Abstract
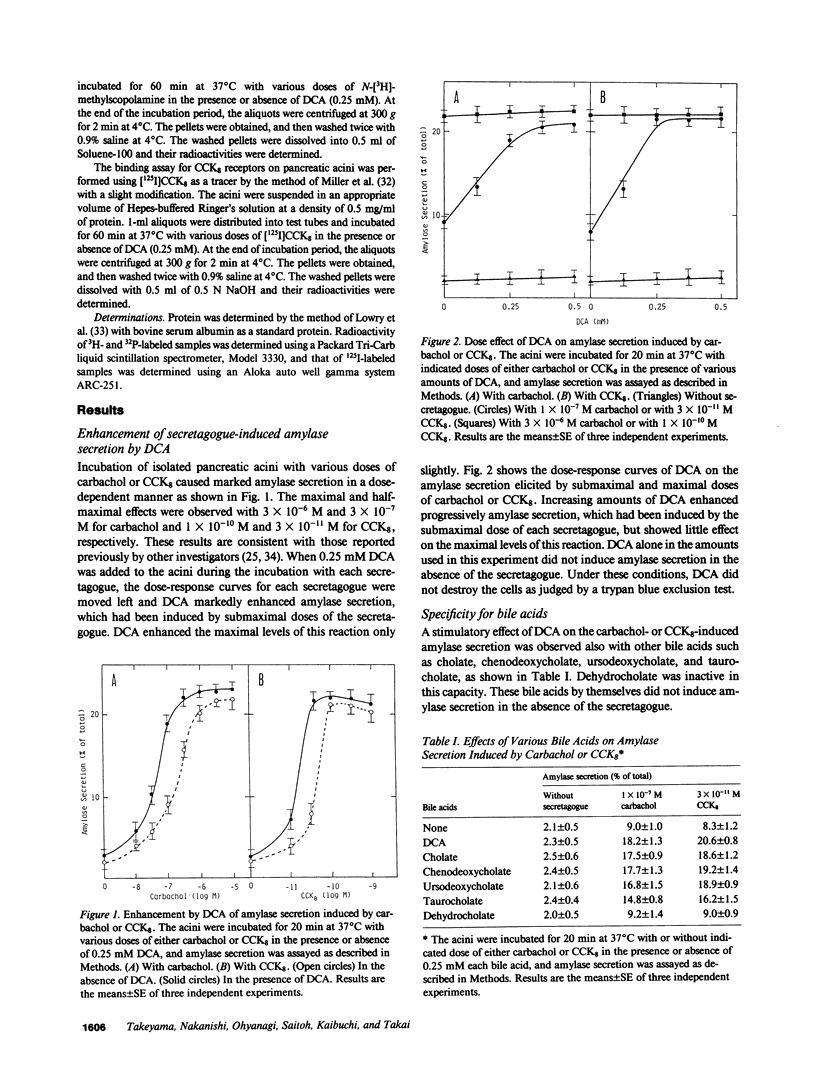
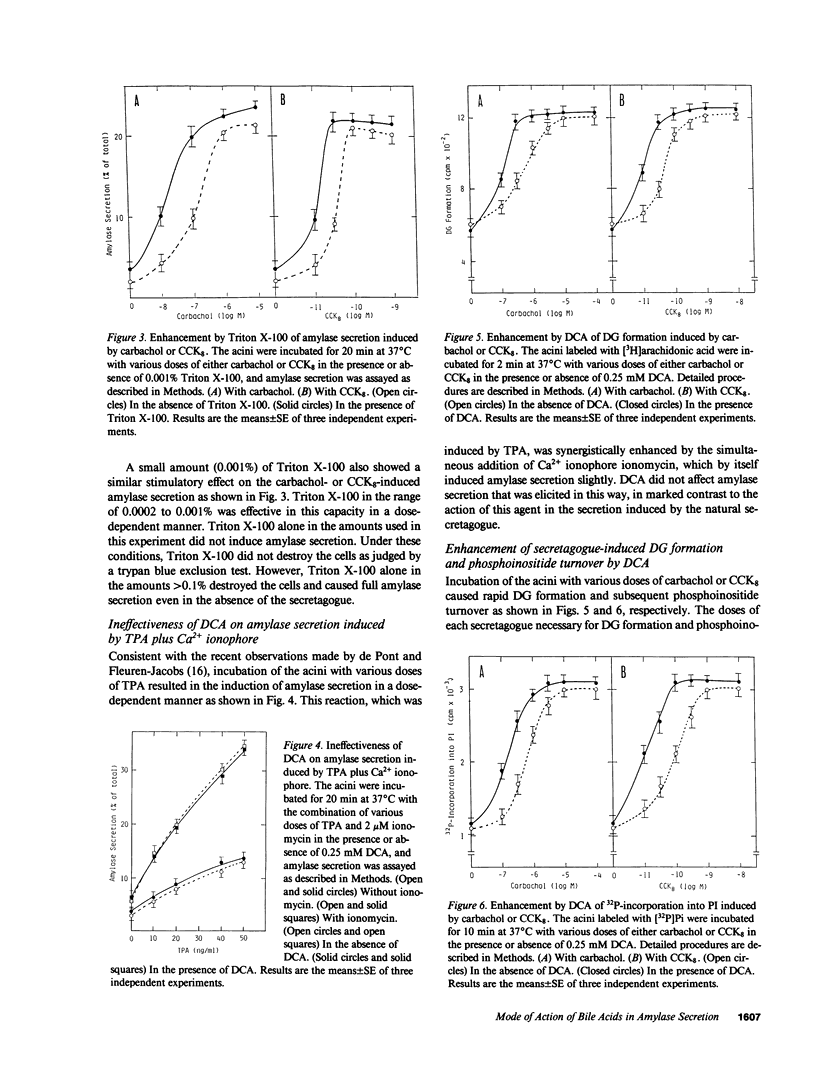
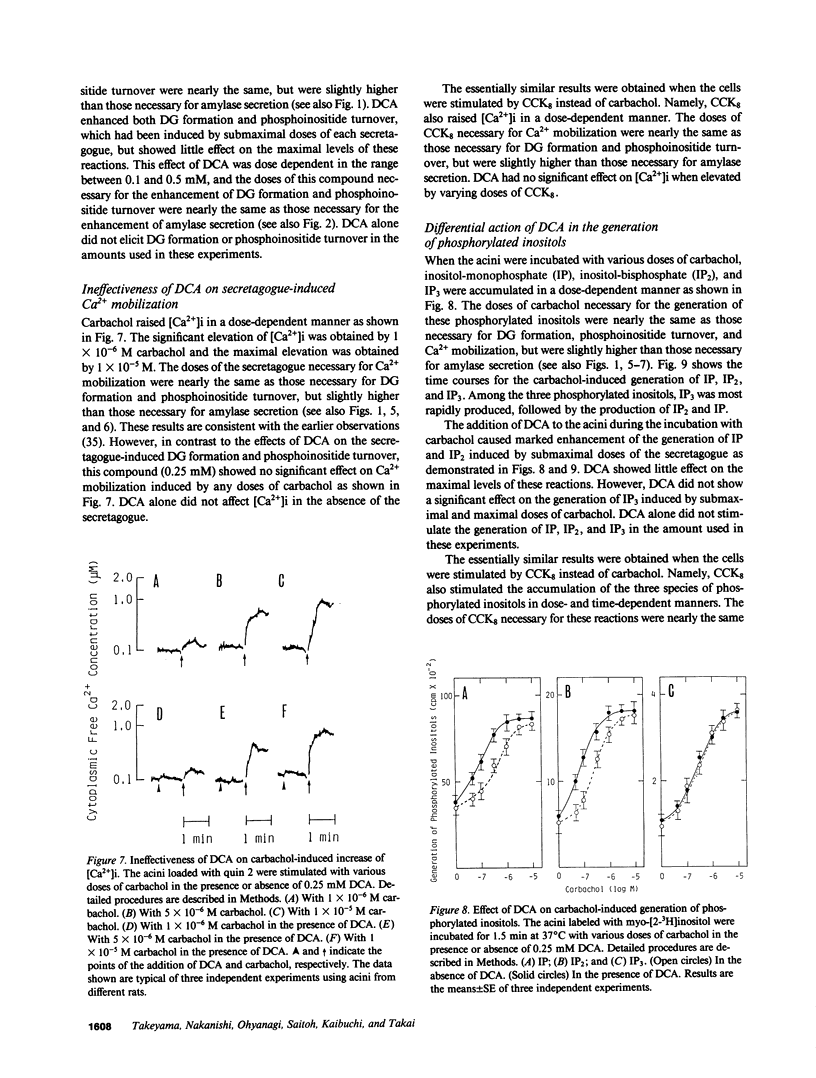
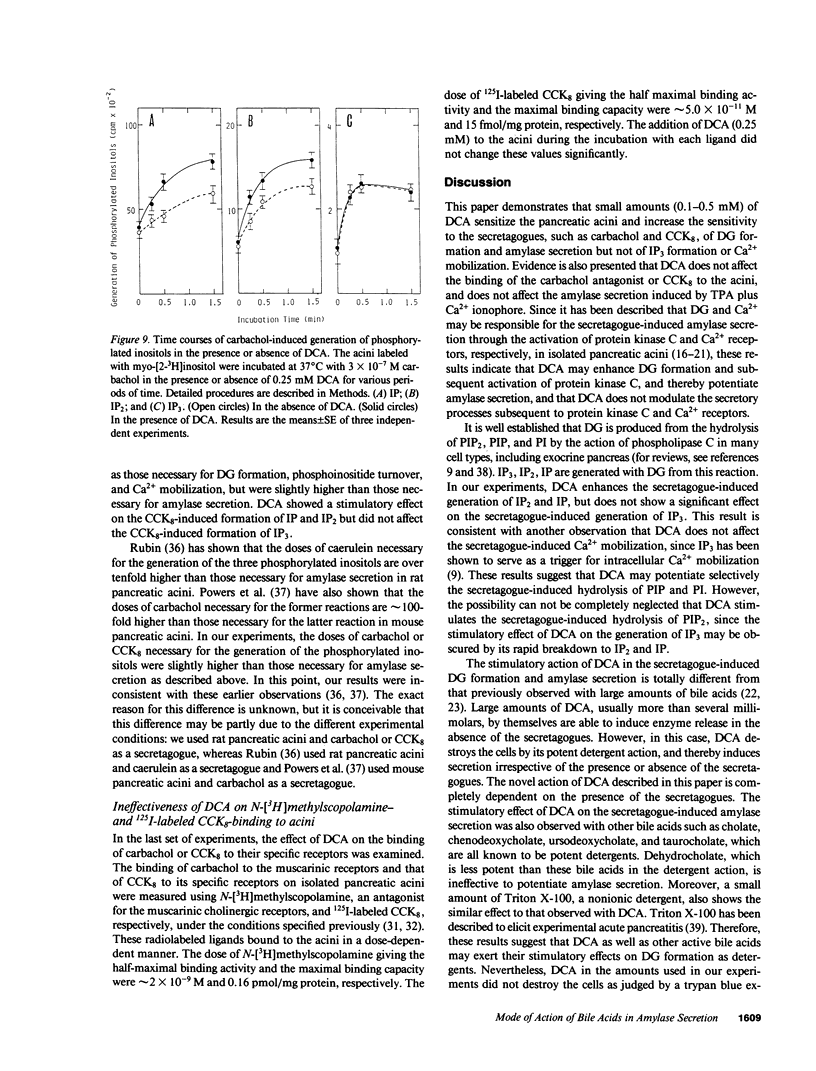
Small amounts (0.1-0.5 mM) of deoxycholate enhanced amylase secretion, which had been induced by submaximal doses of carbachol or cholecystokinin octapeptide, without affecting the maximal levels of these reactions from isolated rat pancreatic acini. Deoxycholate alone did not induce these reactions. The other bile acids such as cholate, chenodeoxycholate, ursodeoxycholate, and taurocholate were also active. Under the similar conditions, deoxycholate enhanced the secretagogue-induced diacylglycerol formation that was derived mainly from the phospholipase C-mediated hydrolysis of phosphatidylinositol and phosphatidylinositol-4-monophosphate. Deoxycholate did not enhance the secretagogue-induced hydrolysis of phosphatidylinositol-4,5-bisphosphate or Ca2+ mobilization. Deoxycholate did not affect amylase secretion, which was induced by the simultaneous addition of protein kinase C-activating 12-O-tetradecanoylphorbol-13-acetate and Ca2+ ionophore ionomycin. Since diacylglycerol and Ca2+ may be responsible for the secretagogue-induced amylase secretion, our results indicate that small amounts of bile acids increase the sensitivity to the secretagogue of diacylglycerol formation and subsequent activation of protein kinase C, and thereby enhance amylase secretion from pancreatic acini.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. C., Schiller W. R. Microcirculatory dynamics in the normal and inflamed pancreas. Am J Surg. 1968 Jan;115(1):118–127. doi: 10.1016/0002-9610(68)90139-6. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Banschbach M. W., Geison R. L., Hokin-Neaverson M. Acetylcholine increases the level of diglyceride in mouse pancreas. Biochem Biophys Res Commun. 1974 Jun 4;58(3):714–718. doi: 10.1016/s0006-291x(74)80476-6. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 and phospholipase C activities of platelets. Differential substrate specificity, Ca2+ requirement, pH dependence, and cellular localization. J Biol Chem. 1980 Nov 10;255(21):10227–10231. [PubMed] [Google Scholar]
- Bockman D. E., Schiller W. R., Anderson M. C. Route of retrograde flow in the exocrine pancreas during ductal hypertension. Arch Surg. 1971 Aug;103(2):321–329. doi: 10.1001/archsurg.1971.01350080237038. [DOI] [PubMed] [Google Scholar]
- Calderon P., Furnelle J., Christophe J. In vitro lipid metabolism in the rat pancreas. III. Effects of carbamylcholine and pancreozymin on the turnover of phosphatidylinositols, 1,2-diacylglycerols and phosphatidylcholines. Biochim Biophys Acta. 1979 Sep 28;574(3):404–422. doi: 10.1016/0005-2760(79)90236-4. [DOI] [PubMed] [Google Scholar]
- Dehaye J. P., Winand J., Poloczek P., Christophe J. Characterization of muscarinic cholinergic receptors on rat pancreatic acini by N-[3H]methylscopolamine binding. Their relationship with calcium 45 efflux and amylase secretion. J Biol Chem. 1984 Jan 10;259(1):294–300. [PubMed] [Google Scholar]
- ELLIOTT D. W., WILLIAMS R. D., ZOLLINGER R. M. Alterations in the pancreatic resistance to bile in the pathogenesis of acute pancreatitis. Ann Surg. 1957 Oct;146(4):669–682. doi: 10.1097/00000658-195710000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halenda S. P., Rubin R. P. Phospholipid turnover in isolated rat pancreatic acini. Consideration of the relative roles of phospholipase A2 and phospholipase C. Biochem J. 1982 Dec 15;208(3):713–721. doi: 10.1042/bj2080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson K. Experimental and clinical studies in aetiologic role of bile reflux in acute pancreatitis. Acta Chir Scand Suppl. 1967;375:102–102. [PubMed] [Google Scholar]
- Irie A., Hunaki M., Bando K., Kawai K. Activation of alpha-amylase in urine. Clin Chim Acta. 1974 Mar 26;51(3):241–245. doi: 10.1016/0009-8981(74)90309-x. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kajikawa N., Kaibuchi K., Matsubara T., Kikkawa U., Takai Y., Nishizuka Y., Itoh K., Tomioka C. A possible role of protein kinase C in signal-induced lysosomal enzyme release. Biochem Biophys Res Commun. 1983 Oct 31;116(2):743–750. doi: 10.1016/0006-291x(83)90587-9. [DOI] [PubMed] [Google Scholar]
- Katakami Y., Kaibuchi K., Sawamura M., Takai Y., Nishizuka Y. Synergistic action of protein kinase C and calcium for histamine release from rat peritoneal mast cells. Biochem Biophys Res Commun. 1984 Jun 15;121(2):573–578. doi: 10.1016/0006-291x(84)90220-1. [DOI] [PubMed] [Google Scholar]
- Kojima I., Lippes H., Kojima K., Rasmussen H. Aldosterone secretion: effect of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Oct 31;116(2):555–562. doi: 10.1016/0006-291x(83)90559-4. [DOI] [PubMed] [Google Scholar]
- Korbová L., Kohout J., Malis F., Balas V., Cízková J., Marek J., Cihák A. Inhibitory effect of various cytostatics and cycloheximide on acute experimental pancreatitis in rats. Gut. 1977 Nov;18(11):913–918. doi: 10.1136/gut.18.11.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller L. J., Rosenzweig S. A., Jamieson J. D. Preparation and characterization of a probe for the cholecystokinin octapeptide receptor, N alpha (125I-desaminotyrosyl)CCK-8, and its interactions with pancreatic acini. J Biol Chem. 1981 Dec 10;256(23):12417–12423. [PubMed] [Google Scholar]
- Ohlsson K., Eddeland A. Release of proteolytic enzymes in bile-induced pancreatitis in dogs. Gastroenterology. 1975 Sep;69(3):668–675. [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Sachs G., Muallem S. Role of free cytosolic calcium in secretagogue-stimulated amylase release from dispersed acini from guinea pig pancreas. J Biol Chem. 1985 Aug 25;260(18):10081–10086. [PubMed] [Google Scholar]
- Peikin S. R., Rottman A. J., Batzri S., Gardner J. D. Kinetics of amylase release by dispersed acini prepared from guinea pig pancreas. Am J Physiol. 1978 Dec;235(6):E743–E749. doi: 10.1152/ajpendo.1978.235.6.E743. [DOI] [PubMed] [Google Scholar]
- Powers R. E., Saluja A. K., Houlihan M. J., Steer M. L. Inositol trisphosphate production and amylase secretion in mouse pancreatic acini. Biochem Biophys Res Commun. 1985 Aug 30;131(1):284–288. doi: 10.1016/0006-291x(85)91800-5. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Burgess G. M., Halenda S. P., McKinney J. S., Rubin R. P. Effects of secretagogues on [32P]phosphatidylinositol 4,5-bisphosphate metabolism in the exocrine pancreas. Biochem J. 1983 May 15;212(2):483–488. doi: 10.1042/bj2120483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. P. Stimulation of inositol trisphosphate accumulation and amylase secretion by caerulein in pancreatic acini. J Pharmacol Exp Ther. 1984 Dec;231(3):623–627. [PubMed] [Google Scholar]
- Schulz I., Stolze H. H. The exocrine pancreas: the role of secretagogues, cyclic nucleotides, and calcium in enzyme secretion. Annu Rev Physiol. 1980;42:127–156. doi: 10.1146/annurev.ph.42.030180.001015. [DOI] [PubMed] [Google Scholar]
- Streb H., Heslop J. P., Irvine R. F., Schulz I., Berridge M. J. Relationship between secretagogue-induced Ca2+ release and inositol polyphosphate production in permeabilized pancreatic acinar cells. J Biol Chem. 1985 Jun 25;260(12):7309–7315. [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Sum P. T., Bencosme S. A., Beck I. T. Pathogenesis of bile-induced acute pancreatitis in the dog. Experiments with detergents. Am J Dig Dis. 1970 Jul;15(7):637–646. doi: 10.1007/BF02236023. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kikkawa U., Kaibuchi K., Nishizuka Y. Membrane phospholipid metabolism and signal transduction for protein phosphorylation. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:119–158. [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe O., Baccino F. M., Steer M. L., Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984 Apr;246(4 Pt 1):G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- Yamanishi J., Takai Y., Kaibuchi K., Sano K., Castagna M., Nishizuka Y. Synergistic functions of phorbol ester and calcium in serotonin release from human platelets. Biochem Biophys Res Commun. 1983 Apr 29;112(2):778–786. doi: 10.1016/0006-291x(83)91529-2. [DOI] [PubMed] [Google Scholar]
- Zawalich W., Brown C., Rasmussen H. Insulin secretion: combined effects of phorbol ester and A23187. Biochem Biophys Res Commun. 1983 Dec 16;117(2):448–455. doi: 10.1016/0006-291x(83)91221-4. [DOI] [PubMed] [Google Scholar]
- de Pont J. J., Fleuren-Jakobs A. M. Synergistic effect of A23187 and a phorbol ester on amylase secretion from rabbit pancreatic acini. FEBS Lett. 1984 May 7;170(1):64–68. doi: 10.1016/0014-5793(84)81369-1. [DOI] [PubMed] [Google Scholar]


