Abstract
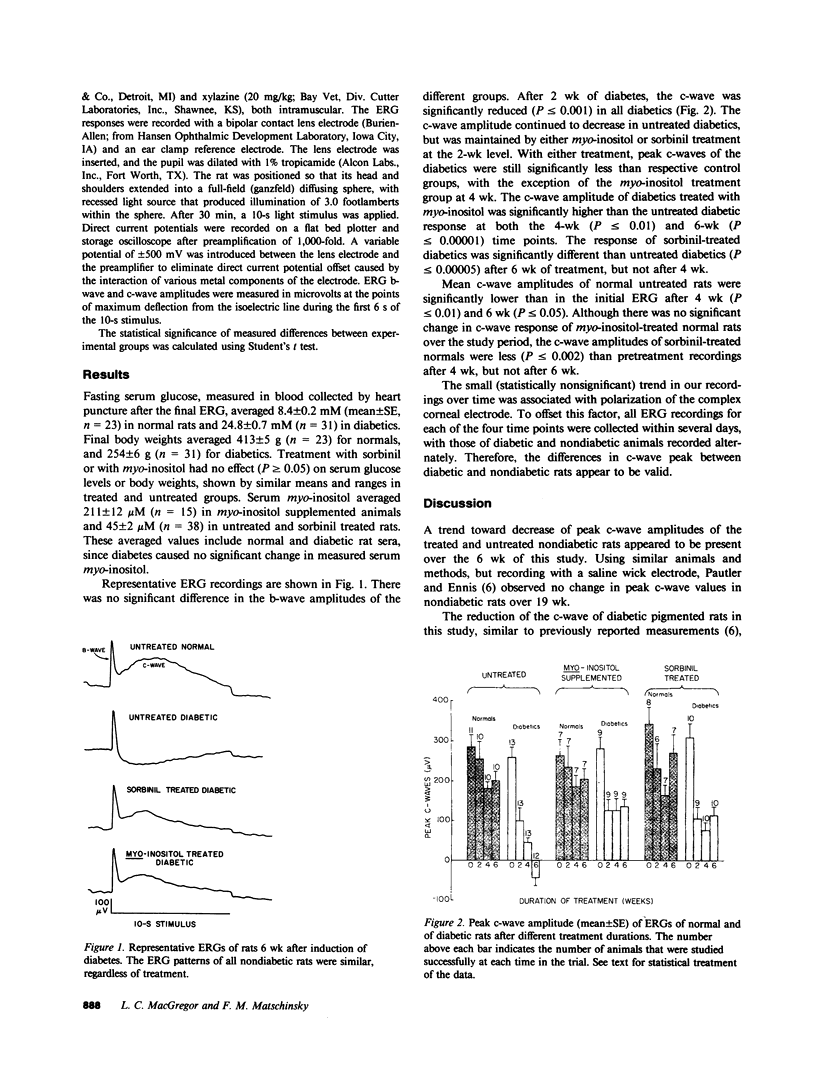
Biochemical abnormalities in the retinal pigment epithelium of experimentally diabetic animals include increased sorbitol and decreased myo-inositol. Diabetes also causes a progressive reduction in the amplitude of the c-wave of the electroretinogram of the pigmented rat. The c-wave is generated by the retinal pigmented epithelium. Myo-inositol administration or treatment with sorbinil, an inhibitor of aldose reductase, arrested the decline in the c-wave. Therefore, hyperglycemia-associated defects in myo-inositol or sorbitol metabolism may be involved in the pathogenesis of the electrophysiological deficit of the diabetic retina. The homogeneous cell layer of the pigment epithelium may be a useful tissue model for studying the pathogenesis of the complications of diabetes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brismar T., Sima A. A. Changes in nodal function in nerve fibres of the spontaneously diabetic BB-Wistar rat: potential clamp analysis. Acta Physiol Scand. 1981 Dec;113(4):499–506. doi: 10.1111/j.1748-1716.1981.tb06928.x. [DOI] [PubMed] [Google Scholar]
- Das P. K., Bray G. M., Aguayo A. J., Rasminsky M. Diminished ouabain-sensitive, sodium-potassium ATPase activity in sciatic nerves of rats with streptozotocin-induced diabetes. Exp Neurol. 1976 Oct;53(1):285–288. doi: 10.1016/0014-4886(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Engerman R., Finkelstein D., Aguirre G., Diddie K. R., Fox R. R., Frank R. N., Varma S. D. Ocular complications. Diabetes. 1982;31(Suppl 1 Pt 2):82–88. doi: 10.2337/diab.31.1.s82. [DOI] [PubMed] [Google Scholar]
- Finegold D., Lattimer S. A., Nolle S., Bernstein M., Greene D. A. Polyol pathway activity and myo-inositol metabolism. A suggested relationship in the pathogenesis of diabetic neuropathy. Diabetes. 1983 Nov;32(11):988–992. doi: 10.2337/diab.32.11.988. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973 Apr 19;288(16):831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- Gillon K. R., Hawthorne J. N. Sorbitol, inositol and nerve conduction in diabetes. Life Sci. 1983 Apr 25;32(17):1943–1947. doi: 10.1016/0024-3205(83)90045-0. [DOI] [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Action of sorbinil in diabetic peripheral nerve. Relationship of polyol (sorbitol) pathway inhibition to a myo-inositol-mediated defect in sodium-potassium ATPase activity. Diabetes. 1984 Aug;33(8):712–716. doi: 10.2337/diab.33.8.712. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Impaired rat sciatic nerve sodium-potassium adenosine triphosphatase in acute streptozocin diabetes and its correction by dietary myo-inositol supplementation. J Clin Invest. 1983 Sep;72(3):1058–1063. doi: 10.1172/JCI111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Sodium- and energy-dependent uptake of myo-inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J Clin Invest. 1982 Nov;70(5):1009–1018. doi: 10.1172/JCI110688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes P. A., Laties A. M. Early morphological alteration of the pigment epithelium in streptozotocin-induced diabetes: increased surface area of the basal cell membrane. Exp Eye Res. 1980 Jun;30(6):631–639. doi: 10.1016/0014-4835(80)90062-7. [DOI] [PubMed] [Google Scholar]
- Kirber W. M., Nichols C. W., Grimes P. A., Winegrad A. I., Laties A. M. A permeability defect of the retinal pigment epithelium. Occurrence in early streptozocin diabetes. Arch Ophthalmol. 1980 Apr;98(4):725–728. doi: 10.1001/archopht.1980.01020030719015. [DOI] [PubMed] [Google Scholar]
- MacGregor L. C., Matschinsky F. M. An enzymatic fluorimetric assay for myo-inositol. Anal Biochem. 1984 Sep;141(2):382–389. doi: 10.1016/0003-2697(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Mayer J. H., Tomlinson D. R. Prevention of defects of axonal transport and nerve conduction velocity by oral administration of myo-inositol or an aldose reductase inhibitor in streptozotocin-diabetic rats. Diabetologia. 1983 Nov;25(5):433–438. doi: 10.1007/BF00282524. [DOI] [PubMed] [Google Scholar]
- Oakley B., 2nd, Green D. G. Correlation of light-induced changes in retinal extracellular potassium concentration with c-wave of the electroretinogram. J Neurophysiol. 1976 Sep;39(5):1117–1133. doi: 10.1152/jn.1976.39.5.1117. [DOI] [PubMed] [Google Scholar]
- Pautler E. L., Ennis S. R. The effect of induced diabetes on the electroretinogram components of the pigmented rat. Invest Ophthalmol Vis Sci. 1980 Jun;19(6):702–705. [PubMed] [Google Scholar]
- Peterson M. J., Sarges R., Aldinger C. E., MacDonald D. P. CP-45,634: a novel aldose reductase inhibitor that inhibits polyol pathway activity in diabetic and galactosemic rats. Metabolism. 1979 Apr;28(4 Suppl 1):456–461. doi: 10.1016/0026-0495(79)90056-8. [DOI] [PubMed] [Google Scholar]
- Tso M. O., Cunha-Vaz J. G., Shih C. Y., Jones C. W. Clinicopathologic study of blood-retinal barrier in experimental diabetes mellitus. Arch Ophthalmol. 1980 Nov;98(11):2032–2040. doi: 10.1001/archopht.1980.01020040884020. [DOI] [PubMed] [Google Scholar]