Abstract
Fast inhibitory signalling in the mammalian brain is mediated by gamma-aminobutyric acid type A receptors (GABAARs), which are targets for anti-epileptic therapy such as benzodiazepines. GABAARs undergo tightly regulated trafficking processes that are essential for maintenance and physiological modulation of inhibitory strength. The trafficking of GABAARs to and from the membrane is altered during prolonged seizures such as in Status Epilepticus (SE) and has been suggested to contribute to benzodiazepine pharmacoresistance in patients with SE. However, the intracellular signalling mechanisms that cause this modification in GABAAR trafficking remain poorly understood. In this study, we investigate the surface stability of GABAARs during SE utilising the low Mg2+ model in hippocampal rat neurons. Live-cell imaging of super ecliptic pHluorin (SEP)-tagged α2 subunit containing GABAARs during low Mg2+ conditions reveals that the somatic surface receptor pool undergoes down-regulation dependent on N-methyl-d-aspartate receptor (NMDAR) activity. Analysis of the intracellular Ca2+ signal during low Mg2+ using the Ca2+-indicator Fluo4 shows that this reduction of surface GABAARs correlates well with the timeline of intracellular Ca2+ changes. Furthermore, we show that the activation of the phosphatase calcineurin was required for the decrease in surface GABAARs in neurons undergoing epileptiform activity. These results indicate that somatic modulation of GABAAR trafficking during epileptiform activity in vitro is mediated by calcineurin activation which is linked to changes in intracellular Ca2+ concentrations. These mechanisms could account for benzodiazepine pharmacoresistance and the maintenance of recurrent seizure activity, and reveal potential novel targets for the treatment of SE.
This article is part of the Special Issue entitled ‘GABAergic Signaling in Health and Disease’.
Keywords: GABAA receptor, Trafficking, Surface stability, Epilepsy, Calcium signaling
Highlights
-
•
We investigate surface stability of GABAARs in an in vitro model of Status Epilepticus.
-
•
Live-cell imaging shows a decrease in surface GABAARs in the cell soma.
-
•
Decrease of somatic GABAARs from the surface is mediated by NMDAR activity.
-
•
In vitro Status Epilepticus induces intracellular Ca2+ changes.
-
•
Calcineurin mediates the decrease of somatic GABAARs from the surface.
1. Introduction
GABAA Receptors (GABAARs) are ligand-gated chloride permeable ion channels which mediate both phasic (synaptic) and tonic (extrasynaptic) inhibitory neurotransmission in the central nervous system (Jacob et al., 2008, Luscher et al., 2011). They assemble from five subunits, the composition of which determines the receptors functional and pharmacological properties and the specific location on the neuronal membrane (Luscher et al., 2011, Jacob et al., 2008). GABAARs containing the γ2 subunit mediate synaptic transmission (in contrast to extrasynaptic receptors located away from the synapse) and are a target for benzodiazepines (Pritchett et al., 1989). The enrichment of GABAARs in subcellular compartments such as the axon initial segment (AIS) has been reported for the α2 subunit and although both α1 and α2 subunits are found at the synapse in dendrites, a minority of GABAARs in the AIS contain the α1 subunit (Panzanelli et al., 2011, Brünig et al., 2002). GABAARs undergo dynamic movement within the cellular membrane. Lateral diffusion facilitates trafficking and assures the appropriate surface localisation of the receptor (Mukherjee et al., 2011), while trafficking to and from the membrane through exocytotic and endocytotic processes allows constant maintenance of the inhibitory synaptic receptor pool (Bogdanov et al., 2006, Kittler et al., 2000, Kittler et al., 2004). Altered neuronal activity causes surface GABAARs to undergo plasticity-induced trafficking changes. These are mediated by alterations in the activity of protein phosphatases and kinases which are linked to changes in intracellular Ca2+ (Muir et al., 2010, Bannai et al., 2009, Saliba et al., 2012, Luscher et al., 2011, Jacob et al., 2008, Petrini et al., 2014).
SE evolves rapidly and dynamically, manifesting as a prolonged and self-sustaining seizure with significant morbidity and mortality (Lothman, 1990, Dodrill and Wilensky, 1990, Sutter et al., 2013). This distinct condition can occur in patients with previous epilepsy or may occur de novo as a result of acute neurological disorders (Trinka et al., 2012). As SE evolves, the patient's response to treatment with benzodiazepines decreases progressively which rapidly results in benzodiazepine pharmacoresistance. This may lead to refractory SE, a pathological state in which seizures are not sopped by first- or second-line anticonvulsant therapies.
To unravel the role of benzodiazepine pharmacoresistance associated with SE patients, studies have addressed whether the trafficking of GABAARs to and from the cellular membrane is altered during models of SE (Naylor et al., 2005, Goodkin et al., 2005, Blair et al., 2004). Interestingly, it has been suggested that GABAARs are subjected to subunit-specific trafficking during prolonged depolarisation. GABAARs containing the synaptic subunits β2/3 and γ2 undergo internalisation whereas those containing the extrasynaptic δ subunit remain unchanged (Goodkin et al., 2008). Despite recent studies, the temporal dynamics of GABAAR trafficking have not been investigated using live-cell imaging. Moreover, whether endocytosis occurs preferentially in distinct compartments such as dendrites or soma remains unclear. It is not known which molecular pathways underlie this subunit-specific trafficking of GABAARs. Furthermore, it remains to be determined whether Ca2+ and its intracellular signalling cascades play a significant role in the modulation of GABAergic inhibition during SE.
To address the molecular mechanisms underlying altered GABAAR trafficking during SE, we used a live-cell imaging approach to examine the surface stability of GABAARs in hippocampal neurons. We induced prolonged epileptiform bursting activity in vitro by exposing neurons to artificial cerebrospinal fluid (aCSF) lacking Mg2+ (Mangan and Kapur, 2004, Sombati and Delorenzo, 1995). Using this model, we show a decrease in somatic surface GABAARs that is dependent on NMDAR activity and the Ca2+-dependent phosphatase, calcineurin. Furthermore, we show that epileptiform activity alters intracellular Ca2+ concentrations, which correlates with the decrease of GABAARs from the surface possibly contributing to pathological signalling during SE.
2. Materials and methods
2.1. Constructs
The N-terminally tagged GABAA α2-SEP DNA was a kind gift from S. Moss (Tufts University, Cambridge, MA) and has been described previously (Tretter et al., 2008).
2.2. Cell culture and transfection
All animal experiments were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986. All efforts were made to minimise animal suffering and to reduce the number of animals used. Dissected hippocampi of P0 rat pups or E18 embryos were immediately placed in ice-cold dissection buffer (HBSS (Invitrogen)) and washed once. Using trypsin (0.25%) tissue was digested for 10 min before trituration in ∼2 ml of attachment medium. Neurons were plated onto poly-l-lysine (Sigma) coated coverslips (500 μg/ml). For nucleofection, hippocampal neurons were nucleofected with GABAA α2SEP plasmid DNA. Neurons were centrifuged and the cell pellet was resuspended in 100 μl transfection buffer (135 mM KCL, 10 mM HEPES-pH 7.3, 2 mM MgCl2, 5 mM EGTA, 0.2 mM CaCl2) and transfected using a single cuvette AMAXA system (Lonza, programme O-003 or AK-009). Neurons were left to develop at 37 °C and 95% O2, 5% CO2 in maintenance medium [Neurobasal (Invitrogen), B27 Supplement (Invitrogen), 0.6% Glucose (Sigma), 2 mM Glutamine (Invitrogen) and Penicillin–Streptomycin] for 14–21 DIV before imaging.
2.3. Live-cell imaging
Live-cell imaging was performed on an upright Olympus microscope (BX51WI) coupled to an EM-CCD camera (Ixon, Andor). Cells were imaged with a water-immersion 60× objective (Olympus). Excitation was provided by an X-cite 120Q light source (Lumen Dynamics). Appropriate filters were used (in nm): Excitation: 470/40; Emission: 525/50; Dichroic: 495, long pass. The image pixel scale was calculated by dividing the camera pixel size (16 μm) by the lens magnification (60×) yielding a pixel size of 0.27 μm. Before constant perfusion with a Cole–Parmer Master-Flex pump (∼4 ml/min), aCSF (126 mM NaCl, 24 mM NaHCO3, 10 mM d-Glucose, 2.5 mM KCL, 2 mM CaCl2, 1 mM MgCl, 1 mM NaH2PO4, 5 mM Sodium Pyruvate) was pre-equilibrated for 20 min with 95% O2 and 5% CO2 to establish a pH of 7.4. Temperature of the waterbath was constantly measured using a digital Thermometer (Hanna Instruments) and maintained at 37 °C. Any focus drift was corrected manually. Protocols were adapted to achieve minimal bleaching conditions. Imaging of SEP-tagged GABAARs was done for 60 min at a rate of one frame every 20 s (180 frames, 48.8 ms exposure, no averaging). For imaging of intracellular Ca2+ using fluo4 (1 μM, Molecular Probes, Invitrogen) hippocampal neurons were incubated for 30 min at 37 °C. After washing twice, fluo4-imaging was done for 60 min (720 frames, 5 ms exposure, no averaging) at 1 frame every 5 s.
2.4. Cell-attached recording
Cell-attached recordings were made on transfected hippocampal neurons at 13 DIV using an Axopatch 200B amplifier (Molecular Devices) and pClamp software. Cells were visualised using an upright Olympus BX50WI microscope equipped with a 40× water-immersion objective and infrared optics. Recording electrodes were pulled from standard-walled borosilicate glass capillaries (Warner Instruments) and filled with aCSF. Gigaseal cell attached recordings were made in voltage-clamp mode at −70 mV; the cells were constantly perfused with aCSF. To block currents during recording NBQX disodium salt (20 μM, Abcam) and dAPV (D-(−)-2-Amino-5-phosphonopentanoic acid, 25 μM, TOCRIS) were added to the perfusion solution.
2.5. Low Mg2+ and drug treatments
To induce epileptiform bursting activity, aCSF without Mg2+ but 2 μM glycine (126 mM NaCl, 24 mM NaHCO3, 10 mM d-Glucose, 2.5 mM KCL, 2 mM CaCl2, 1 mM NaH2PO4, 5 mM sodium pyruvate, 2 μM glycine) was used (Blair et al., 2004). We confirmed previous studies (Robinson et al., 1993, Mangan and Kapur, 2004, DeLorenzo et al., 1998) that low Mg2+ results in cellular burst spiking that is dependent upon glutamateric transmission (Sup. Fig.2). Moreover, action potentials were associated with post-synaptic currents, indicating that the bursting was the result of network activity (Sup. Fig. 2). The mitochondrial substrate sodium pyruvate was supplemented to reduce neuronal death (Kovac et al., 2012). Transfected hippocampal neurons were perfused with control aCSF for 3.3 min (10 frames, baseline), followed by either low Mg2+ treatment or continued perfusion with aCSF with Mg2+ (control) for 60 min. To block NMDAR activity during low Mg2+ treatment, the NMDAR blocker dAPV (25 μM, TOCRIS) was used continuously throughout the low Mg2+ treatment without preincubation. For low Mg2+/NMDA treatment, NMDA (30 μM, TOCRIS) was added to the low Mg2+ medium and applied continuously for 60 min. To block the activity of the Ca2+ dependent phosphatase calcineurin, cells were pre-incubated with calcineurin autoinhibitory peptide (Terada et al., 2003) (50 μM, Calbiochem) for 25 min at 37 °C and imaged (without application of the peptide during perfusion) either during control or low Mg2+ treatment (Muir et al., 2010).
2.6. Image analysis
Intensity analysis of specific regions of interests (ROIs: background, soma, diffuse, clustered) was done in ImageJ 1.43u which allowed the export of raw data to MatlabR2008a Software. Image correction was done in ImageJ software using the plugin StackReg macro (Thévenaz et al., 1998) which corrects for drift in (x,y). Inverted average intensity projection was done in ImageJ by using the Z-stack application from frame 1–10 (0–3 min) and frame 30–60 (10–20 min). Analysis of SEP-imaging raw data was done using Matlab Software through a custom designed code. Background was subtracted from each frame, fluorescence intensity was normalised to the baseline (average value of t = 0–3.33 min) and averaged for each experimental group. The fluorescence intensity values for each specific ROI were analysed in individual loops which allowed separate analysis. Furthermore, the standard error of mean (SEM) was calculated for each time-point and the mean normalised values including error bars were plotted against time. Ca2+ imaging was analysed using ImageJ. Fluorescence intensity in the soma was extracted from one ROI per cell. Baseline for 60 min Ca2+-imaging was the average of the first 10 frames (t = 0–50 s), which corresponds to control conditions.
2.7. Statistical analysis
All experiments were performed on neurons from at least three individual preparations. The software GraphPad Prism was used for statistical tests and to generate bar charts. Data sets were tested to determine if they were normally distributed (KS normality test) before undertaking further statistical analysis. For low Mg2+ only and low Mg2+ NMDA treatments, p values were determined using a Student's t test (two-tailed). Repeat measures ANOVA (for normally distributed data) or Friedman test was used to analyse significance of low Mg2+ induced effects during Ca2+-imaging, low Mg2+/dAPV and low Mg2+/CAIP experiments since there were more than two experimental groups. Appropriate post-hoc tests such as Tukey's for normally distributed data or Dunn's multiple comparison for non-normally distributed data were used. Values are given as mean ± SEM. Error bars represent SEM.
3. Results
3.1. Surface stability of somatic GABAARs in hippocampal neurons is altered during Low Mg2+ treatment
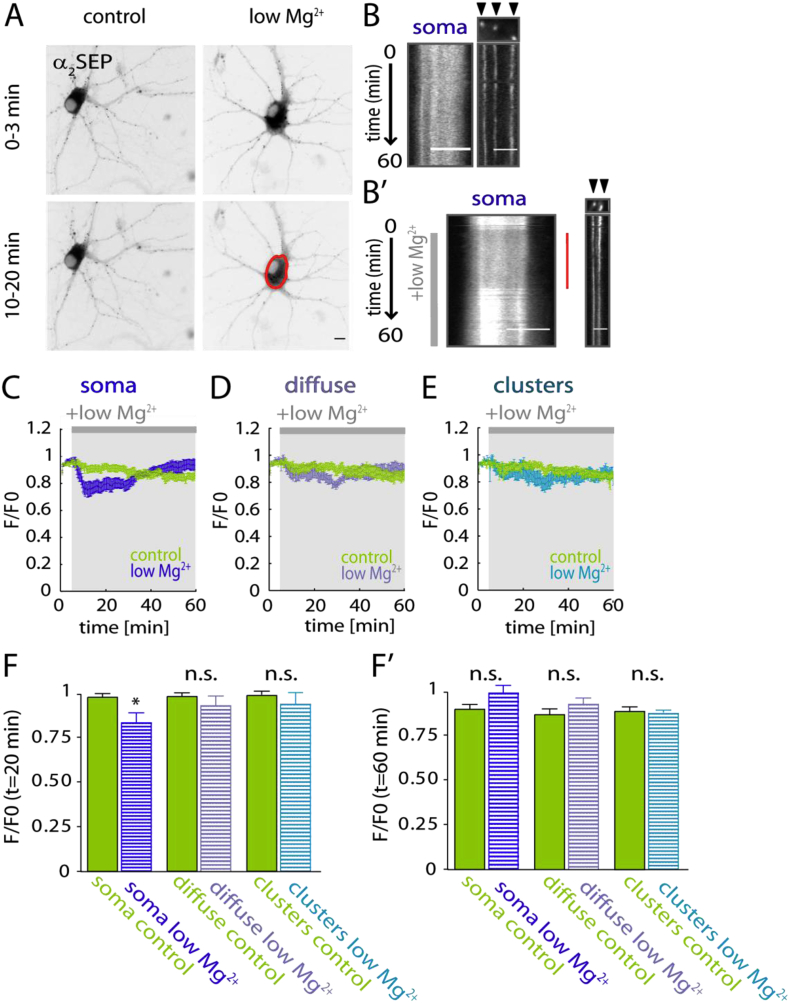
To examine the influence of SE on GABAAR stability and clustering in vitro, we mimicked the characteristic repetitive epileptiform bursting activity of SE by removal of Mg2+ from the extracellular medium of cultured hippocampal rat neurons transfected with SEP-tagged GABAAR α2 subunit (α2SEP) (Sombati and Delorenzo, 1995). Surface GABAARs were imaged via the SEP-tag on the α2 subunit (Muir et al., 2010) (which allows visualisation through high fluorescence in neutral pH, Sup. Fig.1) for 60 min. α2SEP fluorescence was analysed in 3 distinct regions of interests (ROIs): soma, diffuse (extrasynaptic compartment in dendrites) and clusters. At t = 20 min, somatic fluorescence of α2SEP-containing GABAARs was significantly decreased (control F/F0: 0.997 ± 0.02, low Mg2+ F/F0: 0.85 ± 0.05; p = 0.02) indicating that internalisation of GABAARs at the somatic level increases during low Mg2+ treatment (Fig. 1C,F). This could account for a decrease in hippocampal GABAergic inhibition during epileptiform activity. However, α2SEP-fluorescence intensity at the soma was not found to be significantly changed at t = 60 min (control F/F0: 0.92 ± 0.03, low Mg2+ F/F0: 1.01 ± 0.04; p = 0.09) suggesting a biphasic regulation of surface GABAARs during low Mg2+ treatment (Fig. 1C,F'). Interestingly, α2SEP-GABAAR clusters (t = 20 min; control F/F0: 1.02 ± 0.02 low Mg2+ F/F0: 0.96 ± 0.07; p = 0.36) and diffuse (t = 20 min; control F/F0: 1.01 ± 0.02, low Mg2+ F/F0: 0.95 ± 0.05; p = 0.28) fluorescence intensity in the neuronal dendrites during low Mg2+ treatment showed only a minor, non-significant decrease. Our data thus indicates compartmental specificity of low Mg2+ induced decrease of GABAARs from the surface (Fig. 1D,E), with GABAARs primarily endocytosed from the cell soma surface.
Fig. 1.
Somatic surface α2SEP-GABAARs decrease upon low Mg2+ treatment. (A) Representative average intensity projection of α2SEP expression in control and low Mg2+ treated neurons over time (0–3 min and 10–20 min). Scale bar, 10 μm. (B) Kymograph (a line scan vertically projected over time) showing somatic (left; scale bar, 5 μm) and clustered (right; arrow heads indicate clusters; scale bar, 2 μm) α2SEP fluorescence intensity over the movie in control (aCSF) conditions and in the presence of low Mg2+ (grey bar). Red bar on the right indicates a decrease in somatic fluorescence intensity upon low Mg2+ treatment. (C) Average fluorescence intensity of somatic α2SEP GABAAR F/F0: control (green, n = 9 cells) and low Mg2+ (blue, n = 7). (D) Time course of diffuse α2SEP GABAAR F/F0: control (green, n = 9 cells); low Mg2+ (purple, n = 7). (E) Time course of α2SEP GABAAR clusters F/F0: control (green, n = 9 cells) and low Mg2+ (light blue, n = 7). (F) Bar graph of ROI's F/F0: soma (left), diffuse (middle) clusters (right). Significant loss of fluorescence in the soma compared to control at 20 min following low Mg2+ treatment (p = 0.02). Diffuse fluorescence is not altered at 20 min (p = 0.36) after low Mg2+ treatment. Fluorescence intensity of α2SEP GABAAR clusters is unaltered following low Mg2+ treatment (t = 20 min; p = 0.28) compared to control. (F') At 60 min after low Mg2+ treatment somatic (p = 0.09), diffuse (p = 0.85) and clustered (p = 0.42) fluorescence intensity are not significantly altered.*p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Activity of NMDA receptors induces the down-regulation of somatic GABAA receptors from the surface during Low Mg2+ treatment
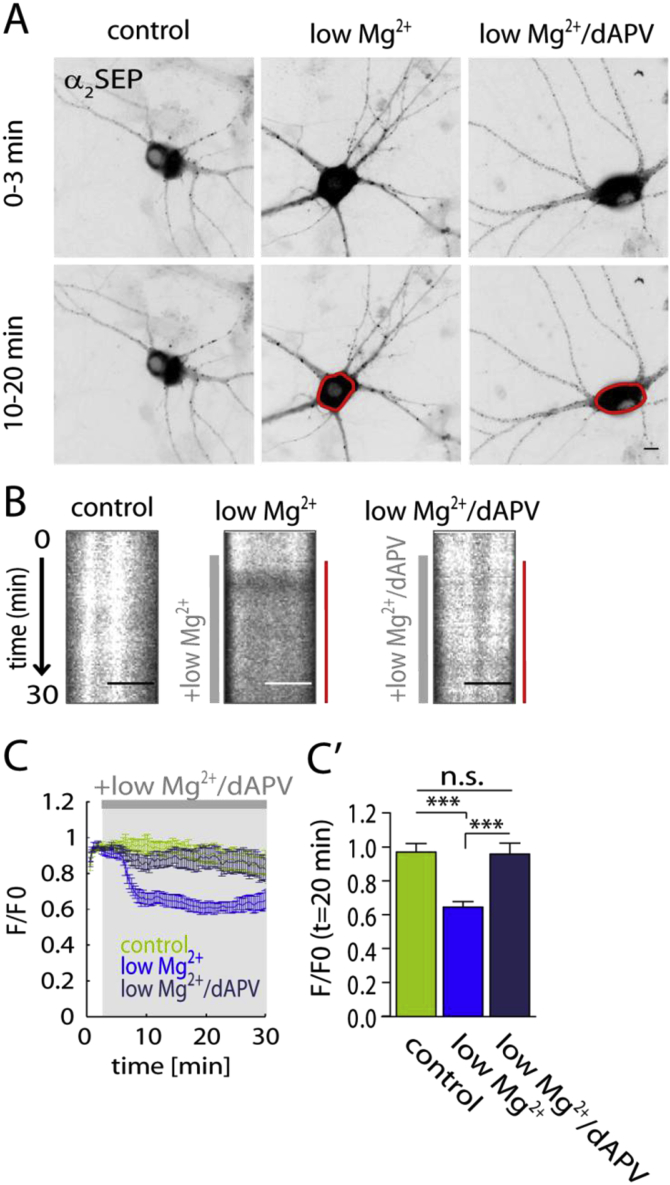
Low extracellular Mg2+ induces epileptiform activity which is abolished by application of the NMDAR antagonist dAPV (Coan and Collingridge, 1985, Coan and Collingridge, 1987, Tancredi et al., 1990, Albowitz et al., 1997, Westerhoff et al., 1995, Gulyás-Kovács et al., 2002, Mangan and Kapur, 2004). Therefore, we tested whether inhibition of NMDAR activity during low Mg2+ treatment blocks the somatic down-regulation of GABAARs (Fig. 2). Low Mg2+ alone induced a significant decrease of α2SEP-GABAAR fluorescence intensity at t = 20 min whereas this loss was inhibited by the co-application of dAPV (t = 20 min; control F/F0: 0.97 ± 0.05, low Mg2+ F/F0: 0.65 ± 0.03 (p < 0.001), low Mg2+/dAPV F/F0: 0.96 ± 0.07 (p > 0.05); one-way ANOVA test, Tukey's multiple comparison post test), confirming that the down regulation of surface GABAARs was dependent on the activation of NMDARs (Fig. 2C,C').
Fig. 2.
NMDARs mediate low Mg2+ induced somatic α2SEP GABAAR surface decrease. (A) Representative images of α2SEP GABAAR fluorescence in control, low Mg2+ and low Mg2+ with dAPV (low Mg2+/dAPV) treated neurons as an average intensity projection over time (0–3 min and 10–20 min). Somatic α2SEP GABAAR loss highlighted in red; scale bar, 10 μm. (B) Kymograph showing somatic (left; scale bar, 5 μm) fluorescence intensity over the movie (duration: 60 min) in control (aCSF) conditions and in the presence of low Mg2+ and low Mg2+/dAPV (grey bar). Red bar on the right indicates decrease in somatic fluorescence intensity upon low Mg2+ treatment and blocking of low Mg2+ induced effect by dAPV (B). (C) Time course of somatic α2SEP GABAAR F/F0: control (green, n = 7 cells), low Mg2+ (blue, n = 8 cells) and low Mg2+/dAPV (dark blue, n = 6 cells). (C') Summary of somatic F/F0 at 20 min after low Mg2+ treatment. Low Mg2+ induces a significant decrease (p < 0.001) in somatic fluorescence intensity, which is inhibited by application of NMDAR blocker dAPV (p < 0.001). ***p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.3. Epileptiform activity evokes intracellular Ca2+ changes that correspond to the temporal dynamics of somatic surface GABAAR decrease
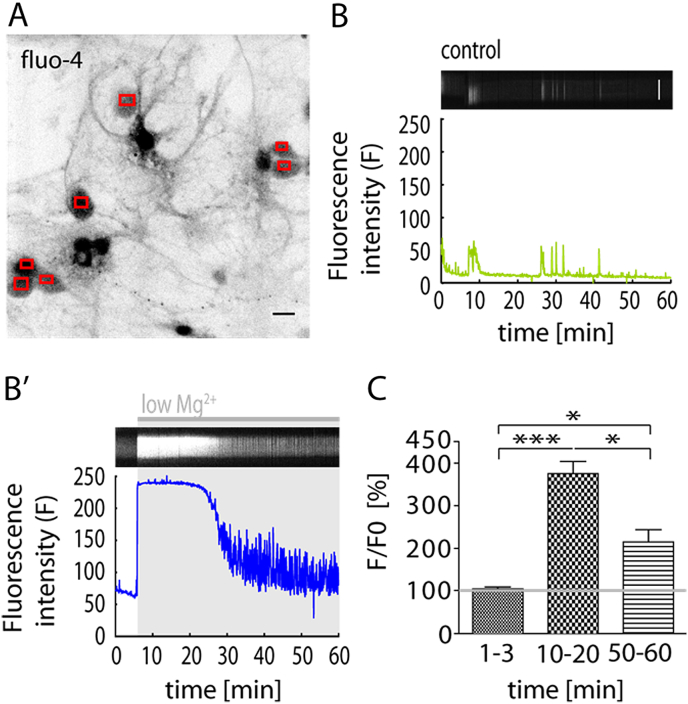
To further explore the mechanisms of NMDAR-driven decrease in surface GABAARs during low Mg2+ treatment, we applied the fluorescent Ca2+ indicator fluo4 in low Mg2+ treated hippocampal neurons. This allowed us to investigate intracellular Ca2+ transients evoked by low Mg2+ treatment. Hippocampal neurons perfused with control aCSF exhibit small Ca2+ transients reflecting spontaneous activity, whereas low Mg2+ perfusion significantly altered intracellular Ca2+ throughout the timeline of 60 min (Fig. 3B,B'). Fluo4 imaging reported intracellular Ca2+ increases rapidly upon early perfusion with low Mg2+ (10–20 min F/F0: 375.8 ± 28.4; p < 0.001, Friedman test and Dunn's multiple comparison post test) and at t = 60 min (F/F0: 215.2 ± 28.8; p < 0.05, Friedman test and Dunn's multiple comparison post test) in comparison to baseline (t = 100–150 s; F/F0: 104.5 ± 4.4), (Fig. 3C). This indicates that low Mg2+ treatment induces an intracellular Ca2+ rise, which is likely to be caused by activation of NMDARs. Interestingly, intracellular Ca2+ concentration drops significantly during the timeline (10–20 min, F/F0: 375.8 ± 28.4; 50–60 min, F/F0: 215.2 ± 28.8; p < 0.05, Friedman test and Dunn's multiple comparison post test) showing that intracellular Ca2+ concentration undergoes alteration on a similar timescale to that of somatic surface GABAAR decrease (Fig. 3C).
Fig. 3.
Low Mg2+ treatment induces intracellular Ca2+ accumulation. (A) Representative average intensity projection of fluo-4 loaded neurons (coloured squares indicate individual cell bodies). (B) Raw fluorescence intensity of spontaneous Ca2+ transients in a neuron (bottom) and correlating kymograph (top; segmented line through somatic ROI) showing fluorescence changes over time under control (aCSF) conditions. (B') Raw fluorescence intensity reporting low Mg2+ (grey bar) induced Ca2+ accumulation (bottom) and kymograph (top) indicating increase in fluorescence intensity. (C) Quantification of increase in intracellular Ca2+ (n = 21 cells). Between 10 and 20 min (averaged data points from min 10 to min 20) after low Mg2+ induction, intracellular Ca2+ is significantly elevated (p < 0.001) compared to baseline (averaged data points from min 0 to min 3). At 50–60 min intracellular Ca2+ has decreased (p < 0.001) compared to 10–20 min and significantly increased (p = 0.02) compared to the baseline. *p < 0.05, ***p < 0.001. Scalebar, 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Dispersion of clustered GABAA receptors is induced by NMDA receptor activation
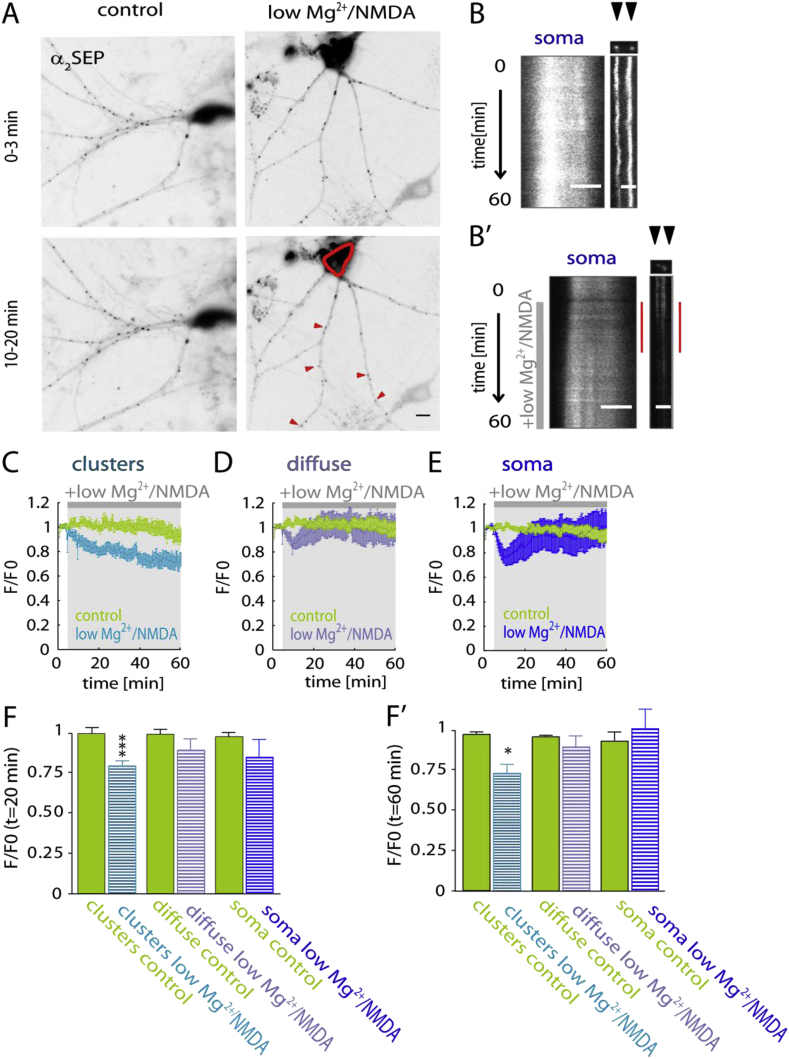
It has been reported that the dispersal of surface GABAAR clusters in neuronal processes is regulated through Ca2+ influx via NMDARs (Muir et al., 2010). Therefore we tested whether further increasing the activation of NMDARs by co-application of low Mg2+ and the agonist NMDA would trigger dispersion of surface GABAAR clusters in proximal dendrites. Indeed, the activation of NMDARs with low Mg2+ in addition to application of the agonist NMDA (low Mg2+/NMDA) caused a loss of α2SEP GABAAR fluorescence intensity in dendritic clusters (Fig. 4A,B,B') at t = 20 min (control F/F0: 1.001 ± 0.04, low Mg2+/NMDA F/F0: 0.799 ± 0.03; p < 0.01) suggesting that surface stability of GABAARs corresponds with the potency of NMDAR activation (Fig. 4C,F). Interestingly, during low Mg2+/NMDA perfusion diffuse (control F/F0: 1.02 ± 0.03, low Mg2+/NMDA F/F0: 0.95 ± 0.09, p = 0.58) and total (data not shown) fluorescence intensity in neuronal processes remains unaltered at t = 20 min (Fig. 4D,F). This indicates dispersion of surface GABAAR upon low Mg2+/NMDA. Somatic GABAAR fluorescence intensity is significantly decreased at t = 10 min after low Mg2+/NMDA (control F/F0: 1.00 ± 0.02, low Mg2+/NMDA F/F0: 0.77 ± 0.059, p < 0.01) treatment, however it is not significantly altered at t = 20 min (control F/F0: 0.98 ± 0.03, low Mg2+/NMDA F/F0: 0.85 ± 0.11, p = 0.34) (Fig. 4 E). Although the decrease in somatic surface GABAARs fluorescence intensity during low Mg2+/NMDA also occurs rapidly after treatment and is of similar size compared to low Mg2+ only treatment, the biphasic recovery phase is shorter in the low Mg2+/NMDA treatment.
Fig. 4.
Clustered α2SEP GABAARs decrease upon low Mg2+/NMDA treatment. (A) Representative average intensity projection of α2SEP GABAAR fluorescence in control and low Mg2+/NMDA treated neurons over time (0–3 min and 10–20 min). Somatic α2SEP GABAAR fluorescence highlighted in red; scale bar, 10 μm. (B) Kymograph showing somatic (left; scale bar, 5 μm) and clustered (right; scale bar, 2 μm) α2SEP GABAAR fluorescence intensity over the movie in control (aCSF) conditions and in the presence of low Mg2+/NMDA (grey bar). Red bar on the right indicates decrease in somatic α2SEP GABAAR fluorescence intensity upon low Mg2+/NMDA treatment. (C) Average fluorescence intensity time course of clustered α2SEP GABAAR F/F0: control (green, n = 7 cells) and low Mg2+/NMDA, (light blue, n = 9). (D) Time course of diffuse α2SEP GABAAR F/F0: control (green, n = 7 cells); low Mg2+, (purple, n = 9). (E) Time course of somatic α2SEP GABAAR F/F0: control (green, n = 7 cells) and low Mg2+ (blue, n = 9). (F) Bar graph of ROI's F/F0: clusters (left), diffuse (middle) soma (right). Significant loss of fluorescence in the clusters compared to control at 20 min following after low Mg2+ treatment (p = 0.0008). Diffuse fluorescence is not altered upon low Mg2+ treatment at 20 min (p = 0.36) after low Mg2+ treatment. Diffuse fluorescence is unaltered following low Mg2+ treatment (t = 20 min; p = 0.58) compared to control. (F') At 60 after low Mg2+ treatment clustered fluorescence intensity is still significantly reduced (p < 0.001), diffuse (p = 0.99) and somatic (p = 0.63) fluorescence are not significantly altered. *p < 0.05, ***p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
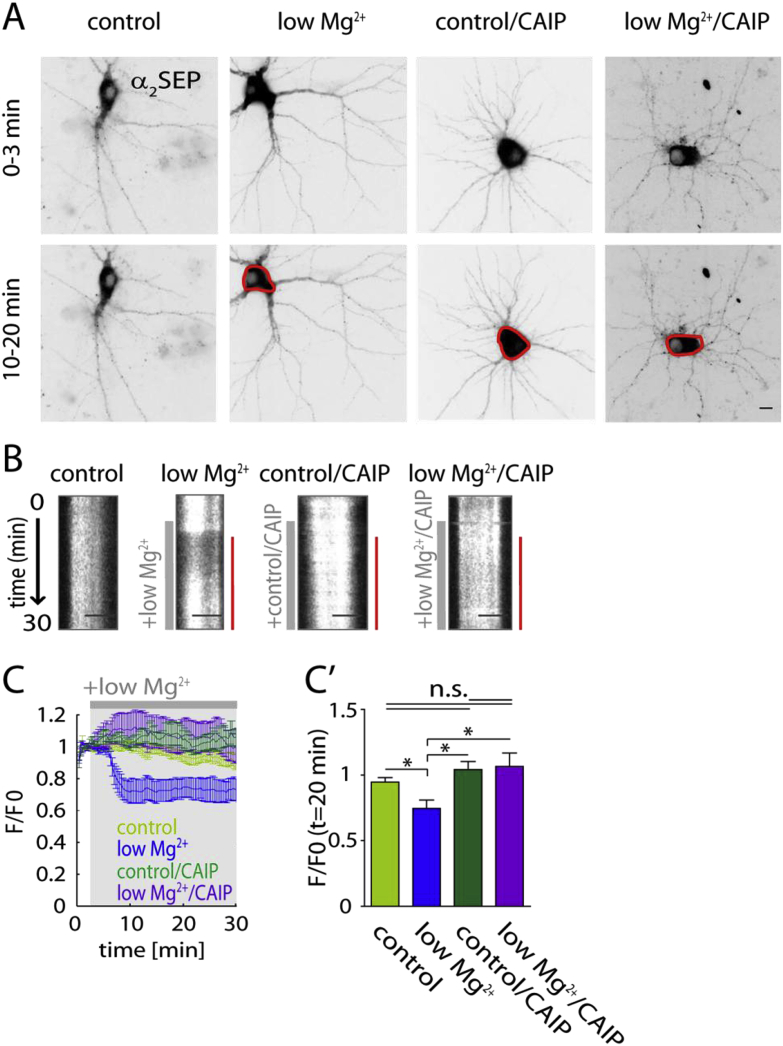
3.5. Calcineurin mediates the decrease of GABAA receptors from the surface during Low Mg2+ induced bursting activity
We next investigated the signalling mechanisms involved in NMDAR mediated GABAAR surface decrease during epileptiform bursting activity. Calcineurin is implicated in activity-dependent regulation of GABAergic inhibition and hence could play an important role in Ca2+ mediated signalling, we therefore analysed its role in GABAAR stability during low Mg2+ bursting (Lu et al., 2000, Wang et al., 2003, Chen and Wong, 1995, Muir et al., 2010). Cells undergoing epileptiform activity showed a decrease in somatic GABAAR fluorescence intensity compared to control. We found that treating cells with a calcineurin autoinhibitory peptide did not significantly affect somatic GABAAR intensity (control F/F0: 1.07 ± 0.10, control/CAIP F/F0: 0.95 ± 0.03; p > 0.05, one-way ANOVA test and Tukey's multiple comparison post test) showing that calcineurin had no effect at t = 20 min in control conditions (Fig. 5C,C'). However, blocking calcineurin activity, inhibited the low Mg2+-induced decrease of surface GABAAR at the soma at t = 20 min significantly (low Mg2+ F/F0: 0.75 ± 0.06, low Mg2+/CAIP F/F0: 1.043 ± 0.06; p < 0.05, one-way ANOVA test and Tukey's multiple comparison post test) (Fig. 5C,C'). These results suggest that calcineurin activation upon Ca2+ influx through NMDARs is directly involved in the decrease of surface GABAAR triggered by epileptiform bursting activity.
Fig. 5.
Calcineurin mediates the decrease of somatic surface GABAARs during low Mg2+ treatment. (A) Representative images of α2SEP GABAAR fluorescence in control, control with CAIP (control/CAIP), low Mg2+ and low Mg2+ with CAIP (low Mg2+/CAIP) treated neurons as an average intensity projection over time (0–3 min and 10–20 min). Scale bar, 10 μm. (B) Kymographs showing somatic (scale bar: 5 μm) fluorescence intensity over the movie (duration: 30 min) in control conditions, control/CAIP and in the presence of low Mg2+ and low Mg2+/CAIP (grey bar). Red bar on the right indicates decrease in somatic fluorescence intensity upon low Mg2+ treatment. (C) Average fluorescence intensity of α2SEP GABAAR F/F0: control (light green, n = 6 cells); low Mg2+ (dark blue, n = 7 cells); control/CAIP (dark green, n = 6); low Mg2+/CAIP (purple, n = 6). (C') Bar graph showing quantification of α2SEP GABAAR F/F0 at t = 20 min. Somatic fluorescence intensity of low Mg2+ treated cells is significantly decreased compared to control at t = 20 min (dark blue bar, p < 0.05). Treatment of low Mg2+ perfused cells with a calcineurin autoinhibitory peptide prevents the change in fluorescence intensity (dark green bar, p < 0.05). Treatment with calcineurin autoinhibitory peptide alone does not significantly alter the fluorescence intensity of GABAAR α2SEP (magenta bar, p > 0.05). *p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
GABAARs are a target for a variety of drugs including benzodiazepines, which are of high clinical relevance for first-line treatment of SE. Therefore it is likely that the modulation of surface stability of GABAARs induces or supports benzodiazepine pharmacoresistance in patients with SE. To identify potential mechanisms facilitating GABAAR internalisation, we performed live-imaging on SEP-tagged GABAARs. The key finding of this study shows epileptiform activity induces activation of calcineurin leading to a decrease in the number of surface GABAARs in the soma. This activity-dependent alteration of inhibitory strength is mediated by activation of NMDARs and is parallelled by an increase in intracellular Ca2+ which in turn is likely to activate calcineurin. These data identify a signalling mechanism underlying surface GABAAR decrease during SE and supports studies that report NMDAR activation regulates GABAergic inhibition (Muir et al., 2010, Bannai et al., 2009, Stelzer et al., 1987).
It is known that internalisation of GABAARs containing α, γ2 or β2/3 subunits is increased during epileptiform activity in vitro using low Mg2+ or high KCl media (Blair et al., 2004, Goodkin et al., 2008, Goodkin et al., 2005). Furthermore, it has been demonstrated that GABAARs are internalised in chemoconvulsant models of SE in vivo (Naylor et al., 2005, Nishimura et al., 2005). Our data supports the hypothesis that GABAAR internalisation occurs during prolonged seizures, by demonstrating that GABAARs which contain the α2 subunits are decreased during low Mg2+ treatment. In the majority of cells this decrease in surface GABAARs was biphasic possibly indicating an adaptational switch in inhibitory strength. Interestingly, we observe that this effect occurs preferentially in the soma but less in dendrites. Compartmental internalisation of GABAARs during SE has not been reported and the mechanisms underlying this differential regulation are unknown, but there are a number of possibilities. Firstly, it remains to be investigated to what extent intracellular Ca2+ buffering systems such as endoplasmic reticulum or mitochondria are contributing to surface stability of GABAARs in neurons undergoing epileptiform activity. Expression of signalling proteins in specific subcellular compartments could explain this effect. Secondly, since this study makes use of experiments based on overexpression of GABAARs, this could account for increased inhibition which could suppress a decrease of GABAARs in dendrites specifically. Omitting Mg2+ in the extracellular medium of cultured hippocampal neurons triggers a sequence of events leading to neuronal death (Kovac et al., 2012, Yoon et al., 2010). To significantly reduce neuronal cell death, mitochondrial substrate sodium pyruvate was added in our experiments. Although we control for this substitution, it would be interesting to test whether mitochondrial ATP production affects GABAAR trafficking during prolonged seizures. Thirdly, correlating the amount of intracellular ATP to the trafficking of GABAARs could contribute to the understanding of compartmentalised trafficking of GABAAR during low Mg2+ treatment.
A comprehensive body of literature describes that low Mg2+ induced epileptiform activity is dependent on increased NMDAR activity (Coan and Collingridge, 1985, 1987; Tancredi et al., 1990, Albowitz et al., 1997, Westerhoff et al., 1995, Gulyás-Kovács et al., 2002, Mangan and Kapur, 2004), therefore we tested whether the somatic surface GABAAR decrease was mediated by NMDAR activity. Our experiments confirm this hypothesis by reporting that the decrease in surface GABAAR was blocked by application of NMDAR blocker dAPV. To our knowledge this is the first study showing a direct regulation of GABAARs by NMDAR activation during low Mg2+ induced epileptiform activity. However, whether the same process underlies the regulation of GABAARs during epileptiform activity induced by different approaches (Goodkin et al., 2008, Laurén et al., 2005) remains to be determined. The observation that NMDAR activation and rises in internal calcium are common to all models of SE (Raza et al., 2004, Rice and DeLorenzo, 1998, Mazarati and Wasterlain, 1999) would suggest that this is a universal mechanism.
Interestingly, the direct activation of NMDARs with low Mg2+ and NMDA induces a potent change in surface stability of GABAAR clusters. The mechanisms underlying this modulation need further investigation, however dephosphorylation of GABAAR γ2 subunits could play a role (Muir et al., 2010). Our data suggest that potency of NMDAR activation correlates with the extent of GABAAR modification, and may explain the synergistic effect of benzodiazepines and NMDAR antagonists in the treatment of SE (Rice and DeLorenzo, 1999, Martin and Kapur, 2008).
Ca2+ influx from the extracellular environment through NMDARs can alter the stability of inhibitory neurotransmitter receptors (Bannai et al., 2009, Muir et al., 2010). Bannai et al. showed that diffusion dynamics of GABAARs too are tuned by Ca2+ entry from the extracellular space. Indeed, here we report an increase in intracellular Ca2+ concentration during low Mg2+ treatment. Moreover, Ca2+ levels correlate with the temporal dynamics of the low Mg2+ induced effect on GABAARs. This indicates that fast Ca2+-signalling could indirectly be altering GABAARs surface stability. Although technically challenging, it would be of major interest to simultaneously dual record Ca2+ dynamics and GABAARs surface stability to better address the relationship of low Mg2+ induced spiking activity and GABAAR trafficking on a single cell level.
Surface stability of GABAARs is regulated by multiple processes which are facilitated by direct or indirect interaction with trafficking proteins (Marsden et al., 2007, Jacob et al., 2008). Our experiments further emphasise the relationship of indirect signalling via intracellular Ca2+ sensing proteins to stabilise neurotransmitter receptors. Downstream effects of Ca2+ sensing proteins such as calmodulin orchestrate a number of target proteins and can trigger selective effects on surface GABAAR stability. It is known that increased phosphorylation of GABAARs contributes to surface stability (Saliba et al., 2012). Terunuma et al. demonstrated deficits in GABAAR phosphorylation during SE mediated by protein kinase C (Terunuma et al., 2008). Opposingly, dephosphorylation induces declustering and increases the diffusion dynamics of GABAARs (Muir et al., 2010). Calcineurin has been shown to interact with GABAARs via the γ2 subunit and it modulates neuronal inhibition. Interestingly, basal and maximal activity of calcineurin is increased (Kurz et al., 2001) and subcellular distribution is altered (Kurz et al., 2003) in SE in vivo. However, it has been poorly investigated whether calcineurin mediates the decrease in surface GABAARs (Wang et al., 2009). We identify calcineurin as a mediator of the decrease in surface GABAAR and therefore, provide a mechanism of inhibitory modulation during SE, and a potential target for therapy. Moreover, this study is the first to demonstrate a decrease in surface GABAARs by live-imaging in hippocampal neurons during SE. The identification of the underlying trafficking mechanism could account for resistance to benzodiazepines and could have additive effects on duration or frequency of seizure activity. Further research is needed to develop more effective therapeutic strategies against SE to which this study will contribute.
Acknowledgements
This work was supported by grants from the UK Medical Research Council, the Wellcome Trust to J.T.K. and UCL School of Life and Medical Sciences Grand Challenge Studentships.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.neuropharm.2014.09.014.
Contributor Information
Matthew C. Walker, Email: m.walker@ucl.ac.uk.
Josef T. Kittler, Email: j.kittler@ucl.ac.uk.
Appendix A. Supplementary data
References
- Albowitz B., König P., Kuhnt U. Spatiotemporal distribution of intracellular calcium transients during epileptiform activity in guinea pig hippocampal slices. J. Neurophysiol. 1997;77(1):491–501. doi: 10.1152/jn.1997.77.1.491. [DOI] [PubMed] [Google Scholar]
- Bannai H. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62(5):670–682. doi: 10.1016/j.neuron.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Blair R.E. Epileptogenesis causes acute and chronic increases in GABAA receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of acquired epilepsy. J. Pharmacol. Exp. Ther. 2004;310(3):871–880. doi: 10.1124/jpet.104.068478. http://www.ncbi.nlm.nih.gov/pubmed/15084648 Available at: (accessed 11.06.14.) [DOI] [PubMed] [Google Scholar]
- Bogdanov Y. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 2006;25(18):4381–4389. doi: 10.1038/sj.emboj.7601309. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1570424&tool=pmcentrez&rendertype=abstract Available at: (accessed 28.05.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brünig I. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 2002;443(1):43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Chen Q.X., Wong R.K. Suppression of GABAA receptor responses by NMDA application in hippocampal neurones acutely isolated from the adult guinea-pig. J. Physiol. 1995;482(Pt 2):353–362. doi: 10.1113/jphysiol.1995.sp020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan E.J., Collingridge G.L. Characterization of an N-methyl-d-aspartate receptor component of synaptic transmission in rat hippocampal slices. Neuroscience. 1987;22(1):1–8. doi: 10.1016/0306-4522(87)90192-8. http://www.sciencedirect.com/science/article/pii/0306452287901928 Available at: (accessed 15.06.14.) [DOI] [PubMed] [Google Scholar]
- Coan E.J., Collingridge G.L. Magnesium ions block an N-methyl-d-aspartate receptor-mediated component of synaptic transmission in rat hippocampus. Neurosci. Lett. 1985;53(1):21–26. doi: 10.1016/0304-3940(85)90091-6. http://www.sciencedirect.com/science/article/pii/0304394085900916 Available at: (accessed 15.06.14.) [DOI] [PubMed] [Google Scholar]
- DeLorenzo R.J., Pal S., Sombati S. Prolonged activation of the N-methyl-D-aspartate receptor-Ca2+ transduction pathway causes spontaneous recurrent epileptiform discharges in hippocampal neurons in culture. Proc. Natl. Acad. Sci. U. S. A. 1998;95(24):14482–14487. doi: 10.1073/pnas.95.24.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodrill C.B., Wilensky A.J. Intellectual impairment as an outcome of status epilepticus. Neurology. 1990;40(5 Suppl. 2):23–27. http://www.ncbi.nlm.nih.gov/pubmed/2185437 Available at: (accessed 11.06.14.) [PubMed] [Google Scholar]
- Goodkin H.P. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J. Neurosci. 2008;28(10):2527–2538. doi: 10.1523/JNEUROSCI.3426-07.2008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2880323&tool=pmcentrez&rendertype=abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin H.P., Yeh J.-L., Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J. Neurosci. 2005;25(23):5511–5520. doi: 10.1523/JNEUROSCI.0900-05.2005. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2878479&tool=pmcentrez&rendertype=abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás-Kovács A. Comparison of spontaneous and evoked epileptiform activity in three in vitro epilepsy models. Brain Res. 2002;945(2):174–180. doi: 10.1016/s0006-8993(02)02751-8. http://www.sciencedirect.com/science/article/pii/S0006899302027518 Available at: (accessed 15.06.14.) [DOI] [PubMed] [Google Scholar]
- Jacob T.C., Moss S.J., Jurd R. Reviews GABA A receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nature. 2008;9(May):331–343. doi: 10.1038/nrn2370. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2709246/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler J.T. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J. Neurosci. 2000;20(21):7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. http://www.jneurosci.org/content/20/21/7972.short Available at: (accessed 11.06.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler J.T. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating γ-aminobutyric acid type A receptor membrane trafficking. Proc. Natl. Acad. Sci. U. S. A. 2004;101(34):12736–12741. doi: 10.1073/pnas.0401860101. http://discovery.ucl.ac.uk/9648/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac S. Prolonged seizure activity impairs mitochondrial bioenergetics and induces cell death. J. Cell Sci. 2012;125(Pt 7):1796–1806. doi: 10.1242/jcs.099176. http://jcs.biologists.org/content/125/7/1796.long Available at: (accessed 28.03.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz J.E. A significant increase in both basal and maximal calcineurin activity in the rat pilocarpine model of status epilepticus. J. Neurochem. 2001;78(2):304–315. doi: 10.1046/j.1471-4159.2001.00426.x. http://doi.wiley.com/10.1046/j.1471-4159.2001.00426.x Available at: (accessed 19.06.14.) [DOI] [PubMed] [Google Scholar]
- Kurz J.E. Status epilepticus-induced changes in the subcellular distribution and activity of calcineurin in rat forebrain. Neurobiol. Dis. 2003;14(3):483–493. doi: 10.1016/j.nbd.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Laurén H.B. Kainic acid-induced status epilepticus alters GABA receptor subunit mRNA and protein expression in the developing rat hippocampus. J. Neurochem. 2005;94(5):1384–1394. doi: 10.1111/j.1471-4159.2005.03274.x. http://www.ncbi.nlm.nih.gov/pubmed/15992369 Available at: (accessed 13.07.14.) [DOI] [PubMed] [Google Scholar]
- Lothman E.W. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40:13–23. [PubMed] [Google Scholar]
- Lu Y.M. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26(1):197–205. doi: 10.1016/s0896-6273(00)81150-2. http://www.sciencedirect.com/science/article/pii/S0896627300811502 Available at: (accessed 15.06.14.) [DOI] [PubMed] [Google Scholar]
- Luscher B., Fuchs T., Kilpatrick C.L. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70(3):385–409. doi: 10.1016/j.neuron.2011.03.024. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3093971&tool=pmcentrez&rendertype=abstract Available at: (accessed 24.05.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan P.S., Kapur J. Factors underlying bursting behavior in a network of cultured hippocampal neurons exposed to zero magnesium. J. Neurophysiol. 2004;91(2):946–957. doi: 10.1152/jn.00547.2003. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2892720&tool=pmcentrez&rendertype=abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden K.C. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J. Neurosci. Off. J. Soc. Neurosci. 2007;27(52):14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. http://www.ncbi.nlm.nih.gov/pubmed/18160640 Available at: (accessed 05.06.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B.S., Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49(2):248–255. doi: 10.1111/j.1528-1167.2007.01384.x. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2844443&tool=pmcentrez&rendertype=abstract Available at: (accessed 13.07.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati A.M., Wasterlain C.G. N-methyl-d-asparate receptor antagonists abolish the maintenance phase of self-sustaining status epilepticus in rat. Neurosci. Lett. 1999;265(3):187–190. doi: 10.1016/s0304-3940(99)00238-4. http://www.sciencedirect.com/science/article/pii/S0304394099002384 Available at: (accessed 13.07.14.) [DOI] [PubMed] [Google Scholar]
- Muir J. NMDA receptors regulate GABAA receptor lateral mobility and clustering at inhibitory synapses through serine 327 on the γ2 subunit. Proc. Natl. Acad. Sci. U. S. A. 2010;107(38):16679–16684. doi: 10.1073/pnas.1000589107. http://discovery.ucl.ac.uk/168536/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J. The residence time of GABAARs at inhibitory synapses is determined by direct binding of the receptor 1 subunit to gephyrin. J. Neurosci. 2011;31(41):14677–14687. doi: 10.1523/JNEUROSCI.2001-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor D.E., Liu H., Wasterlain C.G. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J. Neurosci. 2005;25(34):7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. http://www.ncbi.nlm.nih.gov/pubmed/16120773 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T. Altered expression of GABA(A) and GABA(B) receptor subunit mRNAs in the hippocampus after kindling and electrically induced status epilepticus. Neuroscience. 2005;134(2):691–704. doi: 10.1016/j.neuroscience.2005.04.013. http://www.ncbi.nlm.nih.gov/pubmed/15951123 Available at: [DOI] [PubMed] [Google Scholar]
- Panzanelli P. Distinct mechanisms regulate GABAA receptor and gephyrin clustering at perisomatic and axo-axonic synapses on CA1 pyramidal cells. J. Physiol. 2011;589(20):4959–4980. doi: 10.1113/jphysiol.2011.216028. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3224886&tool=pmcentrez&rendertype=abstract Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini E.M. Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat. Commun. 2014;5:3921. doi: 10.1038/ncomms4921. http://www.ncbi.nlm.nih.gov/pubmed/24894704 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett D.B. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338(6216):582–585. doi: 10.1038/338582a0. Available at: (accessed 30.05.14.) [DOI] [PubMed] [Google Scholar]
- Raza M. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc. Natl. Acad. Sci. U. S. A. 2004;101(50):17522–17527. doi: 10.1073/pnas.0408155101. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=535000&tool=pmcentrez&rendertype=abstract Available at: (accessed 13.07.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A.C., DeLorenzo R.J. NMDA receptor activation during status epilepticus is required for the development of epilepsy. Brain Res. 1998;782(1–2):240–247. doi: 10.1016/s0006-8993(97)01285-7. http://www.ncbi.nlm.nih.gov/pubmed/9519269 Available at: (accessed 13.07.14.) [DOI] [PubMed] [Google Scholar]
- Rice A.C., DeLorenzo R.J. N-methyl-D-aspartate receptor activation regulates refractoriness of status epilepticus to diazepam. Neuroscience. 1999;93(1):117–123. doi: 10.1016/s0306-4522(99)00132-3. http://www.ncbi.nlm.nih.gov/pubmed/10430476 Available at: (accessed 13.07.14.) [DOI] [PubMed] [Google Scholar]
- Robinson H.P. Periodic synchronized bursting and intracellular calcium transients elicited by low magnesium in cultured cortical neurons. J. Neurophysiol. 1993;70(4):1606–1616. doi: 10.1152/jn.1993.70.4.1606. http://jn.physiology.org/content/70/4/1606.abstract Available at: (accessed 14.05.14.) [DOI] [PubMed] [Google Scholar]
- Saliba R.S., Kretschmannova K., Moss S.J. Activity-dependent phosphorylation of GABAA receptors regulates receptor insertion and tonic current. EMBO J. 2012;31(13):2937–2951. doi: 10.1038/emboj.2012.109. http://www.ncbi.nlm.nih.gov/pubmed/22531784 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombati S., Delorenzo R.J. Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J. Neurophysiol. 1995;73(4):1706–1711. doi: 10.1152/jn.1995.73.4.1706. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Slater N.T., ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987;326(6114):698–701. doi: 10.1038/326698a0. Available at: (accessed 05.07.14.) [DOI] [PubMed] [Google Scholar]
- Sutter R. Mortality and recovery from refractory status epilepticus in the intensive care unit: a 7-year observational study. Epilepsia. 2013;54(3):502–511. doi: 10.1111/epi.12064. http://www.ncbi.nlm.nih.gov/pubmed/23294049 Available at: (accessed 28.05.14.) [DOI] [PubMed] [Google Scholar]
- Tancredi V. Low magnesium epileptogenesis in the rat hippocampal slice: electrophysiological and pharmacological features. Brain Res. 1990;511(2):280–290. doi: 10.1016/0006-8993(90)90173-9. [DOI] [PubMed] [Google Scholar]
- Terada H. Inhibition of excitatory neuronal cell death by cell-permeable calcineurin autoinhibitory peptide. J. Neurochem. 2003;87(5):1145–1151. doi: 10.1046/j.1471-4159.2003.02098.x. [DOI] [PubMed] [Google Scholar]
- Terunuma M. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J. Neurosci. 2008;28(2):376–384. doi: 10.1523/JNEUROSCI.4346-07.2008. http://discovery.ucl.ac.uk/134982/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thévenaz P., Ruttimann U.E., Unser M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 1998;7(1):27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Tretter V. The clustering of GABA(A) receptor subtypes at inhibitory synapses is facilitated via the direct binding of receptor alpha 2 subunits to gephyrin. J. Neurosci. 2008;28(6):1356–1365. doi: 10.1523/JNEUROSCI.5050-07.2008. http://www.ncbi.nlm.nih.gov/pubmed/18256255 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinka E., Höfler J., Zerbs A. Causes of status epilepticus. Epilepsia. 2012;53(Suppl. 4):127–138. doi: 10.1111/j.1528-1167.2012.03622.x. [DOI] [PubMed] [Google Scholar]
- Wang A. Calcineurin-mediated GABAA receptor dephosphorylation in rats after kainic acid-induced status epilepticus. Seizure. 2009;18(7):519–523. doi: 10.1016/j.seizure.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Wang J. Interaction of calcineurin and type-A GABA receptor gamma 2 subunits produces long-term depression at CA1 inhibitory synapses. J. Neurosci. Off. J. Soc. Neurosci. 2003;23(3):826–836. doi: 10.1523/JNEUROSCI.23-03-00826.2003. http://www.ncbi.nlm.nih.gov/pubmed/12574411 Available at: (accessed 27.03.14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhoff C.H., Domann R., Witte O.W. Inhibitory mechanisms in epileptiform activity induced by low magnesium. Pflugers Arch. 1995;430(2):238–245. doi: 10.1007/BF00374655. [DOI] [PubMed] [Google Scholar]
- Yoon J.J. Effect of low Mg2+ and bicuculline on cell survival in hippocampal slice cultures. Int. J. Neurosci. 2010;120(12):752–759. doi: 10.3109/00207454.2010.520378. http://www.ncbi.nlm.nih.gov/pubmed/20942591 Available at: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.