Abstract
Objective
To investigate the role of adhesion molecules in C protein-induced myositis (CIM), a murine model for polymyositis (PM).
Methods
CIM was induced in wild type mice, L-selectin-deficient (L-selectin-/-) mice, ICAM-1-deficient (ICAM-1-/-) mice, and both L-selectin- and ICAM-1-deficient (L-selectin-/-ICAM-1-/-) mice. The severity of myositis, inflammatory cell infiltration, and mRNA expression in the inflamed muscles were examined. The effect of dendritic polyglycerol sulfate (dPGS), a synthetic inhibitor that suppresses the function of L-selectin and endothelial P-selectin, was also examined.
Results
L-selectin-/- mice and L-selectin-/-ICAM-1-/- mice developed significantly less severe myositis compared to wild type mice, while ICAM-1 deficiency did not inhibit the development of myositis. L-selectin-/- mice transferred with wild type T cells developed myositis. Wild type mice treated with dPGS significantly diminished the severity of myositis compared to control-treated wild type mice.
Conclusions
These data indicate that L-selectin plays a major role in the development of CIM, whereas ICAM-1 plays a lesser, if any, role in the development of CIM. L-selectin-targeted therapy may be a candidate for the treatment of PM.
Keywords: Polymyositis, C protein-induced myositis, L-selectin, CD8+ T Cells
Introduction
Polymyositis (PM) is a chronic autoimmune inflammatory myopathy. It affects striated muscles and induces varying degrees of muscle weakness, especially in the proximal muscles [1]. Although the pathogenesis of PM has not been elucidated, cytotoxic CD8+ T cells are thought to have a prominent role in the development of myositis [2]. Recently, the C protein-induced myositis (CIM) model was established as an animal model for PM [3]. The skeletal muscle C protein is a myosin-binding protein that regulates muscle filament components [4]. This murine myositis is readily induced by a single immunization with recombinant skeletal muscle C protein fragments in C57BL/6 mice. CIM elicits abundant perforin-positive CD8+ T cells that infiltrate endomysial sites. In addition, CD8+ T cell depletion inhibits the progression of myositis. In the CIM model, inflammatory cytokines, including interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, mediate the induction and development of myositis [3, 5, 6]. This CIM model is primarily utilized to examine the inflammatory phase of PM.
Leukocyte recruitment into inflammatory sites is accomplished by constitutive or inducible expression of multiple adhesion molecules [7-9]. L-selectin (CD62L), which primarily mediates leukocyte capture and rolling on the endothelium, is constitutively expressed by most leukocytes [10]. In addition, L-selectin plays significant roles in the activation of multiple intracellular signaling pathways [11]. ICAM-1 (CD54) is a member of the Ig superfamily that is constitutively expressed on endothelial cells, subsets of leukocytes, fibroblasts, and epithelial cells [12]. It can be transcriptionally up-regulated by several proinflammatory cytokines, such as TNF-α, interferon (IFN)-γ, and IL-1 [12]. L-selectin and ICAM-1 act in concert with each other during leukocyte migration from blood to extravascular tissues where inflammatory responses occur in vivo [13, 14]. However, the relative contributions of L-selectin and ICAM-1 are largely dependent upon the type of inflammatory models being employed. For instance, in the immediate-type hypersensitivity model, deficiency of either L-selectin or ICAM-1 resulted in a reduced immune response to similar degrees. Deficiency of both L-selectin and ICAM-1 did not exhibit a synergistic effect [15]. Introducing defective ICAM-1 into L-selectin-deficient (L-selectin-/-) mice resulted in a profoundly decreased pulmonary fibrosis compared to that observed in mice with a deficiency of a single adhesion molecule in a bleomycin-induced pulmonary fibrosis model [16]. However, wound healing was not inhibited by L-selectin deficiency, while delayed wound healing was observed in ICAM-1-deficient (ICAM-1-/-) mice [17]. Nevertheless, the relative contributions and interaction of L-selectin and ICAM-1 in the CIM model remain unknown.
In this study, we investigated the role of adhesion molecules in CIM by using L-selectin-/- mice, ICAM-1-/- mice, and L-selectin and ICAM-1 doubly deficient (L-selectin-/-ICAM-1-/-) mice. Inhibition of L-selectin ameliorated the severity of myositis. The results of this study indicate that L-selectin contributes to the development of CIM more than ICAM-1 does, and that L-selectin might serve as a therapeutic target for the treatment of polymyositis.
Materials and Methods
Mice
L-selectin-/- mice were produced as described [18]. ICAM-1-/- mice [19] were from the Jackson Laboratory (Bar Harbor, ME). L-selectin-/-ICAM-1-/- mice lacking both L-selectin and ICAM-1 were generated as described [14]. All mice were backcrossed more than 8 generations onto the C57BL/6 genetic background. Female mice used for experiments were 8-10 weeks old. Age-matched C57BL/6 mice (Jackson Laboratory) were used as controls. All mice were housed in a specific pathogen-free barrier facility and screened regularly for pathogens. The Committee on Animal Experimentation of Kanazawa University Graduate School of Medical Science approved all studies and procedures.
CIM induction
To induce CIM, 8-10 week-old female mice were immunized intradermally with 200 μg of murine C protein fragments [3] emulsified in 200 μl of Freund's complete adjuvant (CFA) containing 100 μg of heat-killed Mycobacterium butyricum (Difco, Detroit, MI) [5]. The immunogens were injected at multiple sites in the back and footpads. 0.2-2 μg of pertussis toxin (Seikagaku Corporation, Tokyo, Japan) in phosphate buffered saline (PBS) was injected intraperitoneally at the same time.
Mice were sacrificed at day 14 after immunization with C protein and muscle tissues were harvested. Hematoxylin and eosin (H&E)-stained 10-μm sections of the hamstrings and quadriceps were examined histologically. The histologic severity of myositis in each muscle block was graded as follows [3]: grade 1 = involvement of at least 1 muscle fiber but fewer than 5 muscle fibers; grade 2 = a lesion involving 5–30 muscle fibers; grade 3 = a lesion involving a muscle fasciculus; grade 4 = diffuse, extensive lesions. When multiple lesions with the same grade were found in a single muscle block, 0.5 was added to the grade. We also assessed necrotic muscle areas. Necrotic muscle areas were also quantified by measuring necrotic muscle fibers showing decreased H&E staining and the replacement of muscle fibers by mononuclear cell infiltrates [6].
Immunohistochemical staining
Muscle sections (4 μm in thickness) taken from the left thigh were frozen in cold 2-methylbutane and were stained with anti-CD8a (53-6.7) and anti-CD4 (RM4-5) (BD Biosciences). Muscle sections (4 μm in thickness) taken from the right thigh were fixed in formalin, dehydrated, embedded in paraffin, and were then incubated with rat monoclonal antibodies specific for CD3 (CD3-12; Serotec, Oxford, UK), F4/80 (A3-1: Abcam, Cambridge, UK), and myeloperoxidase (MPO; NeoMarkers, Fremont, CA). Six inflammatory mononuclear cell foci in the serial sections were studied. Stained cells were counted in every focus under high magnification (400×) using a light microscope. The mean score was used for analysis. The stained sections were evaluated by two independent observers who reported comparable results.
Adoptive transfer of spleen T cells
Single-cell splenic leukocyte suspensions of naïve, unimmunized wild type mice were generated by gentle homogenization. CD90.2 mAb-coupled microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) were used to purify T cell populations according to the manufacturer's instructions. The purity of extracted T cells from donor mice was measured using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA) with purities of over 90%. Viable spleen T cells (8 × 106) were transferred intravenously into recipient L-selectin-/- mice. 24 h after adoptive transfer of T cells, recipient L-selectin-/- mice were immunized with C protein fragments and their muscles were harvested 14 days after immunization as described above. PBS-injected mice served as controls.
Real-time reverse transcription-polymerase chain reaction
Total RNAs were extracted from muscle samples using QIAGEN RNeasy spin columns (QIAGEN, Crawley, UK) and real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed using the TaqMan® system (Applied Biosystems, Foster City, CA) on an ABI Prism 7000 Sequence Detector (Applied Biosystems) [20]. TaqMan probes and primers for IL-1β, IL-6, IL-10, IL-12α, IFN- γ, tumor necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were purchased from Applied Biosystems. Relative expression of RT-PCR products was determined using the ΔΔ cycle threshold technique. Each reaction was performed at least in triplicate.
Dendritic polyglycerol sulfates treatment
Dendritic polyglycerol sulfates (dPGS) are multivalent inhibitors of inflammation that inhibit both L-selectin and endothelial P-selectin with high efficacy [21]. dPGS (0.3 mg/mouse) was injected subcutaneously on the shaved neck of wild type mice for 11 consecutive days, beginning 3 days after immunization with C protein. Mice injected subcutaneously with PBS served as controls.
Statistical analysis
The Mann-Whitney U test was used to determine the level of significance of differences in the sample means. The Bonferroni test was used for multiple comparisons. All statistical analysis was performed using Prism software.
Results
L-selectin deficiency ameliorates myositis severity
To determine if there is a role of adhesion molecules in myositis, we assessed the severity of myositis in wild type, L-selectin-/-, ICAM-1-/-, and L-selectin-/-ICAM-1-/- mice (Figure 1A). Histological scores were significantly lower in L-selectin-/- mice compared to wild type mice (P < 0.01) (Figure 1B). Similarly, ICAM-1-/- mice demonstrated a 50% decreased myositis severity compared to wild type mice, although the differences did not reach significance. L-selectin-/-ICAM-1-/- mice displayed significantly lower histological scores compared to wild type mice (P < 0.01). Histological scores of L-selectin-/- and L-selectin-/-ICAM-1-/- mice were also significantly lower than those of ICAM-1-/- mice (P < 0.01 and P < 0.01, respectively). We also assessed necrotic muscle areas to evaluate muscle damage (Figure 1C). Necrotic muscle areas were significantly smaller in L-selectin-/- mice compared to wild type mice (P < 0.01). ICAM-1-/- mice showed a 69% decrease in necrotic muscle areas compared to wild type mice. L-selectin-/-ICAM-1-/- mice displayed significantly decreased necrotic muscle areas comparable to those of L-selectin-/- mice. Necrotic muscle areas of L-selectin-/- and L-selectin-/-ICAM-1-/- mice were also significantly smaller than those of ICAM-1-/- mice (P < 0.01 and P < 0.05, respectively). Histological scores paralleled the increased levels of necrotic muscle areas. L-selectin-/- and L-selectin-/-ICAM-1-/- mice developed little or a minimal degree of myositis. These results suggest that L-selectin contributes profoundly to the development of CIM, whereas the contribution of ICAM-1 is less relevant.
Figure 1.
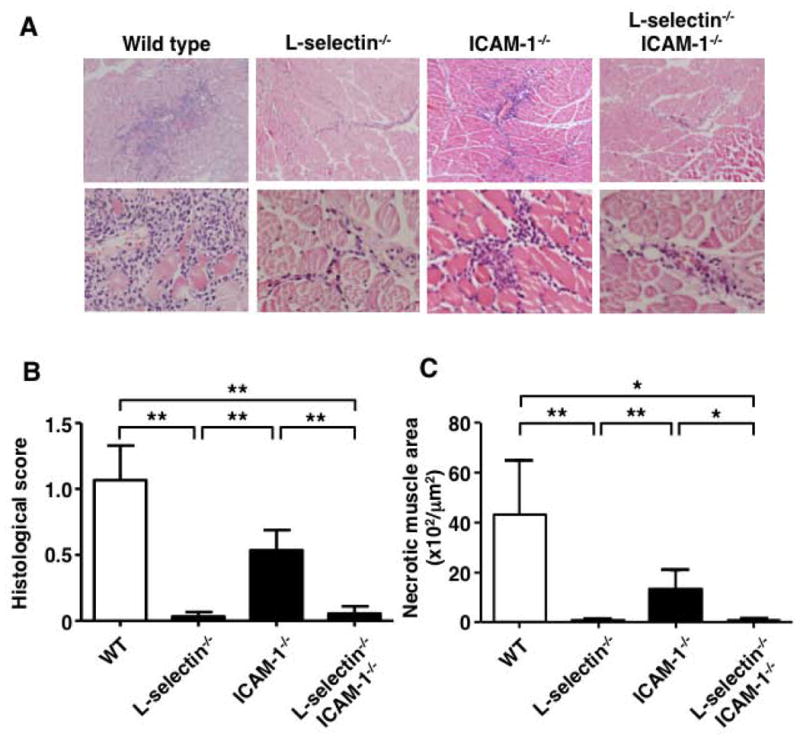
C protein-induced myositis (CIM) in wild type (WT) mice, L-selectin-deficient (L-selectin-/-) mice, ICAM-1-deficient (ICAM-1-/-) mice, and both L-selectin- and ICAM-1-deficient (L-selectin-/-ICAM-1-/-) mice. Mice were sacrificed at day 14 after immunization with C protein and muscle tissues were harvested. A, Representative images of muscle inflammation with hematoxylin and eosin (HE) staining. B-C, Histological score and the area of muscle fiber necrosis in each mouse group. Bars show the mean ± SEM. These results represent those obtained with 8-10 mice of each genotype. **p < 0.01. A, Original magnification: ×100 (upper panels); ×400 (lower panels).
L-selectin deficiency reduces leukocyte recruitment
Since L-selectin-/- mice, but not ICAM-1-/- mice, showed significantly less severe myositis compared to wild type mice, we assessed inflammatory cell infiltration in the inflamed muscles in wild type mice and L-selectin-/- mice (Figure 2A-B). Immunohistological staining revealed that CD3+, CD4+, and CD8+ T cells, F4/80+ macrophages, and MPO+ neutrophils were all significantly reduced in L-selectin-/- mice compared to wild type mice (P < 0.01, respectively). We detected very few B220+ B cells in the inflamed muscles of both wild type mice and L-selectin-/- mice (data not shown). These results suggest that L-selectin plays an important role in the migration of leukocytes into inflamed muscles in the CIM model.
Figure 2.
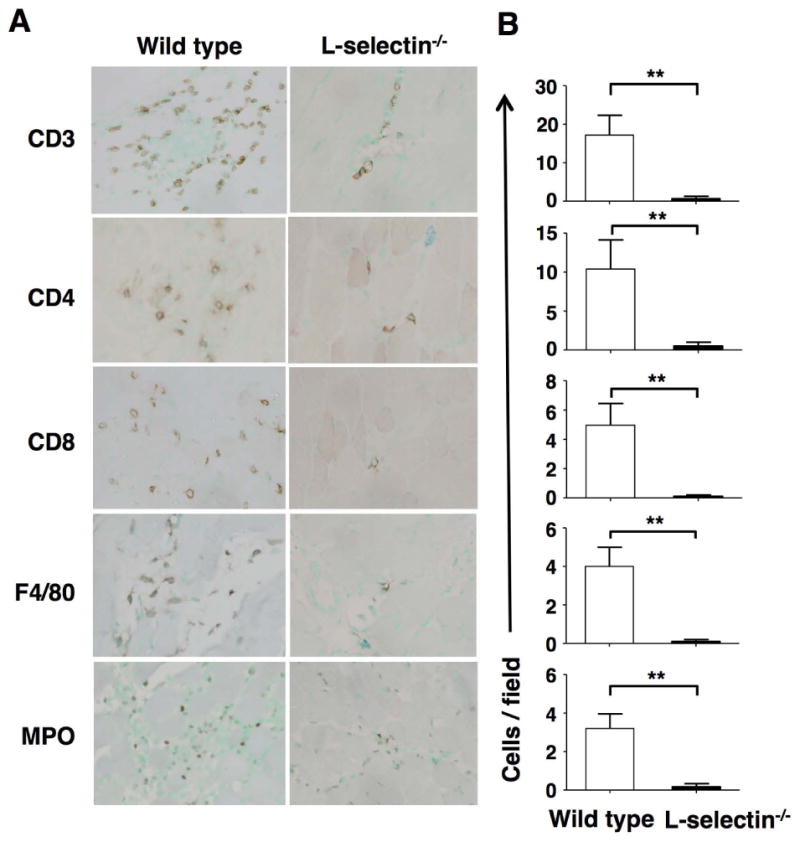
Inflammatory cell infiltration into inflamed muscles of wild type (WT) and L-selectin-/- mice during C protein-induced myositis. Mice were sacrificed at day 14 after immunization with C protein and muscle tissues were harvested. A, Representative immunohistochemical images showing CD3, CD4, CD8, F4/80, and myeloperoxidase (MPO) staining in tissue sections from L-selectin-/- mice and wild type mice. B, Numbers of CD3+, CD4+, and CD8+ T cells, F4/80+ macrophages, and MPO+ neutrophils on day 14. Bars show the mean ± SEM. These results represent those obtained with 8-10 mice of each genotype. **p < 0.01. A, Original magnification: ×400.
L-selectin-/- mice transferred with wild type T cells develop myositis
Although L-selectin is expressed on various leukocyte subsets, CD8+ T cells are considered to be crucial for the induction of CIM. Therefore, we performed adoptive transfer experiments to confirm the importance of L-selectin expression on T cells. As described, L-selectin-/- mice exhibited minimal development of myosis. In contrast, L-selectin-/- mice that were transferred with T cells from naïve, unimmunized wild type mice developed readily apparent myositis. Histological scores of L-selectin-/- mice transferred with wild type T cells were approximately 10-fold higher than those of L-selectin-/- mice and L-selectin-/- mice treated with vehicle (P < 0.01 and P < 0.01, respectively) (Figure 3A-B). Although the histological scores of L-selectin-/- mice transferred with wild type T cells were 52% lower than wild type mice, the difference did not reach significance. Our analysis of cell infiltration showed that both CD4+ and CD8+ T cells were more abundant in L-selectin-/- mice given wild type T cells (Figure 3C). These results indicate that L-selectin expression on T cells is important for leukocyte migration into inflamed muscles and the development of myositis.
Figure 3.
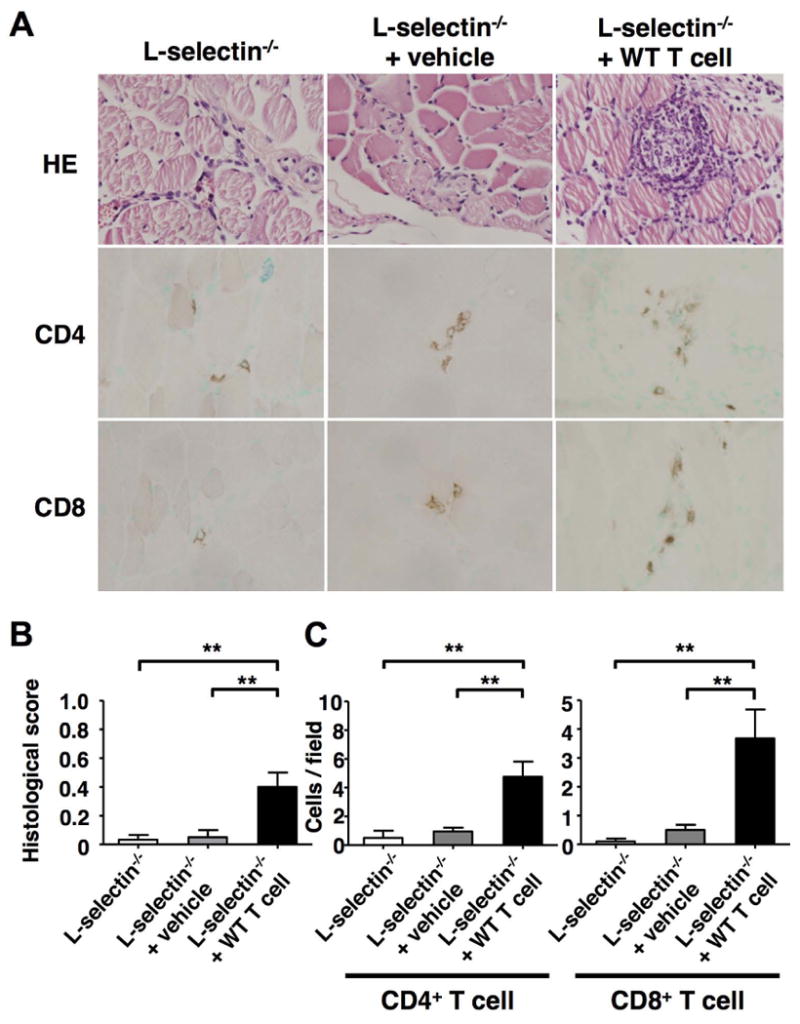
Adoptive transfer of T cells from wild type mice into L-selectin-/- mice. Mice were sacrificed at day 14 after immunization with C protein and muscle tissues were harvested. A, Representative muscle sections stained with hematoxylin and eosin (HE) and immunohistochemical images showing CD4 and CD8 staining from L-selectin-/- mice, L-selectin-/- mice with vehicle, and L-selectin-/- mice transferred with wild type T cells. B, Histological score in each mouse group. C, Numbers of CD4+ and CD8+ T cells. A, Original magnification: ×400 (upper, middle and lower panels). B-C, Bars show the mean ± SEM. These results represent those obtained with 5 mice of each group. **p < 0.01.
Cytokine expression in C-protein induced myositis
We next compared mRNA expression in inflamed muscles between wild type and L-selectin-/- mice by RT-PCR (Figure 4). The mRNA expression levels of IL-6, IL-10, IL-12, IFN-γ, and MCP-1 in L-selectin-/- mice were significantly reduced compared to those in wild type mice (P < 0.05, respectively). IL-1β and TNF-α mRNA expression levels in L-selectin-/- mice were 21% and 43% decreased, respectively, compared to wild type mice, although the expression levels for both cytokines did not reach significance.
Figure 4.
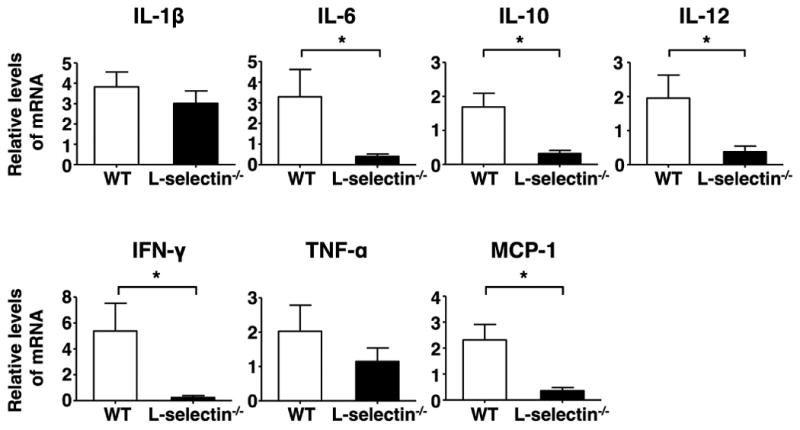
mRNA expression levels in the inflamed muscles from wild type (WT) and L-selection-/- mice on day 14 after immunization with C protein. Bars show the mean ± SEM. These results represent those obtained with 5 or more mice of each genotype. *p < 0.05.
Dendritic polyglycerol sulfate (dPGS) treatment diminished the degree of myositis
Since dPGS is an inhibitor that suppresses the function of adhesion molecules, including L-selectin, wild type mice were treated with dPGS. Wild type mice treated with dPGS showed 52% lower histological scores than PBS-treated control wild type mice (P < 0.05) (Figure 5A-B). Necrotic tissue areas from wild type mice treated with dPGS were significantly smaller than those of PBS-treated control wild type mice (P < 0.05) (Figure 5C). Infiltration of both CD4+ and CD8+ T cells was also significantly decreased by dPGS treatment (Figure 5D).
Figure 5.
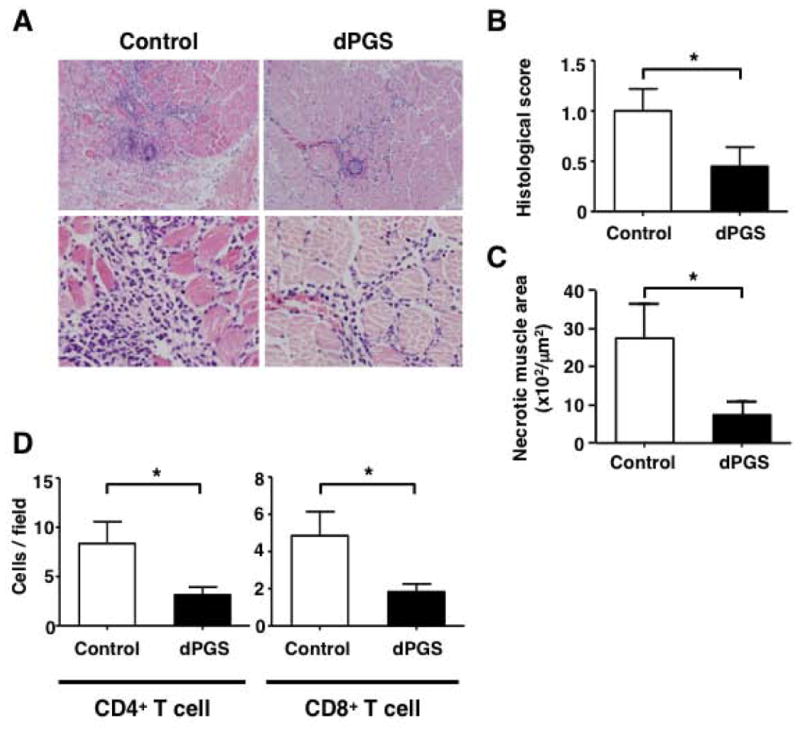
Dendritic polyglycerol sulfate (dPGS) treatment in wild type mice decreased the severity of myositis. dPGS was injected intradermally for 11 consecutive days, beginning 3 days after immunization with C protein. Mice were sacrificed at day 14 after immunization and muscle tissues were harvested. A, Representative images of muscle inflammation with hematoxylin and eosin (HE) staining in wild type mice treated with dPGS and those treated with control. B-C, Histological score and the area of muscle fiber necrosis in each mouse group. D, Numbers of CD4+ and CD8+ T cells on day 14. Bars show the mean ± SEM. These results represent those obtained with 8-10 mice of each genotype. *p < 0.05. A, Original magnification: ×100 (upper panels); ×400 (lower panels).
Discussion
This is the first study to reveal the roles of adhesion molecules in the development of CIM, a murine polymyositis model. Specifically, L-selectin, but not ICAM-1, was critical for the development of myositis. The development of myositis was also prevented by treatment with dPGS, which binds to L-selectin and endothelial P-selectin and prevents leukocytes from binding to inflamed vascular endothelia [21]. Thus, our findings indicate that L-selectin plays a critical role in the development of CIM, and that L-selectin could be a target for the treatment of polymyositis.
The cell adhesion molecules L-selectin and ICAM-1 act cooperatively to mediate optimal leukocyte rolling and the recruitment of leukocytes to inflammatory sites [13, 14, 22-24]. Although the relative contributions of L-selectin and ICAM-1 vary among different models of inflammation, ICAM-1 appears to have a more predominant role than L-selectin in general. However, this study indicates that in the development of CIM L-selectin outreaches ICAM-1 cell adhesive function (Figure 1A-B). Previous reports clearly indicate that CD8+ T cells play a more significant role than CD4+ T cells in the development of CIM. Tang et al. also reported that the percentage of L-selectin+ cells in CD8+ T cells was greater than that of CD4+ T cells, especially in lymphoid tissues, and that L-selectin expression levels on CD8+ T cells were higher than on CD4+ T cells in wild type mice [25]. In contrast, L-selectin deficiency did not affect ICAM-1 expression [16]. Thus, the different expression levels of L-selectin between CD4+ and CD8+ T cells may partly explain the results that L-selectin deficiency had a greater effect on the degree of myositis than ICAM-1. Alternatively, L-selectin is important for anti-viral immunity [26] and anti-tumor activity [27], in which CD8+ cytotoxic T cells have a substantial role. The etiologic role of CD8+ cytotoxic T cells may be another reason why L-selectin is more important than ICAM-1 in the CIM model.
It is assumed that leukocyte infiltration and cytokines participate in the development of CIM. Consistent with this, L-selectin-/- mice exhibited significantly decreased numbers of leukocytes, including CD4+ and CD8+ T cells, F4/80 macrophages, and MPO+ neutrophils, in the inflamed muscles at day 14 following CIM induction (Figure 2A-B). In addition, adoptive transfer of wild type T cells induced myositis in L-selectin-/- mice (Figure 3A-C). These data suggest that L-selectin expression on T cells is required for the induction of myositis via the recruitment of leukocytes into the muscles. However, alternatives should be considered to explain the reduction of myositis severity seen in L-selectin-/- mice. It has been reported that myositis development required both the activation of T cells and conditioning of local muscle tissue as a “seed and soil” model, and CIM regression was due to attenuation of local CFA-induced immune activation [28]. Thus, local immune activation in L-selectin-/- mice might be impaired, leading to reduced myositis severity.
L-selectin expression on leukocytes may be important not only for leukocyte infiltration, but also for subsequent cytokine production. In the CIM model, IL-6 deficiency inhibited the progression of myositis, and IL-6 blockade reduced the severity of myositis [5]. Consistent with this previous report, our study showed that IL-6 mRNA expression in the inflamed muscles of L-selectin-/- mice was significantly decreased compared to wild type mice (Figure 4). Moreover, other inflammatory cytokines, including IL-12 and IFN-γ, were decreased in L-selectin-/- mice as well. The incidence of myositis in IL-1- and TNF-α-deficient mice was significantly lower than wild type mice [3]. Although the differences did not reach significance, IL-1β and TNF-α tended to decrease in L-selectin-/- mice compared to wild type mice in this study (Figure 4). Collectively, L-selectin expression appears to influence cytokine production in inflamed muscles. However, the mechanism how L-selectin is involved in cytokine regulation, needs to be evaluated.
Establishing effective polymyositis therapies has been long awaited. Corticosteroids have been proven to be beneficial, but serious adverse effects can frequently occur. To date, several studies targeting adhesion molecules have been performed. Bimosiamose is a small-molecule and pan-selectin antagonist that targets E-, P- and L-selectin [29]. Inhaled administration of Bimosiamose in asthmatic patients attenuated late asthmatic reactions [30]. Subcutaneous administration of Bimosiamose improved the clinical scores in patients with psoriasis [31]. A humanized anti-L-selectin monoclonal antibody (aselizumab) significantly increased survival time and decreased mortality in a baboon model of hemorrhagic-traumatic shock [32]. However, intravenous administration of aselizumab for multiple traumatized patients resulted in no significant improvement of efficacy in a phase II clinical trial [33]. Also caution should be paid in developing biological therapies targeting adhesion molecules. A humanized anti-CD11a monoclonal antibody, efalizumab, was effective for the treatment of psoriasis, but a long-term follow-up study revealed several fatal cases of progressive multifocal leukoencephalopathy by JC virus [34], leading to voluntary withdrawal of the therapeutic from the market. In this study, the synthetic compound dPGS, which is an inhibitor that suppresses the function of leukocytic L-selectin and endothelial P-selectin, improved myositis severity (Figure 5A-C). Therefore, we assume that a selectin-targeted therapy could still be an option for the treatment of polymyositis.
We are well aware that our study is impacted by a number of limitations. First, the applied murine CIM model of PM only mimics the inflammatory aspect of the human disease. Second, immune responses against C protein have not been reported in human PM patients. Third, we did not conduct functional assays to directly assess muscle weakness in this study. In previous experiments, we attempted several assays, including a rotarod test and measuring serum creatinine kinase. We employed rotarod tests in a previous paper [3]. However, according to our experience, the rotarod test turned out to be less reliable than histologic analyses, because mice learn how to avoid falling off and do not necessarily run consistently. In addition, serum levels of creatinine kinase or other muscle-derived proteins are unreliable parameters. They are often high in normal mice, presumably because of their physical activity [28]. Devices are needed to directly quantify rodent muscle strength to best follow clinical disease course.
In conclusion, immune cell infiltration initiated via selectin-ligand interactions and the production of proinflammatory cytokines, such as IL-6 and chemoattractants for inflammatory cells, like MCP-1, may be a trigger of myositis in CIM. Our findings also indicate that the cell adhesion molecule L-selectin may be a candidate for treating polymyositis, an intractable autoimmune disease.
Acknowledgments
We thank Ms. E. Yoshimoto, M. Matsubara and Y. Yamada for technical assistance.
Financial support: This work was supported by a research grant on intractable diseases from the Ministry of Health, Labor and Welfare of Japan and the Ministry of Education, Culture, Sports, Science and Technology of Japan. In addition, we acknowledge support from the German Research Foundation (DFG) to the collaborative research center SFB 765 and from the United States National Institutes of Health (AI 056363 to TFT).
Footnotes
Conflict of Interest: The authors have declared no conflicts of interest.
References
- 1.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971–82. doi: 10.1016/S0140-6736(03)14368-1. [DOI] [PubMed] [Google Scholar]
- 2.Engel AG, Arahata K, Emslie-Smith A. Immune effector mechanisms in inflammatory myopathies. Research publications - Association for Research in Nervous and Mental Disease. 1990;68:141–57. [PubMed] [Google Scholar]
- 3.Sugihara T, Sekine C, Nakae T, et al. A new murine model to define the critical pathologic and therapeutic mediators of polymyositis. Arthritis Rheum. 2007;56:1304–14. doi: 10.1002/art.22521. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert R, Cohen JA, Pardo S, Basu A, Fischman DA. Identification of the A-band localization domain of myosin binding proteins C and H (MyBP-C, MyBP-H) in skeletal muscle. J Cell Sci. 1999;112(Pt 1):69–79. doi: 10.1242/jcs.112.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Okiyama N, Sugihara T, Iwakura Y, Yokozeki H, Miyasaka N, Kohsaka H. Therapeutic effects of interleukin-6 blockade in a murine model of polymyositis that does not require interleukin-17A. Arthritis Rheum. 2009;60:2505–12. doi: 10.1002/art.24689. [DOI] [PubMed] [Google Scholar]
- 6.Sugihara T, Okiyama N, Suzuki M, et al. Definitive engagement of cytotoxic CD8 T cells in C protein-induced myositis, a murine model of polymyositis. Arthritis Rheum. 2010;62:3088–92. doi: 10.1002/art.27625. [DOI] [PubMed] [Google Scholar]
- 7.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 8.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev immunol. 2004;4:325–35. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 9.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–90. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 10.Tedder TF, Steeber DA, Pizcueta P. L-selectin deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–64. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grailer JJ, Kodera M, Steeber DA. L-selectin: role in regulating homeostasis and cutaneous inflammation. J Dermatol Sci. 2009;56:141–7. doi: 10.1016/j.jdermsci.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1) J Immunol. 1986;137:245–53. [PubMed] [Google Scholar]
- 13.Steeber DA, Tang MLK, Green NE, Zhang XQ, Sloane JE, Tedder TF. Leukocyte entry into sites of inflammation requires overlapping interactions between the L-selectin and intercellular adhesion molecule-1 pathways. J Immunol. 1999;163:2176–86. [PubMed] [Google Scholar]
- 14.Steeber DA, Campbell MA, Basit A, Ley K, Tedder TF. Optimal selectin-mediated rolling of leukocytes during inflammation in vivo requires intercellular adhesion molecule-1 expression. Proc Natl Acad Sci, USA. 1998;95:7562–7. doi: 10.1073/pnas.95.13.7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimada Y, Hasegawa M, Kaburagi Y, et al. L-selectin or ICAM-1 deficiency reduces an immediate-type hypersensitivity response by preventing mast cell recruitment in repeated elicitation of contact hypersensitivity. J Immunol. 2003;170:4325–34. doi: 10.4049/jimmunol.170.8.4325. [DOI] [PubMed] [Google Scholar]
- 16.Hamaguchi Y, Nishizawa Y, Yasui M, et al. Intercellular adhesion molecule-1 and L-selectin regulate bleomycin-induced lung fibrosis. Am J Pathol. 2002;161:1607–18. doi: 10.1016/S0002-9440(10)64439-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagaoka T, Kaburagi Y, Hamaguchi Y, et al. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol. 2000;157:237–47. doi: 10.1016/S0002-9440(10)64534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arbones ML, Ord DC, Ley K, et al. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin (CD62L) deficient mice. Immunity. 1994;1:247–60. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 19.Sligh JE, Jr, Ballantyne CM, Rich SS, et al. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci, USA. 1993;90:8529–33. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda S, Fujimoto M, Matsushita T, Hamaguchi Y, Takehara K, Hasegawa M. Inducible costimulator (ICOS) and ICOS ligand signaling has pivotal roles in skin wound healing via cytokine production. Am J Pathol. 2011;179:2360–9. doi: 10.1016/j.ajpath.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dernedde J, Rausch A, Weinhart M, et al. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc Natl Acad Sci, USA. 2010;107:19679–84. doi: 10.1073/pnas.1003103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaburagi Y, Hasegawa M, Nagaoka T, et al. The cutaneous reverse Arthus reaction requires intercellular adhesion molecule 1 and L-selectin expression. J Immunol. 2002;168:2970–8. doi: 10.4049/jimmunol.168.6.2970. [DOI] [PubMed] [Google Scholar]
- 23.Komura K, Hasegawa M, Hamaguchi Y, et al. UV light exposure suppresses contact hypersensitivity by abrogating ICAM-1 up-regulation at the elicitation site. J Immunol. 2003;171:2855–62. doi: 10.4049/jimmunol.171.6.2855. [DOI] [PubMed] [Google Scholar]
- 24.Matsushita Y, Hasegawa M, Matsushita T, et al. Intercellular adhesion molecule-1 deficiency attenuates the development of skin fibrosis in tight-skin mice. J Immunol. 2007;179:698–707. doi: 10.4049/jimmunol.179.1.698. [DOI] [PubMed] [Google Scholar]
- 25.Tang MLK, Steeber DA, Zhang XQ, Tedder TF. Intrinsic differences in L-selectin expression levels affect T and B lymphocyte subset-specific recirculation pathways. J Immunol. 1998;160:5113–21. [PubMed] [Google Scholar]
- 26.Richards H, Longhi MP, Wright K, Gallimore A, Ager A. CD62L (L-selectin) down-regulation does not affect memory T cell distribution but failure to shed compromises anti-viral immunity. J Immunol. 2008;180:198–206. doi: 10.4049/jimmunol.180.1.198. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Liu F, Wang QJ, Rosenberg SA, Morgan RA. The shedding of CD62L (L-selectin) regulates the acquisition of lytic activity in human tumor reactive T lymphocytes. PLoS One. 2011;6:e22560. doi: 10.1371/journal.pone.0022560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okiyama N, Sugihara T, Oida T, et al. T lymphocytes and muscle condition act like seeds and soil in a murine polymyositis model. Arthritis Rheum. 2012;64:3741–9. doi: 10.1002/art.34629. [DOI] [PubMed] [Google Scholar]
- 29.Kogan TP, Dupre B, Bui H, et al. Novel synthetic inhibitors of selectin-mediated cell adhesion: synthesis of 1,6-bis[3-(3-carboxymethylphenyl)-4-(2-alpha-D- mannopyranosyloxy)phenyl]hexane (TBC1269) J Med Chem. 1998;41:1099–111. doi: 10.1021/jm9704917. [DOI] [PubMed] [Google Scholar]
- 30.Beeh KM, Beier J, Meyer M, Buhl R, Zahlten R, Wolff G. Bimosiamose, an inhaled small-molecule pan-selectin antagonist, attenuates late asthmatic reactions following allergen challenge in mild asthmatics: a randomized, double-blind, placebo-controlled clinical cross-over-trial. Pulm Pharmacol Ther. 2006;19:233–41. doi: 10.1016/j.pupt.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Friedrich M, Bock D, Philipp S, et al. Pan-selectin antagonism improves psoriasis manifestation in mice and man. Arch Dermatol Res. 2006;297:345–51. doi: 10.1007/s00403-005-0626-0. [DOI] [PubMed] [Google Scholar]
- 32.Schlag G, Redl HR, Till GO, Davies J, Martin U, Dumont L. Anti-L-selectin antibody treatment of hemorrhagic-traumatic shock in baboons. Crit Care Med. 1999;27:1900–7. doi: 10.1097/00003246-199909000-00031. [DOI] [PubMed] [Google Scholar]
- 33.Seekamp A, van Griensven M, Dhondt E, et al. The effect of anti-L-selectin (aselizumab) in multiple traumatized patients--results of a phase II clinical trial. Crit Care Med. 2004;32:2021–8. doi: 10.1097/01.ccm.0000142396.59236.f3. [DOI] [PubMed] [Google Scholar]
- 34.Leonardi C, Menter A, Hamilton T, Caro I, Xing B, Gottlieb AB. Efalizumab: results of a 3-year continuous dosing study for the long-term control of psoriasis. Br J Dermatol. 2008;158:1107–16. doi: 10.1111/j.1365-2133.2008.08548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


