Abstract
Primary squamous cell carcinoma of the vagina is an uncommon disease that often exhibits few symptoms before reaching an advanced stage. Topical intravaginal therapies for resolving precancerous and cancerous vaginal lesions have the potential to be non-invasive and safer alternatives to existing treatment options. Two factors limit the testing of this approach: lack of a preclinical intravaginal tumor model and absence of safe and effective topical delivery systems. In this study, we present both an inducible genetic model of vaginal squamous cell carcinoma in mice and a novel topical delivery system. Tumors were generated via activation of oncogenic K-Ras and inactivation of tumor suppressor Pten in LSL-K-RasG12D/+ PtenloxP/loxP mice. This was accomplished by exposing the vaginal epithelium to a recombinant adenoviral vector expressing Cre recombinase (AdCre). As early as 3 weeks after AdCre exposure exophytic masses protruding from the vagina were observed; these were confirmed to be squamous cell carcinoma by histology. We utilized this model to investigate an anticancer therapy based on poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with camptothecin (CPT); our earlier work has shown that PLGA nanoparticles can penetrate the vaginal epithelium and provide sustained CPT release. Particles were lavaged into the vaginal cavity of AdCre-infected mice. None of the mice receiving CPT nanoparticles developed tumors. These results demonstrate a novel topical strategy to resolve precancerous and cancerous lesions in the female reproductive tract.
Keywords: Drug delivery, Polymer nanoparticles, Inducible tumor model, Vaginal squamous cell carcinoma, Camptothecin, KRAS, PTEN
Introduction
Primary vaginal cancer occurs predominantly in post-menopausal women over the age of 60 and accounts for less than 2% of gynecological cancers diagnosed [1, 2]. In the USA in 2010, approximately 780 women died from vaginal cancer and 2,300 new cases were diagnosed [3, 4]. Squamous cell carcinoma, which arises from the epithelial lining of the vaginal tract, accounts for 70–95% of primary vaginal tumors [5]. Adenocarcinoma, sarcoma, and melanoma originating in the vagina occur at a much lower rate. Due to the overall low frequency of vaginal cancer, research into new treatment options is slow and traditional treatment regimes dominate.
Treatment options for primary squamous cell carcinoma of the vagina (SCCVa) depend on the progression of the disease. Most SCCVa begin as a precancerous vaginal intraepithelial neoplasia (VaIN), which can be detected as an abnormality on a pap smear and confirmed by a colposcopy [6]. VaIN and primary vaginal cancer are often asymptomatic until the tumor reaches an advanced stage: only 25% of cases are diagnosed at stage I or II, while the rest are diagnosed at stage III or IV [7]. Treatments for VAIN include laser ablation and topical therapy, neither of which are effective against an invasive tumor [6]. Once cancer has developed, treatments include irradiation (both internal and external) and surgical removal of the cancerous tissue. Unfortunately, curative surgical removal of SCCVa—in which cancerous tissue plus a margin of healthy tissue are resected—is not possible for deeply invasive tumors due to the vagina’s proximity to vital organs and tissues [4]. Systemic chemotherapy is relatively ineffective at treating intravaginal tumors, thus it is used only in combination with surgery or irradiation [6]. Even then, it is avoided in most cases because of the high risk of side-effects of chemotherapy for the elderly patient population [1]. Internal radiation therapy (i.e., brachytherapy) is a widely used alternative; however, a review of 193 cases of SCCVa between 1970 and 2000 found that, despite the reduced risk of side-effects from internal radiation therapy, few patients were treated solely by this method because externally applied radiation is far more efficacious [1]. Among these cases, the rate of major complications due to external radiation therapy ranged from 4% to 21% and included proctitis, fistula, and small-bowel obstruction.
New methods allowing non-invasive localized delivery of cancer therapies in the vagina represent an alternative to traditional systemic approaches. Topical delivery might reduce the need for external radiation therapy or surgery and improve quality of life. To be successful, this approach must be both safe and effective. Some topical treatments exist for VaIN, but they are not approved for SCCVa. For example, imiquimod 5% cream has been used successfully to treat VaIN associated with human papillomavirus (HPV), clearing the VaIN in a majority of patients with low-grade neoplasia, and reducing disease severity in most high-grade cases [8, 9]. The treatment has been well tolerated with few side-effects; however, a large number of SCCVa cases are not associated with HPV. Imiquimod has been used to treat cutaneous squamous cell carcinoma, with mixed results, but it is currently not approved for treatment of SCCVa and there is no guarantee of efficacy against a solid tumor in this environment [10, 11]. VaIN, but not SCCVa, has also been treated successfully with a topical formulation of the chemotherapeutic 5-fluorouracil (5-FU). Unfortunately, the side-effects were severe: even once-weekly application of a 5% 5-FU cream over the course of 10 weeks caused vaginal discharge and bleeding in a majority of cases. More severe side-effects were also observed, including acute ulceration, in a significant number of cases [12, 13]. In summary, there is a clinical need for topical SCCVa treatments that can efficiently deliver anticancer drugs to tumor cells with minimal complications.
The lack of preclinical models that accurately reflect human disease is a barrier to the development of new approaches for chemotherapy of vaginal tumors. In the case of cervical cancer, subcutaneous implantations of tumor cells expressing oncogenic HPV proteins are most common [14, 15]. While an ectopic tumor model has some value in studying systemic and intratumoral therapies, it does not permit evaluation of local delivery in the context of the tissue of origin. A later study attempted to overcome this problem in a cervical cancer model by seeding primary murine lung epithelial cells—which had been transfected ex vivo to express HPVoncogenes—into the chemically disrupted vaginal epithelium of a mouse [16]. But a limitation of this model—and all other ectopic models—is that the tumor is generated from non-native cells, and in some cases requires an immune compromised animal. Recently, transgenic mouse models have been developed as an alternative method to study cancers of the female reproductive tract. In one model of endometrial cancer, transgenic mice with floxed p53 and Pten tumor suppressor genes were generated that also expressed Cre recombinase under the control of the progesterone-receptor promoter [17]. These mice developed endometrial cancer, but were at risk of developing other lesions that would confound the study, because the progesterone receptor is expressed in other tissues such as the ovary, mammary gland, and pituitary [18]. A modification of this system allowed for temporal and site-specific expression of Cre recombinase in loxP-Stop-loxP(LSL)-K-RasG12D/+ PtenloxP/loxP transgenic mice [19]. Cre recombinase was delivered by an adenoviral vector (AdCre), and when expressed induced a loxP-driven inactivating deletion within Pten and removal of a stop cassette blocking transcription of the oncogenic K-RasG12D allele. Since the virus was delivered locally, by injection directly into the ovaries, tumors developed only at the local site. While these transgenic models have substantially increased our understanding of cancer progression, there have been no studies yet of models for evaluating cancers localized to and arising from the vaginal epithelium.
In this present work, we sought: (1) to establish a relevant animal model for intravaginal tumors that reflects the biological characteristics and anatomical localization of human disease; and (2) to evaluate a novel nanoparticle-based approach to treating or preventing tumors. We induced vaginal tumor growth by exposing the vaginal epithelium of LSL-K-RasG12D/+ PtenloxP/loxP mice to a recombinant adenoviral vector expressing Cre recombinase, which causes expression of oncogenic K-Ras and inactivation of tumor suppressor Pten. We then treated the mice intravaginally with PLGA nanoparticles encapsulating camptothecin (CPT) to test the ability of the particles to arrest tumor growth. CPT is a topoisomerase I inhibitor with proven anticancer properties but extremely poor water solubility [20, 21] and instability that arises from lactone ring opening at physiologic pH [22, 23]. We know from past work that incorporation of CPT into polymeric nanoparticles preserves the lactone form and improves delivery to cancer cells [24–26]. Furthermore, poly(lactic-co-glycolic acid) (PLGA) nanoparticles have already proven effective in crossing the vaginal mucosa and penetrating the epithelial layer [27]. We tested the hypothesis that delivery in nanoparticles improves the effectiveness of topically administered CPT.
Materials and methods
Intravaginal tumor model
All animal procedures were approved by the Institutional Animal Care and Use Committee at Yale University. C;129S4-Ptentm1Hwu/J (PtenloxP/loxP) transgenic mice were purchased from Jackson Laboratory (Bar Harbor, ME). These mice have loxP sites flanking exon 5 of the Pten tumor suppressor gene. In the presence of Cre recombinase Pten is inactivated. Strain 01XJ6/LSL-K-RasG12D transgenic mice were obtained from the NCI-Frederick Mouse Repository. LoxP-Stop-LoxP K-ras (LSL-K-RasG12D/+) mice carry a latent point mutant allele of K-Ras that requires Cre-mediated recombination for expression of the oncogenic protein. PtenloxP/loxP mice were crossed with LSL-KrasG12D/+ mice to generate LSL-KrasG12D/+ PtenloxP/loxP progenies. Upon exposure to Cre recombinase, these mice undergo Pten inactivation and K-RasG12D activation.
Genotype was confirmed by PCR analysis of tail DNA. Primers for Pten mutant and wild type: forward 5′ ACT CAA GGC AGG GAT GAG C 3′ and reverse 5′ GCC CCG ATG CAA TAA ATA TG 3′, where the forward primer is intronic and just upstream of the 5′ loxP site, and the reverse primer is at nt 1487–1468. Primers for K-Ras: wild type forward 5′ GTC GAC AAG CTC ATG CGG G 3′; mutant forward 5′ CCATGG CTT GAG TAA GTC TGC 3′; and common reverse 5′ CGC AGA CTG TAG AGC AGC G 3′ [28–30]. The mice were maintained on 2018S Teklad Global 18% Protein Rodent Diet from Harlan Laboratories.
Female mice were injected subcutaneously in the neck scruff with Depo-Provera (medroxyprogesterone acetate, Pfizer) at 2 mg per mouse 5 days prior to infection. Recombinant adenovirus with a cytomegalovirus promoter expressing Cre recombinase (AdCre) or β-galactosidase (Adβ-gal) were obtained from the University of Iowa Gene Transfer Vector Core. The vaginal lumen of each mouse was swabbed with calcium alginate prior to lavage of 10 µl AdCre or Adβ-gal (2.5×1010 pfu/ml in MEM with 20 mM CaCl2) with a micropipette tip. Six weeks after infection mice were euthanized and tissue prepared for histopathological analysis.
Necropsy and histopathology
Mice were euthanized by CO2 asphyxiation. Uteri and vaginas were harvested and fixed in 10% neutral buffered formalin or Bouin’s fixative (Ricca Chemical Corporation, Arlington TX), processed, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin and Masson’s trichrome (MT) by routine methods. Tissues were examined grossly and by light microscopy on an Axio Imager.A1 (Zeiss, Germany) for the macroscopic and microscopic presence of vaginal and uterine tumors, inflammation, and necrosis with the reviewer blind to genetic and experimental manipulation. Digital light microscopic images were taken on a Zeiss Axioskop microscope, AxioCam MRc5 camera, and AxioVision 4.7.1 imaging software (Carl Zeiss Micro Imaging, Inc. Thornwood, NY). Images were optimized in Adobe® Photoshop® CS5 (Adobe Systems Incorporated, San Jose, California).
PCR analysis of Cre-mediated recombination
DNAwas isolated from the reproductive tract of mice using DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). K-Ras recombination was determined using the following primers: forward 5′ GGG TAG GTG TTG GGATAG CTG 3′ and reverse 5′ TCC GAA TTC AGT GAC TAC AGA TGT ACA GAG 3′. Positive K-Ras recombination resulted in a 315-bp band to accompany the 285-bp wild-type band. Recombination of Pten was confirmed by the presence of a 300-bp band using the following primers: forward 5′ ACT CAA GGC AGG GAT GAG C 3′ and reverse 5′ GCT TGA TAT CGA ATT CCT GCA GC 3′ [29].
Particle fabrication
Nanoparticles were fabricated by a single emulsion oil in water method as previously described [26]. Briefly, 100 mg of PLGA (PLGA 50:50, 0.58 dL/g, Lactel) and 10 mg of CPT (Sigma) were dissolved in 2 mL of dichloromethane (J.T. Baker). Blank particles were produced identically, but without the addition of CPT. The polymer/drug mixture was vortexed, added dropwise to 4 mL of 2.5% polyvinyl alcohol (PVA, Sigma), then sonicated on ice three times for 10 s each at an amplitude of 38% (Tekmar Sonic Disruptor, 600 W). The mixture was added to a 50-mL stirring solution of 0.3% PVA, and dichloromethane was allowed to evaporate over 3 h. The nanoparticles were collected by centrifugation at 16,100 relative centrifugal force and washed twice with sterile de-ionized water. Lastly, the particles were suspended in 5 mL water, frozen at −80°C overnight, and lyophilized. Following lyophilization nanoparticles were stored at −20°C under desiccation. All solutions used for particle fabrication were prepared from endotoxin-free materials. Instruments and glassware were rendered free of endotoxin by baking at 250°C for at least 4 h or by treating with PyroClean™ (Alerchek, Inc.). Particles were prepared aseptically.
Particle characterization
Nanoparticle size and morphology were characterized by scanning electron microscopy (SEM). Samples were mounted on carbon tape and sputter-coated with gold under vacuum in an argon atmosphere using a current of 40 mA (Sputter Coater 180auto, Cressington). SEM analysis was carried out using a XL-30 ESEM-FEG (FEI Company) with an acceleration voltage of 5 kV. Particle size was determined by analysis of three representative SEM images per batch using ImageJ software (NIH, Bethesda, MD).
To determine the concentration of CPT loaded within the nanoparticles, 3.5 mg of nanoparticles were dissolved in dimethyl sulfoxide (DMSO, J.T. Baker). A standard curve was created by dissolving CPT in DMSO. Samples and standards were diluted 1:100 in phosphate-buffered saline (PBS) solution containing 0.2% sodium dodecyl sulfate (SDS, Sigma) and 0.01 N hydrochloric acid in PBS. Fluorescence of CPT was read using a plate reader (370 nm excitation, 428 nm emission). Concentration of CPT was determined by linear interpolation from a standard curve of known concentrations.
Release of CPT from nanoparticles
Five milligrams of particles were suspended in 1 mL PBS, pH 7.4 and injected into a dialysis cassette (MWCO 10,000, Pierce). To ensure sink conditions the cassette was placed in 199 mL PBS and incubated at 37°C on a shaker platform. Supernatant samples were taken at various time points over a period of 4 weeks. Samples were prepared for analysis by adding 30 µL of a 1:1:1 DMSO/1 N HCl/SDS (10%) solution to 970 µL sample. The quantity of CPT released into the supernatant was determined using fluorescence, by comparison to a standard curve, as described above.
Treatment of intravaginal tumor
Vaginal tumorigenesis was induced by intravaginal lavage with AdCre in LSL-K-RasG12D/+ PtenloxP/loxP mice as described above. One week after viral infection mice received treatment via intravaginal lavage of free CPT (10 µL at 1.3 mg/mL in PBS with 10% DMSO and 5% Tween 80 (w/v), ~13 µg CPT), buffer only, blank nanoparticles (2 mg particles in 10 µL PBS), or nanoparticles loaded with CPT (2 mg particles in 10 µL PBS, ~120 µg CPT). Due to the poor aqueous solubility of CPT, the free CPT solution was made by first dissolving CPT in DMSO then gradually adding the other buffer components while alternating vortexing with water sonicating. We are able to achieve a solution of 1.3 mg/ml CPT. A precipitate does not form from this solution, but previous work in our lab has shown that this is at or close to the maximum concentration CPT can achieve in aqueous solution. Animals received weekly treatments for a period of 5 weeks. Six weeks post AdCre infection mice were euthanized for tissue histological analysis, which was performed as described above.
Results
Induction of endogenous intravaginal tumors
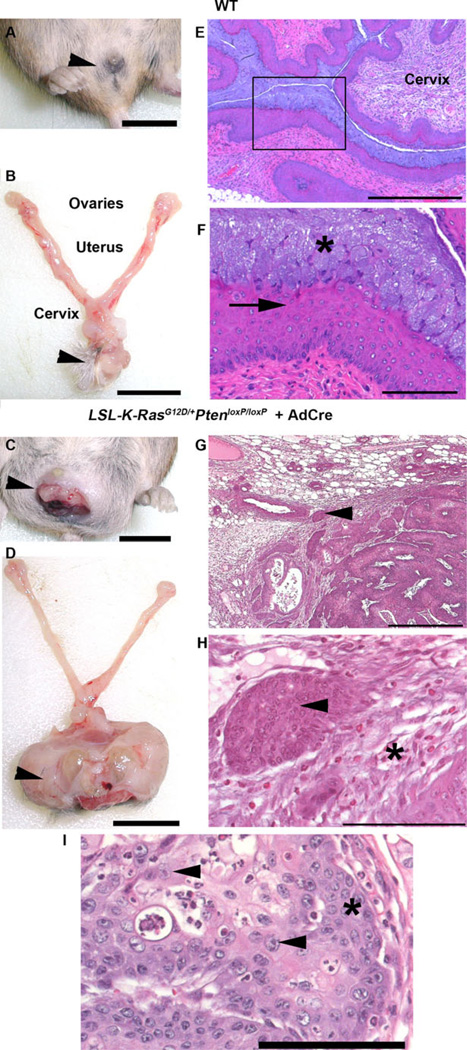
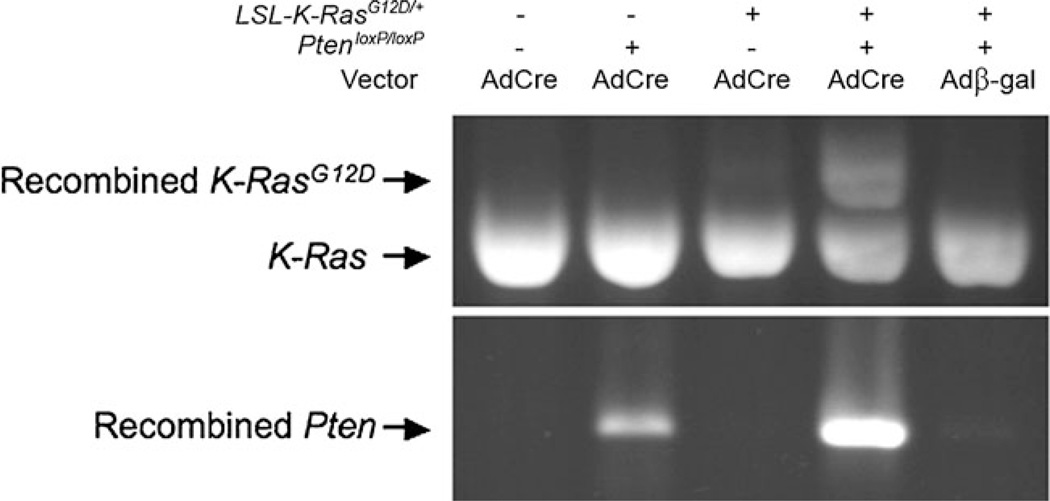
Female transgenic LSL-K-RasG12D/+ PtenloxP/loxP mice, which had been treated with Depo-Provera to induce the diestrus phase of the reproductive cycle, were given intravaginal AdCre, resulting in local activation of oncogenic K-Ras and inactivation of tumor suppressor Pten. Gross and microscopic examination of the vulva (Fig. 1a, b, arrowheads) and reproductive tracts (Fig. 1b) from wild-type (WT) mice were unremarkable. Histopathology of WT vagina (Fig. 1e, f) revealed the expected keratinized stratified squamous epithelium (Fig. 1f, *) with the distinct superficial layer of tall columnar vacuolated epithelial cells (arrow) which slough into the lumen [31]. In contrast over half of the LSL-K-RasG12D/+ PtenloxP/loxP mice that received AdCre developed gross or microscopic evidence of vaginal tumors (Fig. 1c, d, arrowheads). Exophytic tumor-like masses were observed protruding from the vaginal canal of some infected mice as early as 3 weeks post infection (Fig. 1c). Six weeks post infection masses were identified that ranged from microscopic to approximately 2.5 cm3 in volume (Table 1). In all cases, microscopic examination of the vaginal masses confirmed them to be squamous cell carcinoma (Fig. 1g, h). Tumors were characterized by invasive nests and cords of pleomorphic, disorganized, malignant squamous cells (Fig. 1g, h, arrows) surrounded by a desmoplastic fibrous response (Fig. 1h, *) with prominent nucleoli (Fig. 1i, arrowheads) and lack of orderly maturation (Fig. 1i, *). Only one tumor was observed in PtenloxP/loxPmice treated with AdCre, and no tumors developed in the following groups: K-RasG12D or wild-type mice exposed to AdCre, and LSL-K-RasG12D/+ PtenloxP/loxP mice exposed to the control vector Adβ-gal (Table 1). The frequency of tumor development in LSL-K-RasG12D/+ PtenloxP/loxP mice infected with AdCre is statistically significant when compared with AdCre-infected WT, PtenloxP/loxP or K-RasG12D mice (p= 0.023, 0.046, and 0.012, respectively, by Fisher’s exact test). PCR analysis of DNA isolated from reproductive tissue confirmed Cre-mediated recombination of the oncogenic K-RasG12D allele and conditional deletion within Pten (Fig. 2). These data suggest that both recombination events are necessary for intravaginal tumorigenesis, as oncogenic K-RasG12D activation or Pten inactivation alone were not able to generate tumors in the vaginal tract in a vast majority of cases.
Fig. 1.
Vaginal squamous cell carcinoma was induced by activation of KrasG12D and inacti-vation of Pten. In contrast to WT mice with a normal small vulva (a, barrowheads), grossly normal reproductive tract (b), and microscopically normal vagina (e) with stratified squamous epithelium (arrow) covered by a mucosal epithelial layer (f, *), a single intravaginal lavage of AdCre in LSL-K-RasG12D/+PtenloxP/loxP mice resulted in an exophytic vaginal tumor (c, darrowhead) 6 weeks post infection. Histopathologic evaluation of tumors revealed invasion of soft tissue by nests and chords of small, tightly packed malignant squamous cells (g, harrowheads) bordered by a region of demosplastic fibrous connective tissue (h, *). Neo-plastic epithelial cells had prominent nucleoli (iarrowheads) and a lack of orderly growth progression (i, *). Scale barsa, b, c, d=1 cm; e, g=500 µm; f, h, i=100 µm
Table 1.
Frequency of microscopic and gross tumor development and average volume of gross tumors in genotypically different mice infected intravaginally with AdCre or Adβ-gal
| Genotype | Vector | Mice examined | Mice with gross tumor |
Mice with microscopic tumor |
Average gross tumor volume (cm3) |
|---|---|---|---|---|---|
| WT | AdCre | 7 | 0 | 0 | 0 |
| PtenloxP/loxP | AdCre | 10 | 1 | 0 | 0.03 |
| LSL-K-RasG12D/+ | AdCre | 8 | 0 | 0 | 0 |
| LSL-K-RasG12D/+ PtenloxP/loxP | AdCre | 21 | 4 | 7 | 0.4±1.1 |
| LSL-K-RasG12D/+ PtenloxP/loxP | Adβ-gal | 2 | 0 | 0 | 0 |
Fig. 2.
Cre-mediated recombination of KrasG12D and Pten was confirmed by reverse transcriptase PCR
Camptothecin-loaded nanoparticles
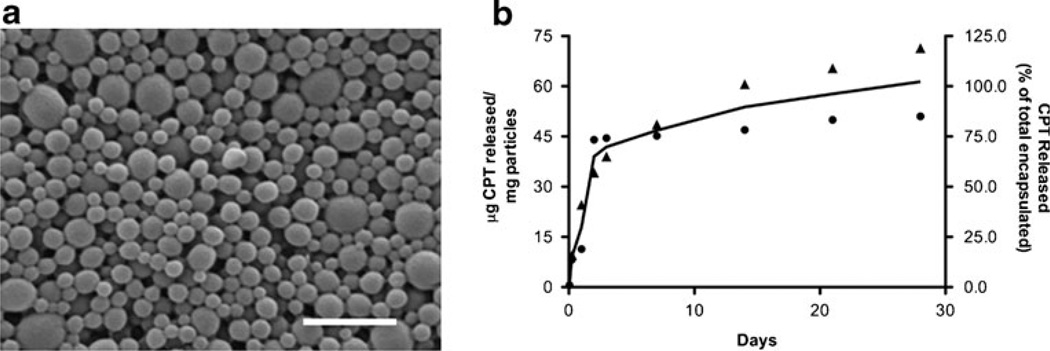
CPT-loaded nanoparticles were fabricated by the single emulsion oil in water method, resulting in a relatively monodisperse population of spherical particles sized 158± 62 nm (Fig. 3a). The amount of CPT loaded in the nanoparticles was 62±14 µg/mg. Particles incubated at physiologic conditions exhibited a burst release of CPT during the first 3 days, after which the release rate was sustained at a more moderate level for the duration of the experiment (Fig. 3b). By 28 days of incubation in vitro, nearly all CPT had been released from the particles.
Fig. 3.
Characterization of CPT-loaded nanoparticles. a A representative scanning electron micrograph of nanoparticles (scale bar=1 µm). The mean diameter of particles is 158±62 nm. b Release of CPT from nanoparticles in phosphate-buffered saline at 37°C over 28 days. Total loading of CPT is 62±14 µg/mg. A biphasic profile is observed with an initial burst release during the first 3 days followed by sustained release. Left axis represents micrograms CPT released from 1 mg of particles. Right axis represents percentage of CPT released from the total loaded within particles. Square and triangle data points represent replicate sets of data. The curve represents the average CPT release profile
Inhibition of intravaginal tumor growth with nanoparticle therapy
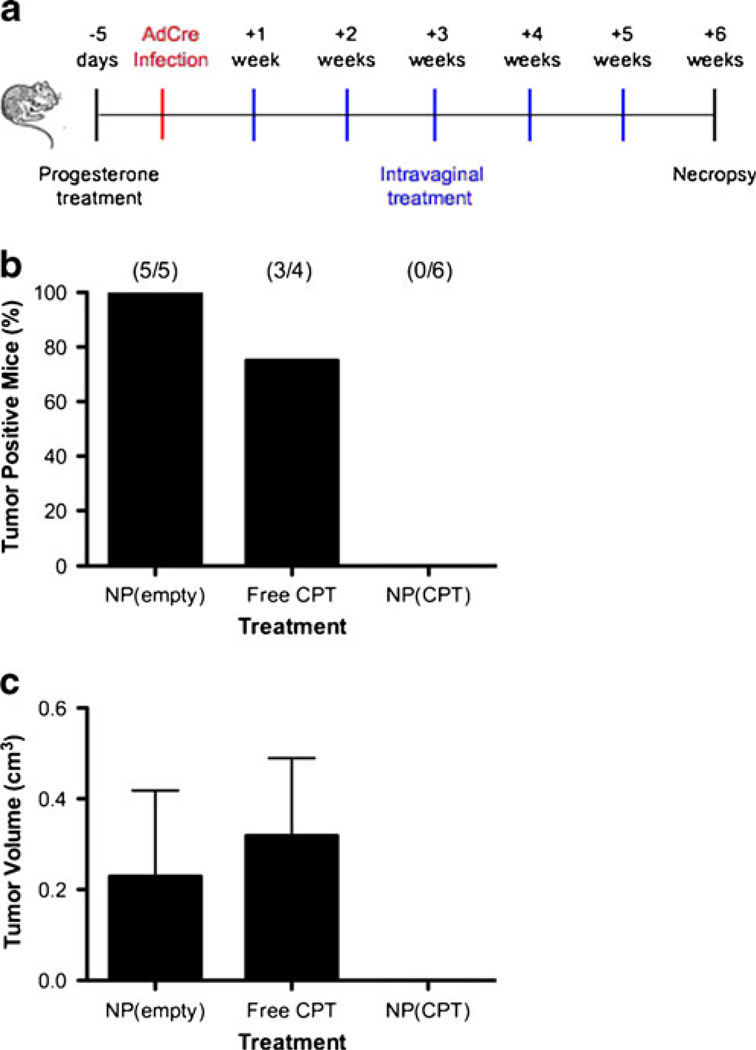
The effectiveness of topical delivery of CPTwas evaluated in LSL-K-RasG12D/+ PtenloxP/loxP mice. One week after initiation of tumorigenesis by introduction of AdCre, mice began receiving a weekly intravaginal lavage of blank nanoparticles [NP(empty)], CPT-loaded nanoparticles [NP(CPT)], or free CPT for a period of 5 weeks (Fig. 4a). None of the animals receiving CPT-loaded nanoparticles developed observable tumors (Fig. 4b); however a single mouse did develop a focus of dysplasia suspicious for early SCCVa. In contrast, 100% of the animals receiving blank particles and 75% of the animals receiving free CPT developed observable tumors. The frequency of mice in which tumor development was prevented in the NP(CPT) group is statistically significant when compared with the NP(empty) and free CPT groups (p=0.002 and 0.033, respectively, by Fisher’s exact test).
Fig. 4.
Treatment of intravaginal tumors by CPT-loaded nanoparticles. a Experimental design for evaluating nanoparticle efficacy against intra-vaginal tumors: (1) Depo-Provera was administer 5 days prior to infection; (2) animals were infected by intravaginal lavage with AdCre to induce tumors; (3) 1 week post infection animals began receiving weekly treatments with blank nanoparticles, free CPT, or CPT-loaded nanoparticles for a period of 5 weeks; (4) 6 weeks post infection animals were euthanized and evaluated for the presence of intravaginal tumors. b Number of mice per group with observable intravaginal tumors after treatment with blank nanoparticles, free CPT, or CPT-loaded nanoparticles. c Average gross tumor volume per group after treatment with blank nanoparticles, free CPT, or CPT-loaded nanoparticles. Volumes do not include mice that did not develop gross tumors
Evaluation of toxicity secondary to nanoparticle therapy
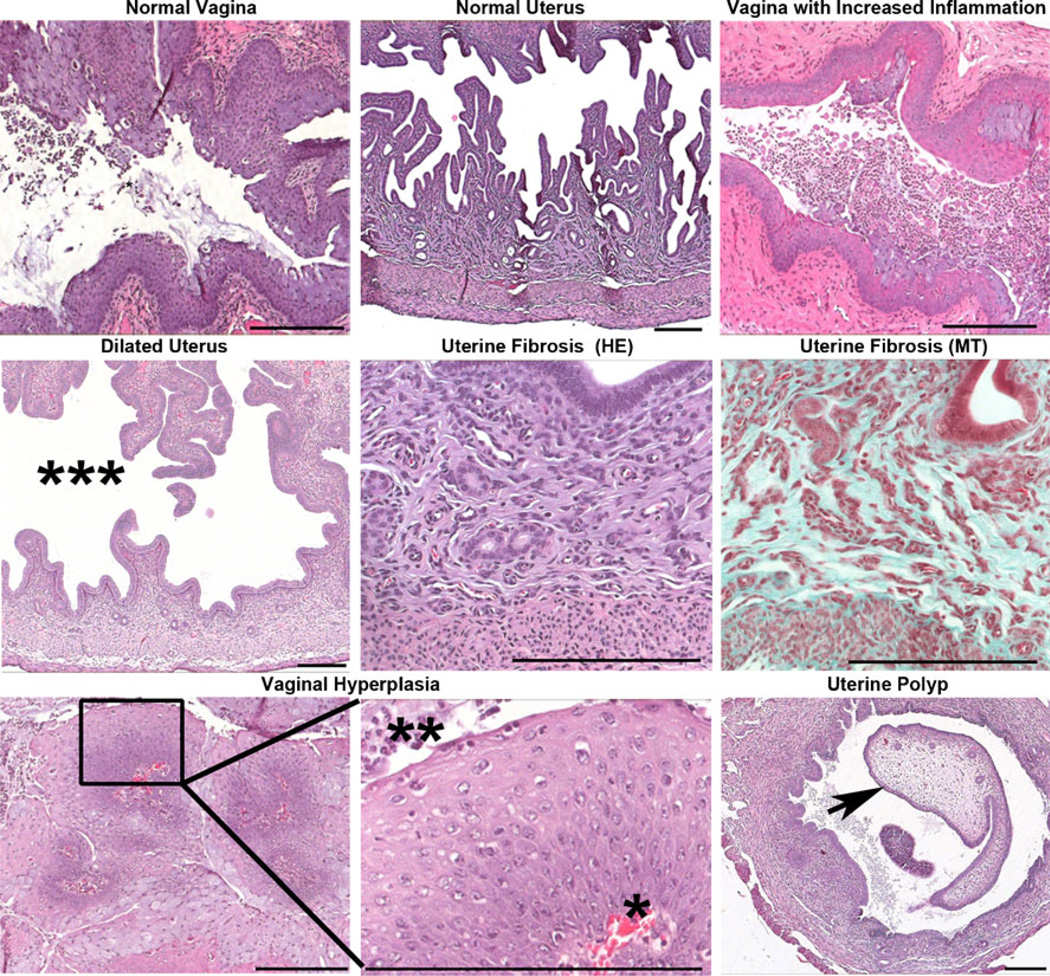
Uteri and vaginas from all experimental and control groups were evaluated for histopathologic changes associated with toxicity, including uterine atrophy, uterine fibrosis, vaginal and uterine polyps, neoplasia, cystic or endometrial hyperplasia, and inflammation beyond normal levels in the vagina and uterus, as indicated by the presence of greater numbers of inflammatory cells (Table 2, Fig. 5). Minimal to moderate numbers of neutrophils and/or eosinophils were present in the vaginas of mice infected with AdCre irrespective of other treatment modalities. LSL-K-RasG12D/+ PtenloxP/loxP mice exhibiting SCCVa tended to have a dilated uterus with minimal to mild uterine fibrosis, cystic and/or endometrial hyperplasia, and a mild increase in vaginal and minimal increase in uterine inflammation. The treatment of AdCre-infected WT mice with free CPT, NP(empty), or NP(CPT) did not result in any significant pathologic changes in the vagina when compared to untreated WT mice infected with AdCre. Additionally, there was no significant inflammation observed in WT mice treated with ether free CPT in PBS/DMSO/Tween 80 buffer or with buffer alone. Endometrial hyperplasia with and without cysts, uterine dilation, and minimal to mild uterine fibrosis are common age-related changes seen in mice and were not considered to be the result of any treatment [31].
Table 2.
Frequency of occurrence of pathological conditions of the uterus and vagina in all experimental and control groups, as determined by histopathologic analysis
| Genotype | Virus | Treatment | Average age (weeks) |
Mice examined |
Vaginal SCC |
Vaginal inflammation |
Uterine inflammation |
Uterine dilation |
Uterine fibrosis |
Cystic/endometrial hyperplasia |
|---|---|---|---|---|---|---|---|---|---|---|
| WT | AdCre | None | 17 | 7 | 0 | 4 | 4 | 4 | 3 | 0 |
| PtenloxF/loxF | AdCre | None | 13 | 10 | 1 | 7 | 1 | 9 | 5 | 1 |
| LSL-KrasG12D/+ | AdCre | None | 12 | 8a | 0 | 8 | 3 | 6 | 1 | 2 |
| LSL-KrasG12D/+ Ptenloxp/loxp | AdCre | None | 13 | 21 | 11 | 18 | 3 | 14 | 14 | 4 |
| LSL-KrasG12D/+ Ptenloxp/loxp | Adβ-gal | None | 15 | 2 | 0 | 2 | 1 | 2 | 0 | 0 |
| LSL-KrasG12D/+ Ptenloxp/loxp | AdCre | NP(empty) | 10 | 6 | 6 | 5 | 0 | 6 | 6 | 4 |
| LSL-KrasG12D/+ Ptenloxp/loxp | AdCre | NP(CPT) | 10 | 6 | 0b | 5 | 3 | 6 | 4 | 4 |
| LSL-KrasG12D/+ Ptenloxp/loxp | AdCre | Free CPT | 11 | 4 | 3 | 4 | 1 | 2 | 2 | 1 |
| LSL-KrasG12D/+ Ptenloxp/loxp | no virus | None | 16 | 2 | 0 | 1 | 0 | 2 | 0 | 0 |
| WT | AdCre | NP(CPT) | 13 | 2 | 0 | 2 | 2 | 2 | 2 | 1 |
| WT | AdCre | NP(empty) | 13 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| WT | no virus | Free CPT | Unknown | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| WT | no virus | Buffer | Unknown | 2 | 0 | 1 | 0 | 0 | 1 | 2 |
Endometrial stromal polyp
Focal hyperplasia/polyp
Fig. 5.
Representative images from histopathologic analysis of the uterus and vagina. Treatment of AdCre-infected wild-type or LSL-K-RasG12D/+ PtenloxP/loxP mice with nanoparticles did not result in pathologic changes beyond those seen in untreated AdCre-infected mice. Pathologic conditions observed after infection with AdCre included: increased vaginal inflammation, uterine dilation (***), and uterine fibrosis. Uterine fibrosis was confirmed by the presence of collagen (blue) by Masson’s trichrome (MT) staining. A single LSL-K-RasG12D/+ PtenloxP/loxP mouse infected with AdCre and treated with NP (CPT) had a focus of squamous hyperplasia with orderly progression of squamous epithelial cells from the basement membrane (*) to the surface (**) in the vagina. Additionally, a single LSL-K-RasG12D/+ mouse infected with AdCre had a uterine polyp (arrow). Scale bars 200 µm
Discussion
In this report we present a model system for studying primary squamous cell carcinoma of the vagina in vivo that is both anatomically relevant and derived from cells native to the experimental animal. We induced tumorigenesis in the vagina by treating LSL-K-RasG12D/+ PtenloxP/loxP mice with AdCre vector by intravaginal lavage, which caused activation of the oncogenic K-RasG12D allele and inactivation of Pten. This model is easily implemented and provides a means of testing new treatment regimens that include topical intravaginal formulations. In addition, this experimental approach eliminates the complications of grafting or implanting foreign cells, which may not behave as they would in their native environment, and of the need for immune compromised animals that are able to accept these cells.
Primary vaginal carcinoma is uncommon, so it has been difficult for researchers to define a set of genetic mutations that lead to this disease. Thus, it is unknown if our model is genotypically the same as naturally occurring SCCVa. It is known, however, that Pten is among the most commonly mutated tumor suppressor genes in all cancers, and occurs at a high frequency (60%) in vulvar precancerous lesions [32, 33]. Like vaginal carcinoma, the vast majority of vulvar carcinomas are of squamous cell origin [33]. In contrast, K-Ras-activating mutations are less common, but K-Ras and Pten have been used in combination in animal models of many different cancers because the effect of both mutations leads to a predictable, aggressive tumor [19, 34– 37]. Regardless of similarity to the natural genotype, the vaginal tumors developed by LSL-K-RasG12D/+ PtenloxP/loxPmice are morphologically indistinguishable from human squamous cell carcinomas of the vagina.
Two distinct types of SCCVa have been identified: those that are HPV-positive and those that are HPV-negative. Between 50% and 75% of primary vaginal carcinomas are HPV-positive, compared to almost 100% of cervical cancer cases [38, 39]. HPV-associated SCCVa is similar to cervical cancer in its protein expression profile, and typically occurs in the youngest SCCVa patients, while HPV is unassociated with SCCVa in the majority of the oldest patients [40, 41]. Our model represents an important tool for studying non-HPV vaginal cancer. Patients could greatly benefit from research into new treatments with few side-effects, as complications from treatment can be especially severe in the elderly patient population. In addition, the non-HPV-associated patient group is likely the subgroup most in need of new SCCVa treatments, because Gardasil® and other HPV vaccines could potentially reduce the frequency of HPV-associated SCCVa in the future [42].
We tested a novel local delivery method for treating intravaginal tumors. Local delivery is especially important in SCCVa, as systemic or intra-arterial chemotherapy has proven to have little benefit to survival, and is accompanied by severe, and sometimes fatal, side-effects [43]. External radiation therapy combined with brachytherapy and/or surgery is currently the best method to treat SCCVa, but this method is also associated with harsh side-effects. In addition, surgical removal of the entire tumor is not always possible because of its proximity to other vital organs [1, 4]. A locally applied topical therapy, such as polymeric nanoparticles encapsulating a therapeutic agent, may prove to be a good alternative or supplement to these procedures. PLGA nanoparticles loaded with CPT were well tolerated and were able to prevent the development of both gross and microscopic SCCVa tumors in 100% experimental animals. The free CPT treatment was effective in only one of four animals.
We evaluated the effectiveness of CPT delivery using the highest reasonable dose for each formulation (free CPT and nanoparticle CPT). Poor aqueous solubility limited the free CPT dose. Even though the highest reasonable dose of free CPT was administered, this dose was nearly tenfold lower than the total amount encapsulated in the dose of nanoparticles. Therefore, we are not yet able to determine the mechanism for enhanced effectiveness of CPT in nanoparticles, which could be due to: (1) the higher dose deliverable in the nanoparticle formulation or (2) more effective delivery of CPT in nanoparticles (on a per milligram basis). It is important to notice, however, that the total CPT released from the nanoparticles in the first hours after treatment is probably lower than the dose of free CPT delivered in PBS: the release profile of CPT from the particles (see Fig. 3b) indicates that only ~10% of encapsulated CPT is released in the first 24 h. CPT is a small hydrophobic molecule, and as such it is likely eliminated from the vagina relatively quickly by the continuous shedding of vaginal mucus [44]. In fact, prior work suggests that hydrophobic molecules interact with exposed hydrophobic domains on mucins, causing the mucins to clump and be cleared more easily than usual [45]. Additionally, it is likely that nanoparticles are more effective because they are able to diffuse through the vaginal mucus layer and penetrate the vaginal epithelium. In a previous study, PLGA nanoparticles loaded with fluorescent agents were shown to penetrate to a depth of 75 µm past the luminal border of the vaginal epithelium. Particles were also detected within the tissue at least 7 days after intravaginal instillation and at an even greater depth [27]. Particles with a low net charge and minimal exposed hydrophobic domains, such as PLGA particles, are able to more easily penetrate the mucus than completely hydrophobic particles [45–47]. This provides a potential explanation for why the PLGA CPT particles were more efficacious in our study than free CPT [45]. We speculate that nanoparticles penetrate the mucus layer, accumulate in the vaginal epithelium, and then slowly release CPT, exposing the tissue to low, constant concentrations of drug. Although this sustained release could be mimicked by more frequent dosing with free drug, we also anticipate that this would increase the risk of side-effects.
Histological evaluation has shown that the addition of nanoparticles to the vagina is nontoxic and does not result in significant pathologic changes. PLGA breaks down by hydrolysis to lactic and glycolic acid, which are natural byproducts of metabolism; importantly, the FDA has approved PLGA materials for a number of clinical applications, including particles for sustained drug delivery [48]. Nanoparticles are also easily incorporated into a topical formulation. This will require less training of the personnel responsible for administering treatment compared to other therapies such as radiation. As discussed earlier, effective topical treatments exist for some forms of VaIN, but not for SCCVa. To our knowledge, this is the first instance of nanoparticles encapsulating chemotherapeutic being used to treat primary SCCVa in a mouse model.
We realize that, because treatment of the tumors in our study occurs at a time point before tumors can be observed in untreated animals, the nanoparticles may be acting to prevent tumor development rather than to treat established tumors. This suggests that CPT-loaded nanoparticles might be useful as an approach to prevent VaIN from developing into SCCVa. More testing must be done, however, to confirm that the particles can also be used as an effective treatment for existing tumors, perhaps in combination with surgery to remove the bulk of the tumor. We are optimistic that the particles will be effective for this application due to their ability to penetrate deep into the vaginal epithelium. If successful, this treatment would greatly benefit patients with non-HPV-associated SCCVa by reducing side-effects associated with treating invasive tumors in an elderly patient population. This is significant because, as mentioned earlier, SCCVa is often not diagnosed until tumors have become invasive and current treatment methods have limited efficacy.
Conclusions
Intravaginal squamous cell carcinoma was induced in LSL-K-RasG12D/+ PtenloxP/loxP mice through lavage of the AdCre virus into the vaginal cavity. This model provides a preclinical tool for evaluating new therapies against vaginal cancers. CPT-loaded nanoparticles delivered topically to the vagina provided significant inhibition of tumor growth in this model. As a result, intravaginal nanoparticle-based therapies hold promise for treating cancers of the female reproductive tract.
Acknowledgments
The authors thank Gordon Terwilliger (Yale University, New Haven, CT) for his technical assistance in retrieving and preparing tissue samples and Dr. Daniela Dinulescu (Boston, MA) for supplying primer sequences for recombination analysis. J.S.B was supported through a National Institutes of Health postdoctoral fellowship (F32 AI072942) and I.A.B. was supported through a Gilliam Fellowship from Howard Hughes Medical Institute. This work was supported by grants to W.M.S. from the National Institutes of Health (R01 EB000487) and to F.J.S. from the James S. McDonnell Foundation.
Footnotes
Ethical standards All experiments performed in this manuscript are in compliance with the current laws of the State of Connecticut and the United States of America.
Contributor Information
Jeremy S. Blum, Department of Biomedical Engineering, Yale University, New Haven, CT 06520, USA
Caroline E. Weller, Department of Biomedical Engineering, Yale University, New Haven, CT 06520, USA
Carmen J. Booth, Section of Comparative Medicine, Yale University School of Medicine, New Haven, CT 06510, USA
Imran A. Babar, Department of Molecular, Cellular, and Developmental Biology, Yale University, New Haven, CT 06511, USA
Xianping Liang, Department of Molecular, Cellular, and Developmental Biology, Yale University, New Haven, CT 06511, USA.
Frank J. Slack, Department of Molecular, Cellular, and Developmental Biology, Yale University, New Haven, CT 06511, USA
W. Mark Saltzman, Email: mark.saltzman@yale.edu, Department of Biomedical Engineering, Yale University, New Haven, CT 06520, USA.
References
- 1.Frank S, Jhingran A, Levenback C, Eifel P. Definitive radiation therapy for squamous cell carcinoma of the vagina. International Journal of Radiation Oncology. 2005;62(1):138–147. doi: 10.1016/j.ijrobp.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Practice & Research Clinical Obstetrics & Gynaecology. 2006;20(2):207–225. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Facts and Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 4.Tjalma W. The role of surgery in invasive squamous carcinoma of the vagina. Gynecol Oncol. 2001;81(3):360–365. doi: 10.1006/gyno.2001.6171. [DOI] [PubMed] [Google Scholar]
- 5.Creasman WT, Phillips JL, Menck HR. The national cancer data base report on cancer of the vagina. Cancer. 1998;83(5):1033–1040. [PubMed] [Google Scholar]
- 6.Society AC, editor. Vaginal Cancer. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 7.Dixit S, Singhal S, Baboo HA. Squamous cell carcinoma of the vagina: a review of 70 cases. Gynecol Oncol. 1993;48:80–87. doi: 10.1006/gyno.1993.1013. [DOI] [PubMed] [Google Scholar]
- 8.Buck HW, Guth KJ. Treatment of vaginal intraepithelial neoplasia (primarily low grade) with iniquimod 5% cream. Journal of Lower Genital Tract Disease. 2003;7(3):290–293. doi: 10.1097/00128360-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Haidopoulos D, Diakomanolis E, Rodolakis A, Vlachos G, Elsheikh A, Michalas S. Safety and efficacy of locally applied imiquimod cream 5% for the treatment of condylomata acuminata of the vulva. Arch Gynecol Obstet. 2003;270(4):240–243. doi: 10.1007/s00404-003-0559-9. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Garcia RF. Imiquimod: an effective alternative for the treatment of invasive cutaneous squamous cell carcinoma. Dermatol Surg. 2005;31:371–374. doi: 10.1111/j.1524-4725.2005.31093. [DOI] [PubMed] [Google Scholar]
- 11.Ondo A, Mings S, Pestak R, Shanler S. Topical combination therapy for cutaneous squamous cell carcinoma in situ with 5-fluorouracil cream and imiquimod cream in patients who have failed topical monotherapy. J Am Acad Dermatol. 2006;55(6):1092–1094. doi: 10.1016/j.jaad.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Murta EFC, Neves Junior MA, Sempionato LRF, Costa MC, Maluf PJ. Vaginal intraepithelial neoplasia: clinical-therapeutic analysis of 33 cases. Arch Gynecol Obstet. 2005;272(4):261–264. doi: 10.1007/s00404-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 13.Krebs HB, Helmkamp BF. Chronic ulcerations following topical therapy with 5-fluorouracil for vaginal human papillomavirusassociated lesions. Obstet Gynecol. 1991;78(2):205–208. [PubMed] [Google Scholar]
- 14.Venuti A, Massa S, Mett V, Vedova LD, Paolini F, Franconi R, et al. An E7-based therapeutic vaccine protects mice against HPV16 associated cancer. Vaccine. 2009;27(25–26):3395–3397. doi: 10.1016/j.vaccine.2009.01.068. [DOI] [PubMed] [Google Scholar]
- 15.Han L, Wang W, Fang Y, Feng Z, Liao S, Li W, et al. Soluble B and T lymphocyte attenuator possesses antitumor effects and facilitates heat shock protein 70 vaccine-triggered antitumor immunity against a murine TC-1 cervical cancer model in vivo. J Immunol. 2009;183(12):7842–7850. doi: 10.4049/jimmunol.0804379. [DOI] [PubMed] [Google Scholar]
- 16.Decrausaz L, Gonçalves A-R, Domingos-Pereira S, Pythoud C, Stehle J-C, Schiller J, et al. A novel mucosal orthotopic murine model of human papillomavirus-associated genital cancers. International Journal of Cancer. 2010 doi: 10.1002/ijc.25561. [DOI] [PubMed] [Google Scholar]
- 17.Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, et al. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Research. 2008;68(14):5619–5627. doi: 10.1158/0008-5472.CAN-08-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soyal SM, Mukherjee A, Lee KYS, Li J, Li H, DeMayo FJ, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 19.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nature Medicine. 2005;11(1):63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 20.O’Leary J, Muggia FM. Camptothecins: a review of their development and schedules of administration. European Journal of Cancer. 1998;34(10):1500–1508. doi: 10.1016/s0959-8049(98)00229-9. [DOI] [PubMed] [Google Scholar]
- 21.Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. Plant antitumor agents I The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata . J Am Chem Soc. 1966;88(16):3888–3890. [Google Scholar]
- 22.Wall ME, Wani MC. Camptothecin and taxol: from discovery to clinic. J Ethnopharmacol. 1996;51:239–254. doi: 10.1016/0378-8741(95)01367-9. [DOI] [PubMed] [Google Scholar]
- 23.Slichenmyer WJ, Rowinsky EK, Donehower RC, Kaufmann SH. The current status of camptothecin analogues as antitumor agents. Journal of the National Cancer Institute. 1993;85:271–291. doi: 10.1093/jnci/85.4.271. [DOI] [PubMed] [Google Scholar]
- 24.Ertl B, Platzer P, Wirth M, Gabor F. Poly(D, L-lactic-co-glycolic acid) microspheres for sustained delivery and stabilization of camptothecin. Journal of Controlled Release. 1999;61:305–317. doi: 10.1016/s0168-3659(99)00122-4. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Jiang Z, Zhang S, Saltzman WM. Poly(ω-pentadecalactone-co-butylene-co-succinate) nanoparticles as biodegradable carriers for camptothecin delivery. Biomaterials. 2009;30(29):5707–5719. doi: 10.1016/j.biomaterials.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawyer AJ, Saucier-Sawyer JK, Booth CJ, Liu J, Patel T, Piepmeier JM, et al. Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors. Drug Delivery and Translational Research. 2010;1(1):34–42. doi: 10.1007/s13346-010-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Mark Saltzman W. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8(6):526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson EL. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes & Development. 2001;15(24):3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32(2):148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- 30.Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, et al. Endogenous oncogenic K-rasG12D stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 31.Davis BJ, Dixon D, Herbet RA. Pathology of the Mouse. Vienna: Cache River Press; 1999. [Google Scholar]
- 32.Leslie NR, Downes CP. PTEN function: how normal cells control it and tumour cells lose it. Biochem J. 2004;382:1–11. doi: 10.1042/BJ20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elmasry K, Gayther S. Genetic mutations in gynaecological cancers. Reviews in Gynaecological and Perinatal Practice. 2006;6(3–4):115–125. [Google Scholar]
- 34.Kim TH, Wang J, Lee KY, Franco HL, Broaddus RR, Lydon JP, et al. The synergistic effect of conditional Pten loss and oncogenic K-ras mutation on endometrial cancer development occurs via decreased progesterone receptor action. Journal of Oncology. 2010;2010:1–10. doi: 10.1155/2010/139087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, et al. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Research. 2010;70(18):7114–7124. doi: 10.1158/0008-5472.CAN-10-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Najbauer J, Yang Y, Iwanaga K, Raso MG, Wislez M, Hanna AE, et al. Phosphatidylinositol 3-kinase mediates bronchioalveolar stem cell expansion in mouse models of oncogenic K-ras-induced lung cancer. PLoS ONE. 2008;3(5):e2220. doi: 10.1371/journal.pone.0002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwanaga K, Yang Y, Raso MG, Ma L, Hanna AE, Thilaganathan N, et al. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Research. 2008;68(4):1119–1127. doi: 10.1158/0008-5472.CAN-07-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hildesheim A, Han CL, Brinton LA, Nasca PC, Richart RM, Jones RB, et al. Sexually transmitted agents and risk of carcinoma of the vagina. International Journal of Gynocologic Cancer. 1997;7:251–255. [Google Scholar]
- 39.Fuste V, del Pino M, Perez A, Garcia A, Torne A, Pahisa J, et al. Primary squamous cell carcinoma of the vagina: human papillomavirus detection, p16INK4A overexpression and clinicopathological correlations. Histopathology. 2010;57(6):907–916. doi: 10.1111/j.1365-2559.2010.03727.x. [DOI] [PubMed] [Google Scholar]
- 40.Hellman K, Alaiya AA, Schedvins K, Steinberg W, Hellström AC, Auer G. Protein expression patterns in primary carcinoma of the vagina. British Journal of Cancer. 2004 doi: 10.1038/sj.bjc.6601944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hellman K, Silfversward C, Nilsson B, Hellstrom AC, Frankendal B, Pattersson F. Primary carcinoma of the vagina: factors influencing the age at diagnosis The Radiumhemmet series 1956—96. International Journal of Gynocologic Cancer. 2004;14:491–501. doi: 10.1111/j.1048-891x.2004.014310.x. [DOI] [PubMed] [Google Scholar]
- 42.Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intra-epithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010;340:c3493. doi: 10.1136/bmj.c3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patton TJ, Kavanagh JJ, Delclos L, Wallace S, Haynie TP, Wharton T, et al. Five-year survival in patients given intra-arterial chemotherapy prior to radiotherapy for advanced squamous carcinoma of the cervix and vagina. Gynecol Oncol. 1991;42:54–59. doi: 10.1016/0090-8258(91)90230-3. [DOI] [PubMed] [Google Scholar]
- 44.Cone RA. Barrier properties of mucus. Advanced Drug Delivery Reviews. 2009;61(2):75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Lai SK, Wang YY, Hida K, Cone R, Hanes J. Nanoparticles reveal that human cervicovaginal mucus is riddled with pores larger than viruses. Proceedings of the National Academy of Sciences. 2009;107(2):598–603. doi: 10.1073/pnas.0911748107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cu Y, Saltzman WM. Mathematical modeling of molecular diffusion through mucus. Advanced Drug Delivery Reviews. 2009;61(2):101–114. doi: 10.1016/j.addr.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cu Y, Saltzman WM. Controlled surface modification with poly (ethylene)glycol enhances diffusion of plga nanoparticles in human cervical mucus. Mol Pharm. 2008;6(1):173–181. doi: 10.1021/mp8001254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saltzman WM. Drug delivery: engineering principles for drug therapy. New York: Oxford University Press; 2001. [Google Scholar]