation of initial antiviral prophylaxis (time point referred to as ‘pre-CMV’) and within 5-14 days of detection of de novo viremia (time point referred to as ‘primary CMV’). Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood samples by density gradient centrifugation using Ficoll-Paque (GE Healthcare) to be used in subsequent assays. All patients had therapeutic levels of calcineurin inhibitors at the time of sampling.
Antigen re-stimulation
Single-pools of overlapping 15-mer peptides for pp65 (JPT, Berlin, Germany) or HLA class I CMV tetramer-matching peptides (A*01 VTEHDTLLY, A*02 NLVPMVATV, B*07 TPRVTGGGAM, B*08 ELRRKMMYM) (IBA Solution for Life Sciences) were used. PBMC were cultured in round-bottom tissue culture tubes (Sarstedt) in the presence or absence (medium alone) of pooled pp65 peptides (1 μg/mL) or HLA class I tetramer matching peptides or the positive control of staphylococcal enterotoxin B (SEB, 1 μg/mL). All stimulations for intracellular cytokine production were performed using 106 cells per condition for 6 h at 37°C with brefeldin-A (10 μg/mL) (Sigma) was added for the final 4 h of culture. Monensin (5 μg/ml) along with brefeldin-A and anti-CD107a-Pacific Blue (Pac Blue) was added at the beginning of culture when CD107a was measured. All cells were collected for flow cytometric analysis with a range of 0.5-1×106 total events collected per condition. All gates for cytokine frequencies were set using the medium alone control and subtracted from peptide re-stimulated samples frequencies. In certain experiments, cells were re-stimulated in vitro as indicated above and labeled with CFSE (0.2 μM; Invitrogen) and cultured for 6 days, with/without pp65 peptides in the presence or absence of exogenous IL-2 (10 IU; Roche). Cell cultures were harvested at day 6, washed, rested overnight in medium alone, and secondary re-stimulation performed in the presence or absence of CMV pp65 peptides for 6 h (according to primary cultures were pulsed/un-pulsed with peptide) and assessed for proliferation via CFSE dilution and cytokine by ICS. Cell fluorescence was analyzed using a LSR Fortessa cytometer equipped with a UV Laser (BD Biosciences). Flow cytometry data were analyzed using FlowJo software (Tree Star).
Flow cytometry
Following in vitro re-stimulation, cells were surface-stained with fluorochrome-labeled antibodies anti-CD3-Alexa-Fluor700, anti-CD8-V500 and anti-CD4-APC Cy7 (BD Biosciences, BioLegend). In addition, HLA class I CMV tetramers-PE labeled (A*01 VTE, A*02 NLV, B*07 TPR, B*08 ELR and B*035 IPS) (Beckman Coulter or IBA Solution for Life Sciences), and Live/Dead Fixable Blue Dead-Cell Stain (Invitrogen) was used for gating on viable cells. In some experiments we used surface-stained with fluorochrome-labeled antibodies anti-PD1-FITC and anti CD160-PE Cy7 (BD Biosciences, BioLegend). Cytofix/Cytoperm (BD Biosciences) reagents were used to fix and permeabilize cells for intracellular cytokine staining (ICS) using anti-IFN-γ-PE Cy7 (or BV605), anti-IL-2-FITC, anti-Granzyme B-PE CF 594, anti-CD107a-Pacific Blue, anti-T-bet-PE or BV655 (BD Biosciences) and anti-Eomes-Alexa-Fluor 660 (eBiosciences).
Statistical Analysis
Statistical analysis was performed using the GraphPad Prism software. As no assumption was made regarding the Gaussian distribution of measured variables, the non-parametric tests of Wilcoxon signed-rank, Mann-Whitney-Wilcoxon, and Spearman’s rank correlation were used. A two-tailed p value of less than 0.05 was considered statistically significant.
RESULTS
Patient characteristics and clinical phenotypes during and following primary CMV infection in D+R− LTRs
Here, we evaluated the peripheral CD8+ T-cell responses in a prospective cohort of 23 D+R− LTRs, whose clinical characteristics are shown in Table I. With close prospective monitoring (see Materials/Methods) we detected primary CMV infection at a median of 149 days posttransplant (~60 days after discontinuation of CMV prophylaxis) in our cohort. We continued prospective monitoring after completion of antiviral therapy and detected relapsing viremia in a subset of 8 LTRs (35%) within the first 6 months of early chronic infection, in contrast to the remaining 15 LTRs who demonstrated immune control. Those patients with relapsing viremia had a median viral load of 2342 copies/ml, occurring at a median 80 days after initial detection of primary infection. Seven of 8 relapsers required additional treatment with antiviral therapy. All episodes of relapsing viremia occurred independent of acute rejection episodes, augmented immunosuppression or other active infections. Also of note is that during acute primary CMV infection, relapsers demonstrated reduced absolute numbers of total lymphocytes and CD8+ lymphocytes compared to controllers (Supplemental Fig. 1). Additionally, there was no clinical evidence of ganciclovir-resistant CMV infection in any LTR relapsers.
Table I.
Patient characteristics and clinical phenotypes following primary CMV infection in D+R− LTRs.
| LTR | Age (yrs) |
Gender | Primary Diagnosis | Immunosuppression at Primary CMV Onseta |
Primary CMV Onsetb |
Relapsing Viremia |
Viral load** (DNA copies/ml) |
|---|---|---|---|---|---|---|---|
| 22 | 60 | F | Idiopathic Pulmonary Fibrosis |
TAC 3.52, MMF 12, Pred 101 | 157 | - | 1220 |
| 24 | 31 | F | Cystic Fibrosis | TAC 1.52, MMF 0.53, Pred 101 | 96 | + | 51100 |
| 25 | 34 | F | Primary Pulmonary Hypertension |
TAC 42, MMF 0.52, Pred 101 | 129 | - | 16900 |
| 28 | 33 | F | Cystic Fibrosis | TAC 62, MMF 0.52, Pred 51 | 210 | - | 1260 |
| 29 | 62 | F | COPD | TAC 2.52, MMF 0.53, Pred 101 | 168 | + | 47700 |
| 30 | 61 | F | Idiopathic Pulmonary Fibrosis |
CSA 175/100, RAPA 11, Pred 51 | 94 | - | 0 |
| 31 | 55 | M | Cystic Fibrosis | TAC 32, AZA 501, Pred 51 | 248 | - | 3930 |
| 33 | 51 | F | Idiopathic Pulmonary Fibrosis |
TAC 22, MMF 0.53, Pred 7.51 | 186 | + | 1090 |
| 34 | 59 | F | COPD | TAC 42, MMF 0.252, Pred 101 | 174 | - | 2710 |
| 35 | 27 | M | Cystic Fibrosis | TAC 12, MMF 0.52, Pred 7.51 | 219 | - | 1258 |
| 36 | 49 | F | Idiopathic Pulmonary Fibrosis |
TAC 42, MMF 0.252, Pred 101 | 167 | + | 9070 |
| 37 | 56 | F | Obliterative Bronchiolitis | TAC 52, MMF 0.54, Pred 101 | 133 | - | 95400 |
| 38 | 56 | M | COPD | TAC 12, MMF 12, Pred 101 | 155 | - | 2010 |
| 40 | 54 | F | COPD | TAC 1.52, MMF 0.54, Pred 151 | 122 | - | 32400 |
| 41 | 64 | F | Bronchiectasis | TAC 42, MMF 0.53, Pred 51 | 184 | - | 23500 |
| 42 | 37 | F | Sarcoidosis | TAC 5.52, MMF 0.54, Pred 12.51 |
138 | - | 19600 |
| 43 | 51 | M | Sarcoidosis | TAC 42, MMF 0.52, Pred 151 | 83 | + | 1490 |
| 45 | 21 | M | Cystic Fibrosis | TAC 2.52, MMF 0.53, Pred 151 | 92 | - | 4840 |
| 46 | 59 | M | COPD | TAC 1.52, MMF 12, Pred 201 | 37 | + | 49500 |
| 48 | 47 | M | Idiopathic Pulmonary Fibrosis |
TAC 22, MMF 0.252, Pred 101 | 162 | + | 27331 |
| 50 | 64 | M | Idiopathic Pulmonary Fibrosis |
TAC 0.52, MMF 0.53, Pred 101 | 127 | - | 14194 |
| 51 | 41 | F | Pulmonary Hypertension | TAC 22, MMF 0.52, Pred 7.51 | 214 | - | 1675 |
| 53 | 35 | F | Cystic Fibrosis | TAC 22, MMF 0.52, Pred 101 | 125 | + | 47913 |
| Summaryd | 48.13 | F 65.2% | 148.70 | + 34.7% | 19830.04 |
TAC, tacrolimus (dose in milligrams with superscript times per day); MMF, mycophenolate mofetil (dose in grams); Pred, prednisone (dose in milligrams); CSA, cyclosporine (dose in milligrams); RAPA, sirolimus (dose in milligrams); AZA, azathioprine (dose in milligrams).
Days post-transplant.
Days post-primary infection. TAC 22, MMF 0.52, Pred 7.51
Values represent medians or percent of indicated group.
Viral load at time of sampling
Increased CD8+Eomes+ T-cells and reduced T-bet:Eomes CD8+ T-cell ratio in high-risk LTRs with relapsing viremia following primary CMV infection
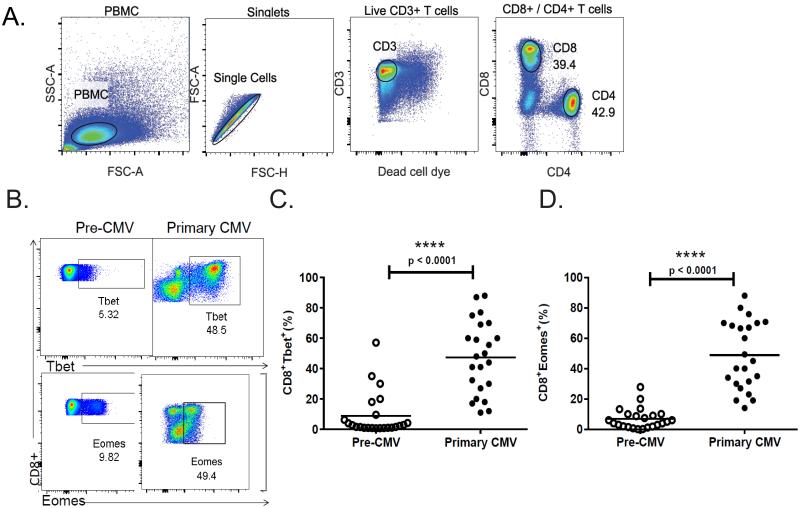
We previously demonstrated T-bet is significantly induced in CD8+ T-cells during primary CMV infection (10). To further characterize the development of CD8+ T-cell effector responses to CMV, we evaluated protein expression of another T-box transcription factor, Eomesodermin, along with T-bet during a CMV-naïve post-transplantation period prior to discontinuation of antiviral prophylaxis and during primary infection. Using the PBMC gating strategy shown in Fig. 1A, we observed a marked increase in the frequencies of CD8+ T-cells expressing Eomes and T-bet, during primary CMV infection compared with pre-CMV time point (Fig. 1B). Analysis of the entire cohort revealed significant increases in CD8+T-bet+ (Fig. 1C) and CD8+Eomes+ (Fig. 1D) during primary infection.
Figure 1. Significant induction of T-bet and Eomes in peripheral CD8+ T cells during primary CMV infection.
(A) Representative flow cytometric plots (LTR#35) of gating strategy used to analyze the numbers of PBMC, CD8+ or CD4+ T cells from prospective cohort of 23 D+R− LTRs patients. Gating was done on PBMC cells with doublet exclusion, than gating on live/CD3+ T cells, and then on subsets of CD4+ or CD8+ T cell populations. Numbers indicate frequencies of populations gated. Plots are representative of 23 D+R− LTRs patients analyzed from individuals during CMV infection. (B) Representative flow cytometric plots showing the intracellular protein expression of T-bet (LTR#45) and Eomes (LTR#38) during a CMV-naïve post-transplantation period prior to discontinuation of prophylaxis (pre-CMV-left panels) and at the time of primary infection (primary CMV-right panels). Pooled data showing the frequencies of PBMC CD8+Tbet+ (C), and CD8+Eomes+ (D), during pre-CMV (empty dots) and primary CMV infection (filled dots) from 23 patients. Bars represent median values and p-values were calculated using the Wilcoxon signed-rank test.
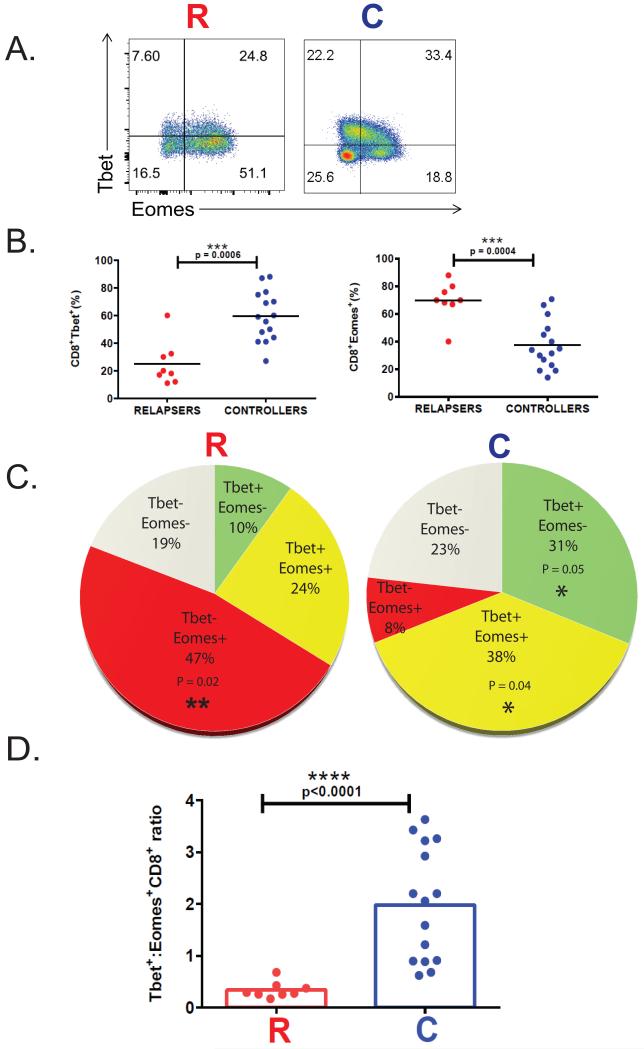
Next, we compared acute CD8+ T-bet and Eomes expression in relapsers versus controllers. Relapsers demonstrated significantly higher frequencies of CD8+Eomes+ T-cells compared to controllers, and in contrast to reduced frequencies of CD8+T-bet+ T-cells (Fig 2 A-B). We also observed variable frequencies of single T-bet+- and/or Eomes+-CD8+ T-cells among the two clinical phenotypes during primary infection (Fig. 2C), with controllers demonstrating increased frequencies of T-bet+CD8+ T-cells and T-bet+Eomes+CD8+ T-cells versus relapsers. In contrast, we observed significantly increased frequencies of T-bet-Eomes+ CD8+ T-cells in relapsers compared to controllers. Next, we determined that the T-bet:Eomes CD8+ T-cell ratio differentiated the two clinical phenotypes, with controllers demonstrating significantly increased T-bet:Eomes CD8+ T-cell ratios (median 1.98 compared to 0.34 in relapsers, p<0.0001, Fig 2D). Together, these data show the balance of induction of T-bet, relative to Eomes in the CD8+ T-cell pool differentiates high-risk LTR clinical phenotypes.
Figure 2. Increased CD8+Eomes+ T cells and reduced T-bet:Eomes CD8+ T-cell ratio in high-risk LTRs with relapsing viremia following primary CMV infection.
(A) Representative flow cytometric plots showing the intracellular protein expression of the CD8+Tbet+ and CD8+Eomes+ transcription factors during primary infection from an LTR relapser (LTR#29) (left panel) and LTR controller patient (LTR#37) (right panel). The gating strategy of the flow cytometric plots was done on PBMC cells with doublet exclusion, then gating on live/CD3+ T cells, and CD3+Tbet+ or CD3+Eomes+, than the subsets of CD8+Tbet+ and CD8+Eomes+ T cell populations, and the numbers indicate frequencies of each cells gated (%). Plots are representative of 23 D+R− LTRs patients analyzed from individuals during CMV infection. (B) Pooled data showing the frequencies of PBMC CD8+Tbet+ (left panel) and CD8+Eomes+ (right panel) transcription factors expression during primary CMV infections from cohort, in those with relapsing viremia (n=8 ‘relapsers’) - (red dots) versus those without (n=15 ‘controllers’) - (blue dots). (C) Pooled data showing pie charts of cumulative frequencies of single T-bet and/or Eomes expression in the CD8+T cell pool in the LTR cohort during primary CMV from controllers (n=15) (right pie chart) and relapsers (n=8) (left pie chart). (D) Pooled data showing the T-bet+:Eomes+ CD8+T cell ratio in relapsers (red columns) versus controllers (blue columns) from the total LTR cohort. Bars represent median values and p-values were calculated using the Wilcoxon signed-rank test or the Mann–Whitney-Wilcoxon t-test where appropriate.
Acute CD8+ T-cell granzyme B loading and pp65-specific IFN-γ/CD107a responses are driven by T-bet and differentiate controllers from relapsers during early chronic CMV infection
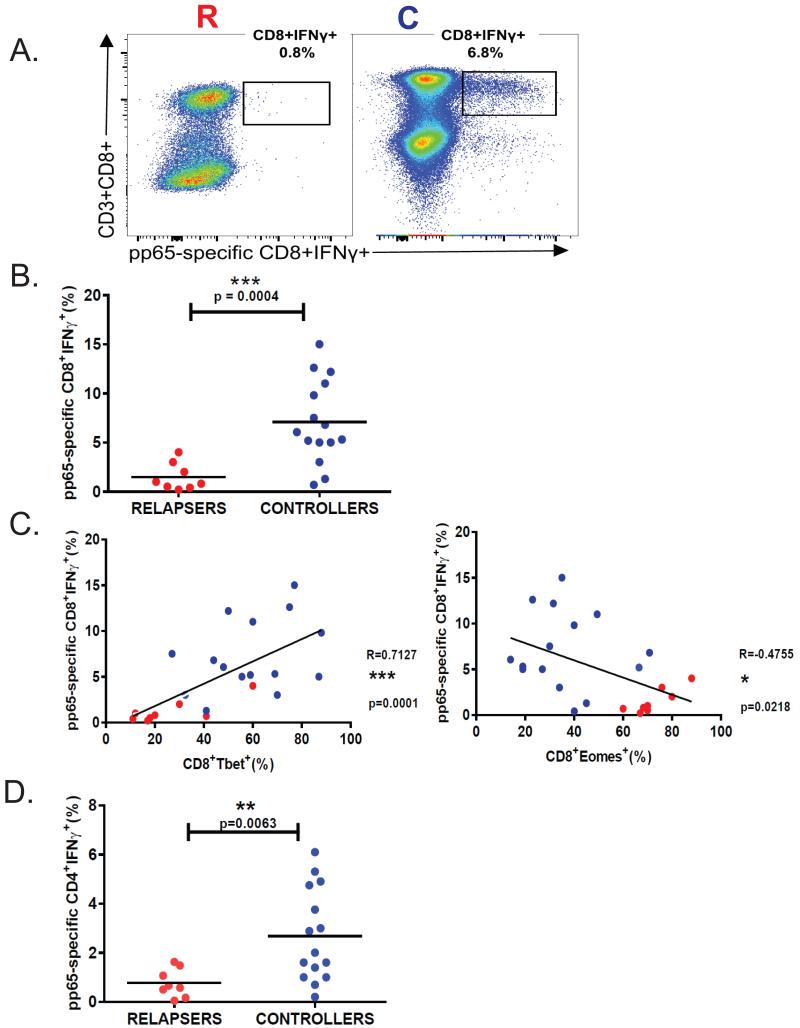
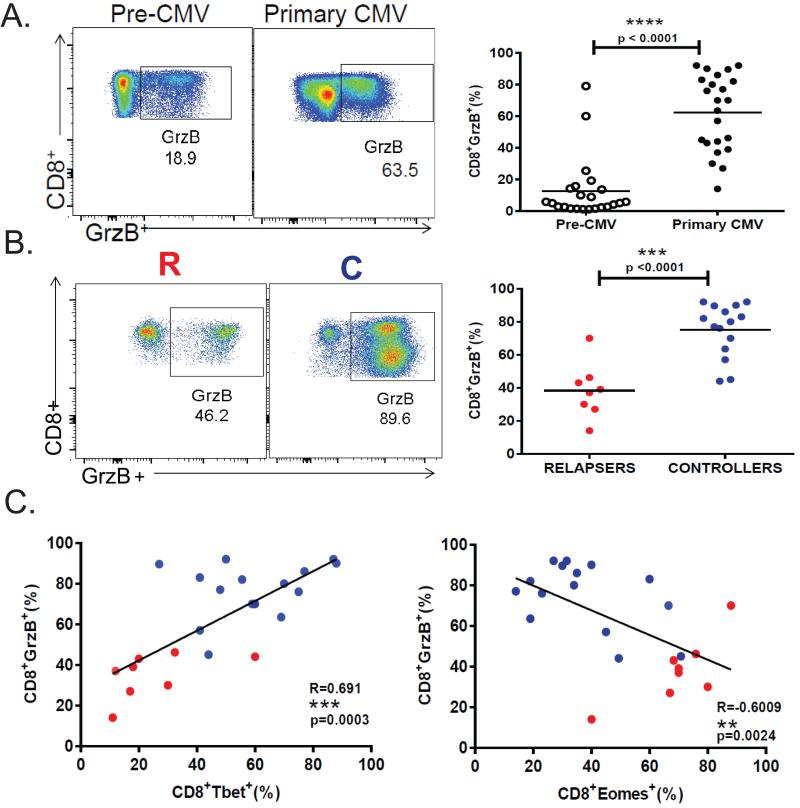
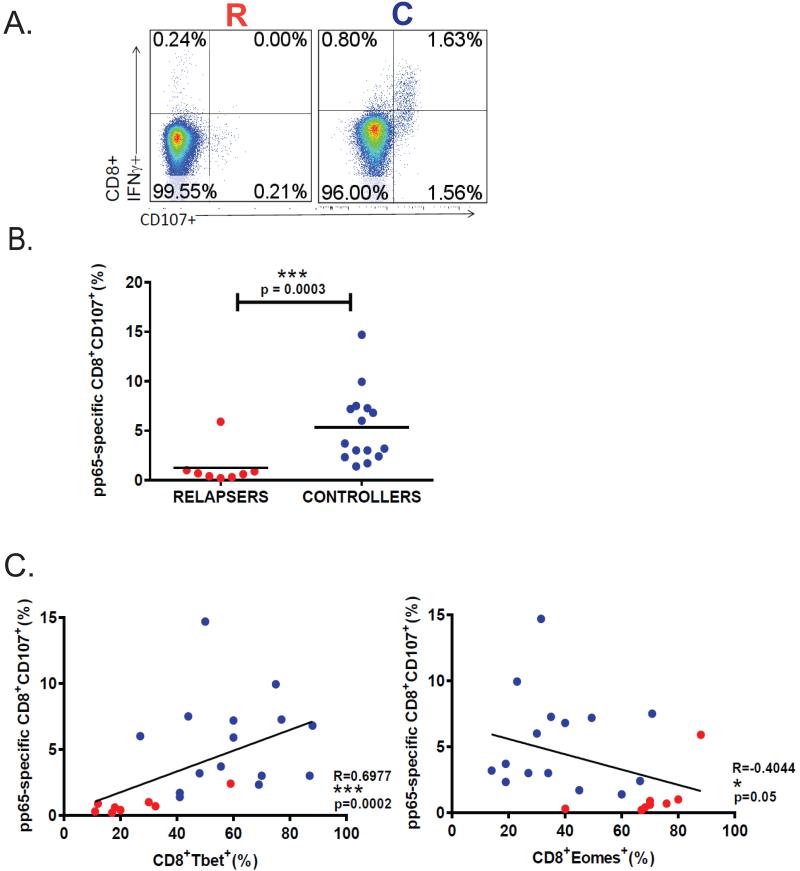
Cytotoxic T-cell effector function is critical for viral control during chronic infection(14, 15). Therefore, we examined the relationship between T-bet and Eomes induction and CD8+ T-cell function during acute primary infection. We confirmed our previous findings of a positive correlation between acute T-bet+ and pp65-specific IFN-γ+CD8+ T-cell responses, but in contrast, observed an inverse correlation between acute Eomes+ and pp65-specific IFN-γ+CD8+ T-cell responses (Fig. 3). We also found the frequencies of CD4+ T-cells from LTR relapsers producing IFN-γ(Fig. 3D) in response to pp65 peptides were significantly reduced compared to LTR controllers during acute primary infection. Additionally, the CD8+ T-cell loading of the cytotoxic molecule granzyme B (GrzB) during acute primary infection is significantly increased compared to pre-CMV (Fig. 4A), and levels are increased in controllers compared to relapsers (Fig. 4B). We compared acute CD8+T-bet+ levels with acute CD8+GrzB+ levels and observed a significant correlation during primary CMV infection (Fig. 4C-left panel), though unexpectedly, we observed an inverse relationship between CD8+GrzB+ and CD8+Eomes+ frequencies (Fig. 4C). Next, we assessed CD107a mobilization as a surrogate for cytotoxic molecule degranulation(16) in response to the major CMV antigen pp65 (Fig. 5). We found significant co-expression of CD107a with IFN-γ and increased frequencies of CMV pp65-specific CD8+CD107a+ cells during primary infection in LTR controllers compared to relapsers (Fig. 5A-B). We then performed scatterplot analysis of CD8+T-bet+ and pp65-specific CD8+CD107+ frequencies and observed a significant correlation (Fig. 5C). In contrast, we observed an inverse correlation between CD8+Eomes+ and pp65-specific CD8+CD107+ frequencies (Fig. 5C). We should also mention that in a minority (~25%) of patients, CD8+T-bethigh and CD8+T-betint populations could be elucidated, with increased CMV-specific IFN-γ+ and CD107a+ frequencies associated with the T-bethi cells in controllers, but not in relapsers (Supplemental Fig. 2). Taken together, these data indicate that acute primary GrzB loading in CD8+ T-cells and pp65-specific CD8+CD107+ and IFN-γ+ responses are positively correlated with T-bet, but not Eomes, and differentiate the capacity for durable immune viral control in high-risk LTRs during early chronic CMV infection.
Figure 3. CMV pp65-specific CD8+IFN-γ+ effector responses reciprocally correlate with T-bet and Eomes expression and differentiate the capacity for viral control in high-risk LTRs.
(A) Representative flow cytometric plots showing ICS of the pp65-specific CD3+CD8+IFNγ+ during primary CMV infection from a relapser (LTR#33) (left panel) and a controller patient (LTR#35) (right panel). (B)Pooled data showing the frequencies of CMV pp65-specific CD8+IFNγ+ (minus medium alone) during primary infection from 23 patients cohort with LTR relapsers (red dots) versus controllers (blue dots). Bars represent median values and p-values were calculated using the Mann–Whitney-Wilcoxon test.(C)Evaluation of the relationship between CMV pp65-specific CD8+IFNγ+ and CD8+Tbet+ frequencies (left panel) or CD8+Eomes+ frequencies (right panel) during primary CMV infection with LTR relapsers (red dots) versus controllers (blue dots). (D) LTR relapsers have reduced frequencies of pp65-specific CD4+IFN-γ+ T-cells during acute primary CMV infection. Comparison of pp65-specific CD4+IFNγ+ frequencies (minus medium alone) from entire cohort, showing relapsers (red dots) and controllers (blue dots). Bars represent median values and p-values were calculated using the Mann–Whitney t-test. Correlation coefficients (R) and p-values were calculated using Spearman rank correlation test.
Figure 4. Induction of granzyme B in CD8+ T cells during primary CMV reciprocally correlates with T-bet and Eomes and differentiates LTR phenotypes.
(A) Representative flow cytometric plots (LTR#42) showing CD8+ intracellular protein expression of granzyme B (GrzB) during a CMV-naïve post-transplantation period prior to discontinuation of prophylaxis (pre-CMV-left panels) and at primary infection (primary CMV-middle panels). Pooled data (right panel) showing the frequencies of CD8+GrzB+ during pre-CMV (empty dots) and primary CMV infections (filled dots) from the patient cohort. (B)Representative flow cytometric plots showing the intracellular protein expression of CD8+GrzB+ cells during primary infection from a relapser (LTR#48) (left panel) and a controller patient (LTR#31) (middle panel) and pooled data (right panel) showing the frequencies of PBMC CD8+GrzB+ during primary CMV in LTR relapsers (red dots) versus controllers (blue dots). Bars represent median values and p-values were calculated using the Mann-Whitney-Wilcoxon or Wilcoxon signed-rank test where appropriate. (C) Evaluation of the relationship between CD8+GrzB+ and CD8+Tbet+ (left panel) or CD8+Eomes+ (right panel) during primary CMV infections from LTR cohort with relapsers (red dots) versus controllers (blue dots) by scatter plot analysis. Correlation coefficients (R) and (p) values were calculated using Spearman rank correlation test.
Figure 5. CMV pp65-specific CD8+CD107a+ responses reciprocally correlate with T-bet and Eomes and differentiate LTR relapser versus controller phenotypes.
(A) Representative flow cytometric plots showing pp65-specific CD8+IFNγ+ and CD8+CD107a+ responses during primary infection from relapser (LTR#48) (left panel) and controller patients (LTR#51) (right panel) from the cohort. (B) Pooled data showing the frequencies of CD8+CD107a+ cells (minus medium alone) during primary CMV infection with relapsers (red dots) versus controllers (blue dots). Bars represent median values and p-values were calculated using the Mann-Whitney-Wilcoxon test.(C)Evaluation of pp65-specific CD8+CD107+ versus CD8+Tbet+ (left panel) or CD8+Eomes+ (right panel) during primary CMV in relapsers (red dots) versus controllers (blue dots) by scatter plot analysis. Correlation coefficients (R) and p-values were calculated using Spearman rank correlation test.
LTR relapsers demonstrate impaired CMV pp65-specific proliferative and CD4+IL-2+ responses compared to controllers during acute primary CMV infection
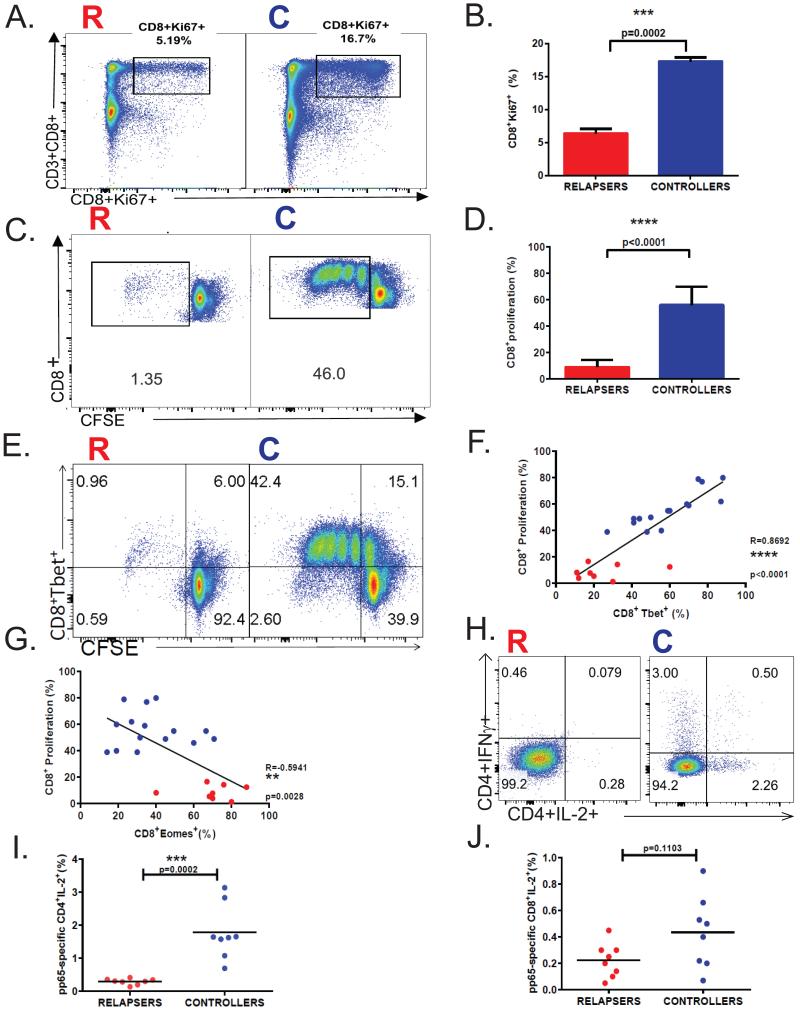
Proliferative expansion of antigen-specific CD8+ T-cells during acute viral infection is critical to establish a functional effector pool to promote viral clearance and establish T-cell memory. Therefore, we asked whether acute proliferative responses differed between LTR relapsers and controllers. First, we evaluated ex vivo Ki-67 protein, a marker for proliferation associated with ribosomal RNA transcription in CD8+ T-cells during acute infection. We detected significantly increased Ki-67+ cells in CD8+ T-cells in controllers compared to relapsers (Fig. 6A-B). We then assessed day 6 CMV-specific proliferation using CFSE dilution in response to pp65-pooled peptides, and found significantly higher pp65-specific proliferation in LTR controllers compared to relapsers (Fig. 6C-D). Additionally, we detected increased T-bet expression in proliferating pp65-specific CD8+ T-cells in LTR controllers over relapsers (Fig. 6E), and a positive correlation between CD8+T-bet+ levels and CD8+ pp65-specific proliferative responses (Fig. 6F), but in striking contrast, a reciprocal relationship with CD8+Eomes+ levels (Fig. 6G). Further, we analyzed CMV plasma viral loads and found that CD8+ pp65-specific proliferation was unrelated to the level of viremia at the time of sampling during acute infection (Supplemental Fig. 3). Lastly, there were no significant differences in calcineurin inhibitor levels during acute infection between relapsers and controllers (data not shown). Collectively, our data demonstrate impaired CMV-specific CD8+ T-cell proliferative responses in LTR relapsers versus controllers, and provide evidence that acute CD8+T-bet+ levels are significantly coupled to CMV-specific CD8+ T-cell proliferative capacity.
Figure 6. LTR relapsers demonstrate impaired CMV pp65-specific proliferative responses compared to controllers during acute primary CMV infection.
(A) Representative flow cytometric plots showing CD3+CD8+Ki-67+ responses during primary infection from a relapser (LTR#33) (left panel) and a controller patient (LTR#45) (right panel). (B)Pooled data showing the frequencies of CD8+Ki-67+ during primary CMV infection with relapsers (red column) versus controllers (blue column). Bars represent median values ± SEM and p-values were calculated using Mann-Whitney t-test.(C)Representative flow cytometric plotsof day-6 proliferative responses using CFSE dilution in response to CMV pp65 pooled-peptides during primary infection from a relapser (LTR#53) (left panel) and a controller patient (LTR#38) (right panel) from the LTR cohort.(D)Pooled data showing the frequencies of pp65-specific CD8+ day-6 proliferation (CFSE dilution minus medium alone) during primary CMV infection from relapsers (red column) versus controllers (blue column). Bars represent median values and p-values were calculated using the Mann–Whitney t-test. (E)Representative flow cytometric plotsof day-6 pp65-specific proliferation by CFSE dilution of CD8+Tbet+ during primary infection from a relapse (LTR#43) (left panel) and a controller patient (LTR#40) (right panel). (F)Pooled data showing the correlation of pp65-specific CD8+ proliferation and CD8+Tbet+ or (G) CD8+Eomes+ during primary CMV infection in relapsers (red dots) versus controllers (blue dots) from the LTR cohort. Correlation coefficients (R) and p-values were calculated using Spearman rank correlation test. (H) Representative flow cytometric plotsshowing the frequencies of blood CD4+IFNγ+ and/or CD4+IL-2+ cells in response to pp65 pooled-peptides during primary infection from a relapser (LTR#53) (left panel) and a controller patient (LTR#51) (right panel).(I)Pooled data showing pp65-specific CD4+IL-2+ frequencies (minus medium alone) in relapsers (n=8; red dots) versus controllers (n=8; blue dots). (J)Pooled data showing pp65-specific CD8+IL-2+ frequencies (minus medium alone) in relapsers (n=8; red dots) versus controllers (n=8; blue dots).
The T-cell growth factor, IL-2, is a key driver of T-cell proliferation and a central target of calcineurin inhibitor therapies such as cyclosporine A and tacrolimus. Based on our findings of impaired pp65-specific CD8+ T-cell proliferation during primary infection, we hypothesized that CD4+ T-cells, the major source of IL-2, were impaired in their ability to secrete IL-2 in response to pp65-antigen. We found the frequencies of CD4+ T-cells from LTR relapsers producing IL-2 (Fig. 6H-I) and/or IFN-γ (Fig. 3D, Fig. 6H) in response to pp65 peptides were significantly reduced compared to LTR controllers during acute primary infection concomitant with pp65-specific CD8+ proliferative responses. In contrast, the frequencies of CD8+ T-cells from LTR relapsers producing IL-2 were lower but not significantly different than LTR controllers (Fig. 6J). Additionally, the frequencies of CD4+ T-cells from LTR relapsers producing IL-2 and/or IFN-γ in response to the superantigen, SEB, were significantly reduced compared to LTR controllers during primary infection indicative of a more global functional immune defect (data not shown). Together, our findings identify concomitant CMV- specific CD4+ T-cell defects in LTR relapsers during acute CMV that likely contribute to an impaired ability to mount CMV-specific CD8+ proliferation.
Antigen and exogenous IL-2 rescues impaired CMV-specific CD8+proliferation, the CD8+ T-bet:Eomes ratio, and effector function
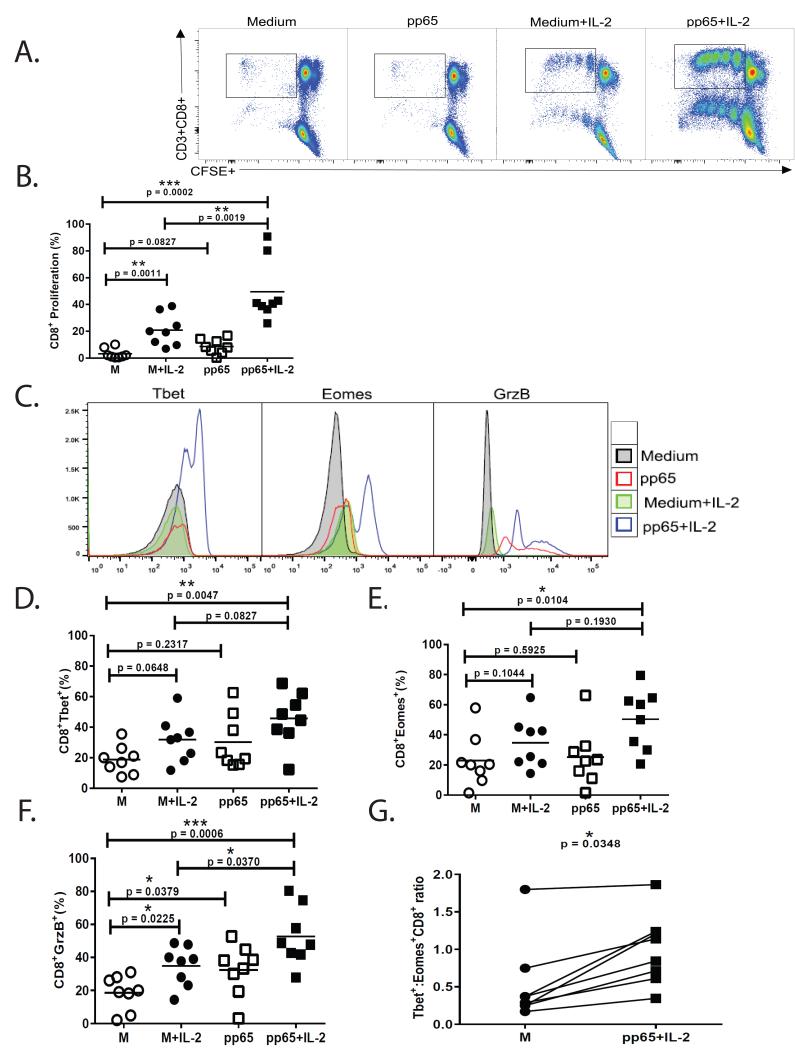
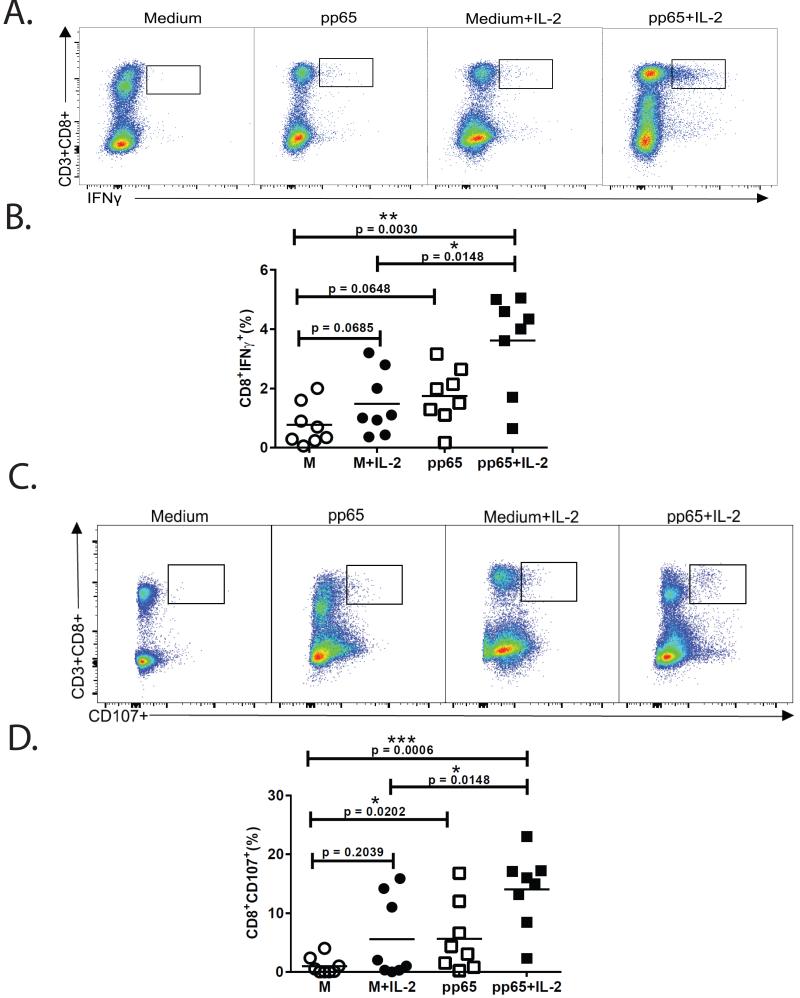
Previous studies have shown adoptive T-cell therapy can be effective in controlling active CMV infection (17). Successful ex vivo expansion of CMV- specific cells from autologous patient PBMCs could provide broad HLA-restricted specificities to a major antigen such as pp65 compared to single HLA-restricted, matched donor-derived cells. Because we found impaired CMV-specific CD4+IL-2+ responses in relapsers, we asked whether reduced CD8+ CMV-specific proliferative responses and T-bet induction could be rescued in vitro with low dose exogenous IL-2 in the presence or absence of antigen. The addition of exogenous IL-2 (10 IU) to day-6 cultures significantly enhanced CD8+ pp65-specific proliferative responses measured by CFSE dilution (Fig. 7A-B). We also measured T-bet, Eomes and Grz B in CD8+ T-cells at day-6 and found enhanced expression in cultures pulsed with pp65 antigen and IL-2 (Fig. 7C-F). Moreover, the T-bet:Eomes ratio was significantly increased in relapser CD8+ T-cells in cultures pulsed with pp65 peptide in the presence of IL-2 compared to cultures in medium and/or IL-2 alone (Fig. 7G). Next, using the same primary culture conditions, we performed secondary re-stimulation after 6 days and found significant increases in CD8+ pp65-specific IFN-γ+ and CD107a+ frequencies, most demonstrably in cultures pulsed with pp65 and IL-2 (Fig. 8A-D). Collectively, these data demonstrate that impaired CD8+ T-cell responses in LTR relapsers can be rescued most efficiently in vitro in the presence of pp65 antigen and exogenous IL-2, leading to enhanced CD8+ T-bet:Eomes balance and CMV- specific proliferation and effector function.
Figure 7. Antigen and exogenous IL-2 rescues impaired CMV-specific CD8+ proliferative responses and increases the CD8+ T-bet:Eomes ratio and granzyme B loading.
(A) Representative flow cytometric plots of CD3+CD8+ day-6 proliferation (by CFSE dilution) in cultures with/without pp65 peptides, in the presence or absence of exogenous IL-2 (10 IU). Shown are representative results from 8 relapsers patients (LTR#46). (B)Pooled data showing day-6 CD8+proliferation (CFSE dilution) during primary CMV infection from 8 relapser patients under culture conditions described in (A). Bars represent median values and p-values were calculated using the Wilcoxon signed-rank test.(C)Representative flow cytometric histograms of intracellular expression of transcription factors T-bet (left panel), Eomes (middle panel) and Granzyme B (right panel) in cultures of a relapser patient (LTR#33), where medium alone (grey filled histograms), pp65 peptide-stimulated (red line histograms), medium+IL-2 (green filled histograms), and pp65 peptides+IL-2 (blue line histograms) are shown. Pooled data showing the frequencies of day-6 CD8+Tbet+ (D), CD8+Eomes+ (E), and CD8+GrzB+(F) during primary CMV infection from LTR relapsers under the culture conditions described. Bars represent median values and p-values were calculated using the Wilcoxon signed-rank test.(G)Comparison of theT-bet:Eomes ratio in CD8+ T-cells from LTR relapsers (n=8) in day-6 cultures in medium alone (M) compared to those pulsed with pp65 peptides in the presence of IL-2, using the Wilcoxon signed-rank test.
Figure 8. Antigen and exogenous IL-2 treatment rescues impaired pp65-specific CD8+IFNγ+ and CD8+CD107+ responses.
(A) Representative flow cytometric plotsof CD3+CD8+IFNγ+ frequencies in relapser patient following secondary re-stimulation, under the primary culture conditions shown. At day-6, primary cultures were harvested, rested overnight, and secondary re-stimulation (6 h) performed in the presence or absence of pp65 peptides (according to whether present in primary cultures) immediately followed by ICS.(B)Pooled data showing the frequencies of CD8+IFN-γ+ frequencies following secondary re-stimulation as above, according to primary culture conditions. Bars represent median values and p-values were calculated using the Wilcoxon signed-rank test.(C)Representative flow cytometric plotsof CD3+CD8+CD107+ frequencies under the same conditions as described in (A). (D) Pooled data showing the frequencies of CD8+CD107+ frequencies following secondary re-stimulation as above, according to primary culture conditions. Bars represent median values and p-values were calculated using the Wilcoxon signed-rank test.
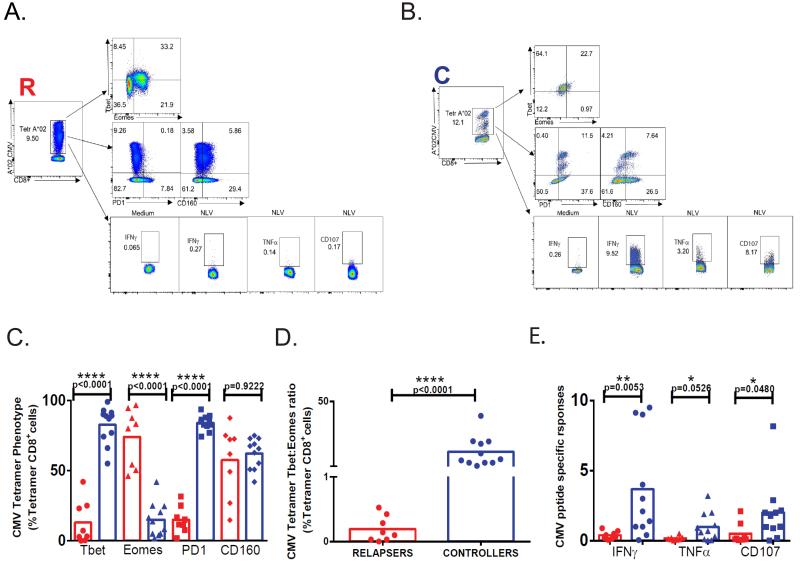
LTR Relapsers have similar frequencies of CMV tetramer+CD8+ T cells compared to LTR controllers, yet demonstrate a reduced T-bet:Eomes ratio and impaired effector function during acute primary CMV infection
Lastly, we wished to assess whether LTR relapsers had detectable CMV-specific tetramer+CD8+ T cells compared to LTR controllers. In our cohort, we had 19 patients who were evaluable using five CMV-specific class I tetramers. As shown in Table II, while CMV tetramer frequencies were quite variable, overall median frequencies were similar between LTR controllers and relapsers, with several relapsers demonstrating high frequencies during acute primary infection. To further characterize these responses, we evaluated expression of T-bet, Eomes, and the co-inhibitory receptors, PD-1 and CD160 in CD8+tetramer+ T cells. As shown in Fig. 9A-D, tetramer+ cells expressed T-bet>Eomes from controllers whereas tetramer+ cells from relapsers expressed Eomes>T-bet and thus overall, significantly different T-bet:Eomes ratios (Fig. 9D). Somewhat unexpectedly, PD-1 expression in tetramer+ cells was increased in controllers compared to relapsers, whereas no differences were detected in CD160 expression (Fig. 9A, B, C). However, we did not detect double positive PD-1 and CD160 expression in tetramer+ cells (data not shown) as has been reported in chronic HIV infection (18). To evaluate function, we performed in vitro restimulation using the corresponding tetramer-specific CMV peptides and measured IFN-γ, TNF-α, and CD107. With this approach, we detected significant increased frequencies of CMV single peptide-specific IFN-γ+ and CD107a+ cells from controllers over relapsers, and borderline significance for TNF-α+ responses (Fig. 9A, B, E).
Table II.
CMV CD8+tetramer+ T cell frequencies during primary CMV infection
| CD8+ Tetramer (%) LTR Controllers |
A*01 VTE |
A*02 NLV |
B*07 TPR |
B*08 ELR |
B*035 IPS |
|---|---|---|---|---|---|
| 22 | 1.81 | 12.3 | |||
|
|
|||||
| 25 | 1.22 | ||||
|
|
|||||
| 28 | 1 | ||||
|
|
|||||
| 30 | 0.06 | 0.02 | |||
|
|
|||||
| 31 | 1.97 | ||||
|
|
|||||
| 34 | 2.36 | 0.13 | |||
|
|
|||||
| 35 | NTM* | ||||
|
|
|||||
| 37 | 12.6 | ||||
|
|
|||||
| 38 | NTM | ||||
|
|
|||||
| 40 | NTM | ||||
|
|
|||||
| 41 | 0.85 | 4.26 | |||
|
|
|||||
| 42 | NTM | ||||
|
|
|||||
| 45 | 2.6 | 0.17 | |||
|
|
|||||
| 50 | 14.5 | ||||
|
|
|||||
| 51 | 1.48 | 1.08 | |||
| Mean | 1.30 | 3.47 | 5.56 | 0.13 | 0.17 |
| 4.61 | |||||
|
|
|||||
| CD8+ Tetramer (%) LTR Relapsers |
A*01 VTE |
A*02 NLV |
B*07 TPR |
B*08 ELR |
B*035 IPS |
|
| |||||
|
|
|||||
| 24 | 0.2 | ||||
|
|
|||||
| 29 | 0.8 | ||||
|
|
|||||
| 33 | 9.47 | ||||
|
|
|||||
| 36 | 0.36 | 0.24 | |||
|
|
|||||
| 43 | 3.17 | ||||
|
|
|||||
| 46 | 0.56 | 18.1 | |||
|
|
|||||
| 48 | 17.3 | ||||
|
|
|||||
| 53 | 2.78 | 0.08 | |||
|
|
|||||
| Mean | 0.98 | 13.39 | 0.80 | 5.40 | |
|
|
|||||
| 4.82 | |||||
|
|
|||||
NTM=no tetramer matched
Figure 9. LTR Relapsers have similar frequencies of CMV tetramer+ CD8+ T cells compared to LTR controllers, yet demonstrate a reduced T-bet:Eomes ratio and impaired effector function during acute primary CMV infection.
(A) Representative flow cytometric plots of A*02 CMV CD8+tetramer+ phenotypic analysis for T-bet, Eomes (top panel) PD1 and CD160 (middle panel) percentages and frequencies of tetramer-matched CMV peptide-specific re-stimulation responses for IFN-γ, TNF-α and CD107a (bottom panel) in representative relapser and (B) controller LTR. (C) Pooled data showing the percent tetramer+ cells expressing T-bet, Eomes, PD1 and CD160 and (D) the T-bet:Eomes ratio for tetramer+ cells in relapsers (n=8; red) versus controllers (n=11; blue). (E) Pooled data showing frequencies of tetramer-matched CMV peptide-specific responses for IFN-γ, TNF-α and CD107a in relapsers (n=8; red) versus controllers (n=11; blue). Bars represent median values and p-values were calculated using the Mann-Whitney-Wilcoxon t-test.
DISCUSSION
Here, we show high-risk D+R− LTRs are a heterogeneous group in their acquisition of peripheral CD8+ T-cell effector responses during acute primary CMV infection, and that notably, distinct CD8+ immune parameters differentiate patients who subsequently establish durable viral immune control compared to those who develop relapsing viremia during early chronic infection. Our results demonstrate that while the T-box factors T-bet and Eomes are both significantly induced and partially co-expressed during acute primary CMV infection in CD8+ T-cells, the relative balance or the CD8+T-bet:Eomes ratio, along with the pattern of T-box expression in the CD8+ T-cell pool, differentiates high-risk LTR controllers versus relapsers. Moreover, we provide similar evidence in CMV CD8+tetramer+ cells, underscoring the importance of the T-bet:Eomes ratio. Our findings are consistent with two recent studies demonstrating induction of T-bet and Eomes in CD8+ T-cells during primary CMV infection in renal transplant patients and differential T-box expression patterns in the CD8+ T-cells of CMV- seropositive individuals (13, 19). However, our study provides new evidence that early T-bet and Eomes CD8+ expression patterns differ in high-risk patients with respect to their capacity to establish durable CMV control. These findings are also consistent with a recent study by Hersperger et al. (20) showing higher levels of T-bet expression in HIV-specific memory CD8+ T-cells from elite controllers compared to chronically-infected progressors. However, because our study measured acute CD8+ effector responses versus memory responses, this might account for some differences. For example, we detected distinct T-bethi and T-betint CD8+ T-cell populations in the minority of patients, raising the question of whether this phenotype is more common in memory populations. Indeed, we previously demonstrated acute blood CMV-specific CD8+ effectors transition from a CD45low to a predominantly CD45high phenotype from acute into early chronic CMV infection with resolution of viremia, indicating other phenotypic changes can occur(21), (22). Together, our data show the T-bet:Eomes ratio and T-box transcription factor expression patterns in the acute CD8+ T-cell pool and CMV CD8+tetramer+ cells differentiates the capacity for viral control during early chronic infection.
Previous murine studies have demonstrated the importance of the T-box transcription factors T-bet and Eomes in regulating functional CD8+ T-cell responses important for control of chronic viral infection(23-26). In addition to the relative balance of T-bet:Eomes, we found these T-box factors are related to CD8+ function during acute infection, with T-bet being positively correlated with GrzB loading and CMV-specific IFN-γ, CD107a, and proliferative responses, whereas Eomes has a reciprocal relationship to these parameters. Our results with respect to Eomes and function were somewhat unexpected, as Eomes expression has been shown to be important for memory renewal in mice(26). However, our data indicate higher levels of Eomes during acute primary viral infection are associated with impaired effector function. While the mechanism(s) for this remain to be elucidated, Eomes+CD8+ T-cells have recently been shown to express higher levels of programmed death-1 (PD-1)(26). Interestingly, we did not observe increased PD-1 expression in conjunction with Eomes during acute primary infection, though this may reflect increased PD-1 expression due to activation during viremia. Therefore, other factors, including other co-inhibitory molecules or mechanisms (27, 28) might be important negative regulation of CMV-specific proliferation and effector function in relapser patients. Indeed, our CMV tetramer studies further demonstrate that impaired CMV-specific effector responses are not for lack of CMV cells, rather, cells with an impaired function and altered T-bet:Eomes balance compared to cells from controllers with functional responses. Our data also indicates at least part of the impaired CD8+ function observed in relapsers is due to significantly reduced proliferative capacity, resulting in poor differentiation and reduced acquisition of effector function. Surprisingly, these impaired CMV-specific proliferative responses could be restored, most efficiently in the presence of antigen and exogenous IL-2, and were accompanied by increased T-bet:Eomes ratio and enhanced effector function. Collectively, our data indicate that the acquisition of granzyme B loading, CMV-specific IFN-γ secretion, CD107a mobilization and proliferation in CD8+ T-cells is coupled to T-bet induction and reciprocally related to Eomes induction during acute primary CMV infection. Indeed, these findings are reminiscent of an earlier study showing a coupling of perforin and HIV-specific proliferation capacity in elite nonprogressors compared to progressors during chronic infection(29). Furthermore, our data suggest that early clinical monitoring of T-box transcription factor expression patterns and CMV-specific CD8+ T-cell effector and proliferative responses can differentiate high-risk LTR clinical phenotypes, and may be a useful tool to predict those at highest risk for relapsing CMV viremia.
It is also noteworthy that the heterogeneity in the capacity for pp65-specific CD8+ T-cell in vitro proliferation we observed in our high-risk patients was unrelated to the absolute viral load. There are other examples of viral infections in humans in which in vitro T-cell proliferative responses are impaired or absent during viremia including HIV virus(30), measles virus(31), hepatitis B virus(32), Dengue virus (33), as well as CMV infection(34). Moreover, CMV is known to down regulate MHC class I expression(35), in addition to other mechanisms of immune evasion(36, 37) that might contribute to impaired T-cell proliferation. Notably, we observed concomitantly reduced frequencies of pp65-specific CD4+IL-2+ T-cells in relapsers in association with impaired in vitro proliferative responses consistent with a previous report by Tilton et al. (38) that demonstrated reduced IL-2 production in patients during HIV viremia. Importantly, we show that exogenous IL-2 restored pp65-specific proliferative responses in relapsers, confirming that IL-2 responsiveness in CD8+ T-cells remains intact in these patients, despite poor CMV-specific responses. This finding also raises the possibility that LTR relapsers are more significantly impacted by calcineurin inhibitor therapy, which directly targets IL-2 mRNA synthesis, compared to LTR controllers despite similar trough levels. To this end, we found SEB-reactive CD8+ T-cell responses were also reduced in LTR relapsers, supporting the concept of more prominent global immunosuppression in these patients, despite a lack of differences in their immunosuppressive drug regimens. Together, while we observe concomitantly impaired CMV-specific proliferation and IL-2 production in LTR relapsers, other factors such as viremia, CMV immune evasion, or unknown factors may also play important roles in regulating the capacity to expand effector cells during acute infection.
Recently, several groups have advanced efforts to provide third-party, HLA-matched, viral-specific memory cells as adoptive cellular immunotherapy for viral infections such as CMV and other viruses in susceptible solid and hematopoietic transplant recipients(17, 39-43). In our studies we found, unexpectedly, that impaired in vitro CMV-specific proliferative and effector responses could be rescued with pp65 antigen and exogenous IL-2 over 6 days, thus raising the potential for further exploration of ex vivo expansion of autologous CMV-specific T-cells with enhanced effector function for adoptive immunotherapy therapy. While this therapy might carry an increased risk of transferring alloreactive viral-specific T-cells, the potential benefit of adoptive cell therapy could offset this risk, particularly in patients who develop drug-resistant CMV, which is more common in the D+R− population(44, 45). This autologous adoptive therapy approach for example, could provide pp65-specific CD8+ T-cells with multiple host HLA-restrictions, and could likely be expanded to other major antigens, such as IE-1. Moreover, autologous viral-specific cells may be more durable than 3rd party donor cells. Nonetheless, a careful risk/benefit analysis would need to be performed when considering such adoptive therapy strategies in LTRs, and perhaps be best initially explored in high-risk patients failing conventional antiviral therapy.
There are several caveats to our studies. We acknowledge that there are potential confounding factors that may impact the capacity for immune control during early chronic CMV infection. However, we did not find significant differences between relapsers and controllers in our cohort in regard to immunosuppression, duration of primary infection, or other posttransplant clinical parameters. Interestingly however, relapsers in our cohort were found to be relatively lymphopenic, in addition to impaired functional responses, thus potentially indicating an overall higher level of immunosupression not captured in drug levels or dosing. Also, while our studies focused on T-cell responses to pp65, we acknowledge the total effector response to CMV may be considerably larger, including a significant contribution by CD4+ T-cells(46, 47). Indeed, a broader CD8+ response is supported by our findings of striking induction of T-bet, Eomes and GrzB during primary infection compared to pre-CMV levels. However, because our previous studies found pp65-specific >IE1-specific CD8+ effector responses during primary infection during acute infection(21), we focused on pp65 responses. Finally, we recognize that our cohort size is somewhat small, however, the findings in this study could provide the foundation for a larger prospective study to test the clinical utility of these CMV immune parameters to assist in antiviral decision-making in high-risk LTRs. Nonetheless, despite these potential limitations, our study provides important evidence on the role of T-cell immunity and the capacity of high-risk D+R− LTRs to establish durable viral control following primary CMV infection.
In summary, we report that high-risk D+R− LTRs represent a heterogeneous group of patients with respect to their acquisition of CD8+ T-cell T-bet:Eomes balance, granzyme B loading, CMV-specific proliferative capacity, IFN-γ/CD107a secretion, and IL-2/IFN-γ production by CD4+ T-cells. We demonstrate that these immune parameters during acute primary CMV infection differentiate LTR clinical phenotypes with respect to the capacity for establishing durable immune control during early chronic infection, following antiviral therapy for primary infection. Furthermore, we show that impaired CMV cellular immunity can be restored in vitro with antigen and exogenous IL-2, raising the potential for autologous adoptive cell therapy as a therapeutic strategy in high-risk patients failing therapy. Together, our findings provide plausible immune correlates to support the pursuit of future studies aimed at determining whether immune monitoring is useful to prospectively risk-stratify high-risk LTRs and assist in antiviral decision-making, and ultimately, as a tool to advance personalized antiviral therapies directed toward optimal CMV control.
Supplementary Material
Key Points.
CD8 T-bet:Eomes balance differentiates capacity for CMV immune control and correlates with effector function
CD8 CMV-specific proliferation drives early T-bet and effector function and differentiates the capacity for durable immune control
Acknowledgments
Funding
This work was supported by National Institute of Health grants R01-AI079175 (J.F.M.)
Footnotes
Author contribution statement
I.P. acquisition of the data or the analysis and interpretation of such information, writing the article or substantial involvement in its revision prior to submission.
M.R.P. acquisition of the data or the analysis and interpretation, writing the article or substantial involvement in its revision prior to submission.
P.D.S. acquisition of the data or the analysis and design of the study.
J.B.O. acquisition of the data or the analysis and design of the study.
J.F.M. acquisition of the data or the analysis and interpretation of such information, writing the article or substantial involvement in its revision prior to submission, involvement in the conception, hypotheses delineation, and design of the study.
Conflict of interest Disclosure
The material in this manuscript has not been previously reported, nor is under consideration for publication elsewhere. None of the authors have a conflicting financial interest to disclose.
REFERENCES
- 1.Fishman JA. Infection in solid-organ transplant recipients. The New England journal of medicine. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 2.Ljungman P. Beta-herpesvirus challenges in the transplant recipient. The Journal of infectious diseases. 2002;186(Suppl 1):S99–S109. doi: 10.1086/342962. [DOI] [PubMed] [Google Scholar]
- 3.Zamora MR. Cytomegalovirus and lung transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2004;4:1219–1226. doi: 10.1111/j.1600-6143.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 4.Balthesen M, Messerle M, Reddehase MJ. Lungs are a major organ site of cytomegalovirus latency and recurrence. Journal of virology. 1993;67:5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusen RD, Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Kirk R, Lund LH, Rahmel AO, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report--2013; focus theme: age. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2013;32:965–978. doi: 10.1016/j.healun.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Snyder LD, Finlen-Copeland CA, Turbyfill WJ, Howell D, Willner DA, Palmer SM. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. American journal of respiratory and critical care medicine. 2010;181:1391–1396. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 8.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation. 2003;75:2064–2068. doi: 10.1097/01.TP.0000069234.04901.A3. [DOI] [PubMed] [Google Scholar]
- 9.Kerschner H, Jaksch P, Karigl G, Popow-Kraupp T, Klepetko W, Puchhammer-Stockl E. Cytomegalovirus DNA load patterns developing after lung transplantation are significantly correlated with long-term patient survival. Transplantation. 2009;87:1720–1726. doi: 10.1097/TP.0b013e3181a60b4e. [DOI] [PubMed] [Google Scholar]
- 10.Pipeling MR, John ER, Orens JB, Lechtzin N, McDyer JF. Primary cytomegalovirus phosphoprotein 65-specific CD8+ T-cell responses and T-bet levels predict immune control during early chronic infection in lung transplant recipients. The Journal of infectious diseases. 2011;204:1663–1671. doi: 10.1093/infdis/jir624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. The Journal of experimental medicine. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nature immunology. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 13.Hertoghs KM, Moerland PD, van Stijn A, Remmerswaal EB, Yong SL, van de Berg PJ, van Ham SM, Baas F, ten Berge IJ, van Lier RA. Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. The Journal of clinical investigation. 2010;120:4077–4090. doi: 10.1172/JCI42758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. Journal of virology. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nature immunology. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 16.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods in cell biology. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 17.Sukdolak C, Tischer S, Dieks D, Figueiredo C, Goudeva L, Heuft HG, Verboom M, Immenschuh S, Heim A, Borchers S, Mischak-Weissinger E, Blasczyk R, Maecker-Kolhoff B, Eiz-Vesper B. CMV−, EBV− and ADV− specific T cell immunity: screening and monitoring of potential third-party donors to improve post-transplantation outcome. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2013;19:1480–1492. doi: 10.1016/j.bbmt.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Peretz Y, He Z, Shi Y, Yassine-Diab B, Goulet JP, Bordi R, Filali-Mouhim A, Loubert JB, El-Far M, Dupuy FP, Boulassel MR, Tremblay C, Routy JP, Bernard N, Balderas R, Haddad EK, Sekaly RP. CD160 and PD-1 co-expression on HIV-specific CD8 T cells defines a subset with advanced dysfunction. PLoS pathogens. 2012;8:e1002840. doi: 10.1371/journal.ppat.1002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith C, Elhassen D, Gras S, Wynn KK, Dasari V, Tellam J, Tey SK, Rehan S, Liu YC, Rossjohn J, Burrows SR, Khanna R. Endogenous antigen presentation impacts on T-box transcription factor expression and functional maturation of CD8+ T cells. Blood. 2012 doi: 10.1182/blood-2012-03-420182. [DOI] [PubMed] [Google Scholar]
- 20.Hersperger AR, Martin JN, Shin LY, Sheth PM, Kovacs CM, Cosma GL, Makedonas G, Pereyra F, Walker BD, Kaul R, Deeks SG, Betts MR. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood. 2011;117:3799–3808. doi: 10.1182/blood-2010-12-322727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pipeling MR, West EE, Osborne CM, Whitlock AB, Dropulic LK, Willett MH, Forman M, Valsamakis A, Orens JB, Moller DR, Lechtzin N, Migueles SA, Connors M, McDyer JF. Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection. J Immunol. 2008;181:546–556. doi: 10.4049/jimmunol.181.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagannathan P,C, Osborne M, Royce C, Manion MM, Tilton JC, Li L, Fischer S, Hallahan CW, Metcalf JA, McLaughlin M, Pipeling M, McDyer JF, Manley TJ, Meier JL, Altman JD, Hertel L, Davey RT, Jr., Connors M, Migueles SA. Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. Journal of virology. 2009;83:2728–2742. doi: 10.1128/JVI.02128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paley MA, Wherry EJ. TCF-1 flips the switch on Eomes. Immunity. 2010;33:145–147. doi: 10.1016/j.immuni.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nature immunology. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 28.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu S, Connors M. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature immunology. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 30.McNeil AC, Shupert WL, Iyasere CA, Hallahan CW, Mican JA, Davey RT, Jr., Connors M. High-level HIV-1 viremia suppresses viral antigen-specific CD4(+) T cell proliferation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:13878–13883. doi: 10.1073/pnas.251539598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whittle HC, Dossetor J, Oduloju A, Bryceson AD, Greenwood BM. Cell-mediated immunity during natural measles infection. The Journal of clinical investigation. 1978;62:678–684. doi: 10.1172/JCI109175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boni C, Bertoletti A, Penna A, Cavalli A, Pilli M, Urbani S, Scognamiglio P, Boehme R, Panebianco R, Fiaccadori F, Ferrari C. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. The Journal of clinical investigation. 1998;102:968–975. doi: 10.1172/JCI3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathew A, Kurane I, Green S, Vaughn DW, Kalayanarooj S, Suntayakorn S, Ennis FA, Rothman AL. Impaired T cell proliferation in acute dengue infection. J Immunol. 1999;162:5609–5615. [PubMed] [Google Scholar]
- 34.Carney WP, Hirsch MS. Mechanisms of immunosuppression in cytomegalovirus mononucleosis. II. Virus-monocyte interactions. The Journal of infectious diseases. 1981;144:47–54. doi: 10.1093/infdis/144.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Jones TR, Wiertz EJ, Sun L, Fish KN, Nelson JA, Ploegh HL. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11327–11333. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kavanagh DG, Koszinowski UH, Hill AB. The murine cytomegalovirus immune evasion protein m4/gp34 forms biochemically distinct complexes with class I MHC at the cell surface and in a pre-Golgi compartment. J Immunol. 2001;167:3894–3902. doi: 10.4049/jimmunol.167.7.3894. [DOI] [PubMed] [Google Scholar]
- 37.Yewdell JW, Hill AB. Viral interference with antigen presentation. Nature immunology. 2002;3:1019–1025. doi: 10.1038/ni1102-1019. [DOI] [PubMed] [Google Scholar]
- 38.Tilton JC, Luskin MR, Johnson AJ, Manion M, Hallahan CW, Metcalf JA, McLaughlin M, Davey RT, Jr., Connors M. Changes in paracrine interleukin-2 requirement, CCR7 expression, frequency, and cytokine secretion of human immunodeficiency virus-specific CD4+ T cells are a consequence of antigen load. Journal of virology. 2007;81:2713–2725. doi: 10.1128/JVI.01830-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 40.Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, Kennedy-Nasser AA, Leung KS, Gee AP, Krance RA, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ, Gee AP, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nature medicine. 2006;12:1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 42.Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, Mohty M, Or R, Maschan M, Schumm M, Hamprecht K, Handgretinger R, Lang P, Einsele H. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116:4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 43.Bollard CM, Gottschalk S, Torrano V, Diouf O, Ku S, Hazrat Y, Carrum G, Ramos C, Fayad L, Shpall EJ, Pro B, Liu H, Wu MF, Lee D, Sheehan AM, Zu Y, Gee AP, Brenner MK, Heslop HE, Rooney CM. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:798–808. doi: 10.1200/JCO.2013.51.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limaye AP, Raghu G, Koelle DM, Ferrenberg J, Huang ML, Boeckh M. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. The Journal of infectious diseases. 2002;185:20–27. doi: 10.1086/338143. [DOI] [PubMed] [Google Scholar]
- 45.Eid AJ, Arthurs SK, Deziel PJ, Wilhelm MP, Razonable RR. Emergence of drug-resistant cytomegalovirus in the era of valganciclovir prophylaxis: therapeutic implications and outcomes. Clinical transplantation. 2008;22:162–170. doi: 10.1111/j.1399-0012.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 46.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. The Journal of experimental medicine. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan N, Bruton R, Taylor GS, Cobbold M, Jones TR, Rickinson AB, Moss PA. Identification of cytomegalovirus-specific cytotoxic T lymphocytes in vitro is greatly enhanced by the use of recombinant virus lacking the US2 to US11 region or modified vaccinia virus Ankara expressing individual viral genes. Journal of virology. 2005;79:2869–2879. doi: 10.1128/JVI.79.5.2869-2879.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.