Abstract
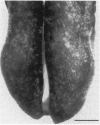
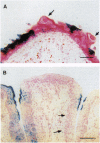
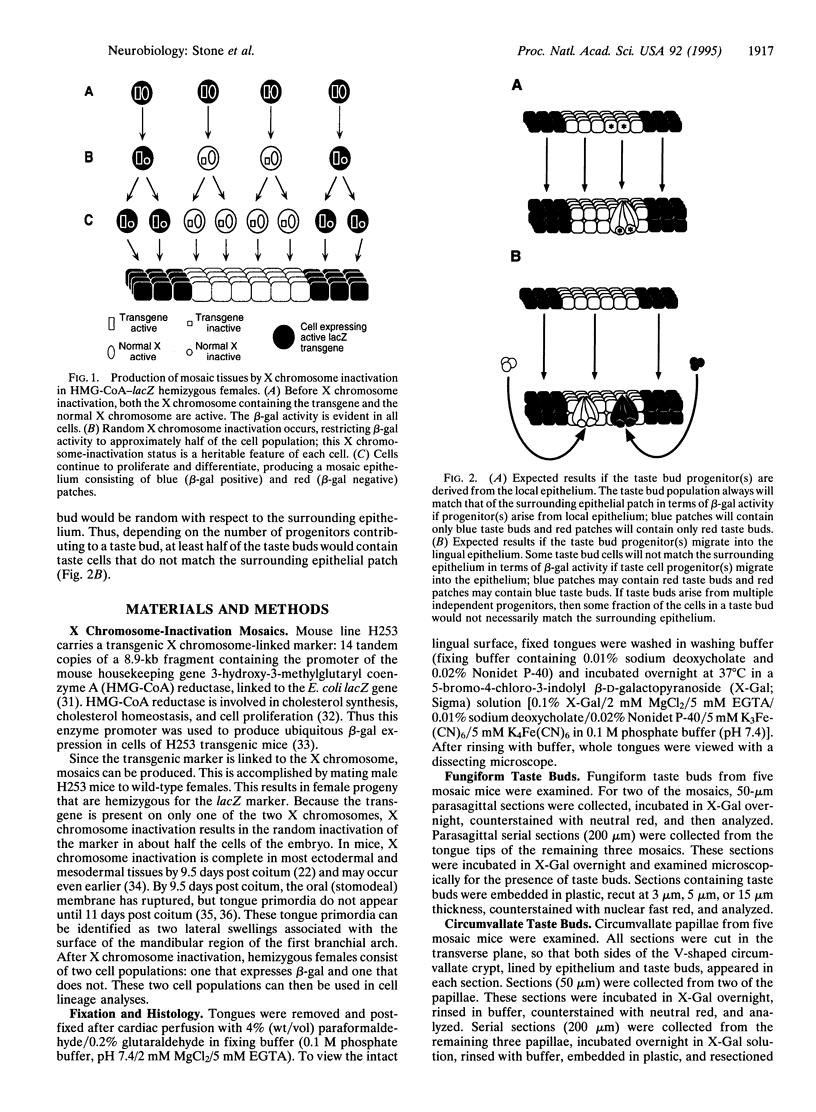
Except for taste bud cells, all sensory receptor cells and neurons have been shown to originate from neurogenic ectoderm (i.e., neural tube, neural crest, or ectodermal placodes). Descriptive studies on taste buds indicate that they, however, may arise from local epithelium. To determine whether taste receptor cells originate from neurogenic ectoderm or from local epithelium, the tongues of X chromosome-inactivation mosaic mice were examined. Results of this analysis show that taste bud cells and their surrounding epithelium always match in terms of the mosaic marker. This suggests that taste cells and epithelial cells arise from a common progenitor and that taste receptor cells originate from local tissue elements. Since taste buds are widespread in the oropharynx, they lie in epithelium derived from both ectoderm and endoderm. Therefore, taste receptor cells can be induced in tissue from two different germ layers. Thus in terms of tissues of origin, taste receptor cells are unlike other cells with neuronal characteristics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler D. A., West J. D., Chapman V. M. Expression of alpha-galactosidase in preimplantation mouse embryos. Nature. 1977 Jun 30;267(5614):838–839. doi: 10.1038/267838a0. [DOI] [PubMed] [Google Scholar]
- Beidler L. M., Smallman R. L. Renewal of cells within taste buds. J Cell Biol. 1965 Nov;27(2):263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R. M., Stern I. B. The development of the human taste bud during the foetal period. J Anat. 1967 Sep;101(Pt 4):743–752. [PMC free article] [PubMed] [Google Scholar]
- Collazo A., Fraser S. E., Mabee P. M. A dual embryonic origin for vertebrate mechanoreceptors. Science. 1994 Apr 15;264(5157):426–430. doi: 10.1126/science.8153631. [DOI] [PubMed] [Google Scholar]
- Conger A. D., Wells M. A. Radiation and aging effect on taste structure and function. Radiat Res. 1969 Jan;37(1):31–49. [PubMed] [Google Scholar]
- Epstein C. J., Smith S., Travis B., Tucker G. Both X chromosomes function before visible X-chromosome inactivation in female mouse embryos. Nature. 1978 Aug 3;274(5670):500–503. doi: 10.1038/274500a0. [DOI] [PubMed] [Google Scholar]
- FARBMAN A. I. ELECTRON MICROSCOPE STUDY OF THE DEVELOPING TASTE BUD IN RAT FUNGIFORM PAPILLA. Dev Biol. 1965 Feb;11:110–135. doi: 10.1016/0012-1606(65)90040-0. [DOI] [PubMed] [Google Scholar]
- Farbman A. I., Mbiene J. P. Early development and innervation of taste bud-bearing papillae on the rat tongue. J Comp Neurol. 1991 Feb 8;304(2):172–186. doi: 10.1002/cne.903040203. [DOI] [PubMed] [Google Scholar]
- Farbman A. I. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980 Jul;13(4):349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Gautier C., Mehtali M., Lathe R. A ubiquitous mammalian expression vector, pHMG, based on a housekeeping gene promoter. Nucleic Acids Res. 1989 Oct 25;17(20):8389–8389. doi: 10.1093/nar/17.20.8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Hosley M. A., Hughes S. E., Morton L. L., Oakley B. A sensitive period for the neural induction of taste buds. J Neurosci. 1987 Jul;7(7):2075–2080. doi: 10.1523/JNEUROSCI.07-07-02075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosley M. A., Hughes S. E., Oakley B. Neural induction of taste buds. J Comp Neurol. 1987 Jun 8;260(2):224–232. doi: 10.1002/cne.902600206. [DOI] [PubMed] [Google Scholar]
- Kusakabe M., Yokoyama M., Sakakura T., Nomura T., Hosick H. L., Nishizuka Y. A novel methodology for analysis of cell distribution in chimeric mouse organs using a strain specific antibody. J Cell Biol. 1988 Jul;107(1):257–265. doi: 10.1083/jcb.107.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Monk M., Harper M. I. Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature. 1979 Sep 27;281(5729):311–313. doi: 10.1038/281311a0. [DOI] [PubMed] [Google Scholar]
- Noden D. M. Spatial integration among cells forming the cranial peripheral nervous system. J Neurobiol. 1993 Feb;24(2):248–261. doi: 10.1002/neu.480240210. [DOI] [PubMed] [Google Scholar]
- Noden D. M. Vertebrate craniofacial development: the relation between ontogenetic process and morphological outcome. Brain Behav Evol. 1991;38(4-5):190–225. doi: 10.1159/000114388. [DOI] [PubMed] [Google Scholar]
- Northcutt R. G., Catania K. C., Criley B. B. Development of lateral line organs in the axolotl. J Comp Neurol. 1994 Feb 22;340(4):480–514. doi: 10.1002/cne.903400404. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Schmidt G. H., Wilkinson M. M., Wood M. J., Monk M., Reid A. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985 Feb 21;313(6004):689–691. doi: 10.1038/313689a0. [DOI] [PubMed] [Google Scholar]
- Rastan S. Timing of X-chromosome inactivation in postimplantation mouse embryos. J Embryol Exp Morphol. 1982 Oct;71:11–24. [PubMed] [Google Scholar]
- Roper S. D. The cell biology of vertebrate taste receptors. Annu Rev Neurosci. 1989;12:329–353. doi: 10.1146/annurev.ne.12.030189.001553. [DOI] [PubMed] [Google Scholar]
- Roper S. D. The microphysiology of peripheral taste organs. J Neurosci. 1992 Apr;12(4):1127–1134. doi: 10.1523/JNEUROSCI.12-04-01127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. H., Blount M. A., Ponder B. A. Immunochemical demonstration of the clonal organization of chimaeric mouse epidermis. Development. 1987 Jul;100(3):535–541. doi: 10.1242/dev.100.3.535. [DOI] [PubMed] [Google Scholar]
- Tam P. P., Tan S. S. The somitogenetic potential of cells in the primitive streak and the tail bud of the organogenesis-stage mouse embryo. Development. 1992 Jul;115(3):703–715. doi: 10.1242/dev.115.3.703. [DOI] [PubMed] [Google Scholar]
- Tam P. P., Williams E. A., Tan S. S. Expression of an X-linked HMG-lacZ transgene in mouse embryos: implication of chromosomal imprinting and lineage-specific X-chromosome activity. Dev Genet. 1994;15(6):491–503. doi: 10.1002/dvg.1020150608. [DOI] [PubMed] [Google Scholar]
- Tan S. S., Breen S. Radial mosaicism and tangential cell dispersion both contribute to mouse neocortical development. Nature. 1993 Apr 15;362(6421):638–640. doi: 10.1038/362638a0. [DOI] [PubMed] [Google Scholar]
- Tan S. S., Williams E. A., Tam P. P. X-chromosome inactivation occurs at different times in different tissues of the post-implantation mouse embryo. Nat Genet. 1993 Feb;3(2):170–174. doi: 10.1038/ng0293-170. [DOI] [PubMed] [Google Scholar]
- Vischer H. A. The development of lateral-line receptors in Eigenmannia (Teleostei, Gymnotiformes). I. The mechanoreceptive lateral-line system. Brain Behav Evol. 1989;33(4):205–222. doi: 10.1159/000115929. [DOI] [PubMed] [Google Scholar]
- Vischer H. A. The development of lateral-line receptors in Eigenmannia (Teleostei, Gymnotiformes). II. The electroreceptive lateral-line system. Brain Behav Evol. 1989;33(4):223–236. doi: 10.1159/000115930. [DOI] [PubMed] [Google Scholar]
- Winklbauer R., Hausen P. Development of the lateral line system in Xenopus laevis. I. Normal development and cell movement in the supraorbital system. J Embryol Exp Morphol. 1983 Aug;76:265–281. [PubMed] [Google Scholar]