Abstract
Purpose
Multiple prospective Radiation Therapy Oncology Group (RTOG) protocols have evaluated bladder-preserving combined-modality therapy (CMT) for muscle-invasive bladder cancer (MIBC), reserving cystectomy for salvage treatment. We performed a pooled analysis of long-term outcomes in patients with MIBC enrolled across multiple studies.
Patients and Methods
Four hundred sixty-eight patients with MIBC were enrolled onto six RTOG bladder-preservation studies, including five phase II studies (RTOG 8802, 9506, 9706, 9906, and 0233) and one phase III study (RTOG 8903). Overall survival (OS) was estimated using the Kaplan-Meier method, and disease-specific survival (DSS), muscle-invasive and non–muscle-invasive local failure (LF), and distant metastasis (DM) were estimated by the cumulative incidence method.
Results
The median age of patients was 66 years (range, 34 to 93 years), and clinical T stage was T2 in 61%, T3 in 35%, and T4a in 4% of patients. Complete response to CMT was documented in 69% of patients. With a median follow-up of 4.3 years among all patients and 7.8 years among survivors (n = 205), the 5- and 10-year OS rates were 57% and 36%, respectively, and the 5- and 10-year DSS rates were 71% and 65%, respectively. The 5- and 10-year estimates of muscle-invasive LF, non–muscle-invasive LF, and DM were 13% and 14%, 31% and 36%, and 31% and 35%, respectively.
Conclusion
This pooled analysis of multicenter, prospective RTOG bladder-preserving CMT protocols demonstrates long-term DSS comparable to modern immediate cystectomy studies, for patients with similarly staged MIBC. Given the low incidence of late recurrences with long-term follow-up, CMT can be considered as an alternative to radical cystectomy, especially in elderly patients not well suited for surgery.
INTRODUCTION
Although radical cystectomy (RC) remains the usual treatment for muscle-invasive bladder cancer (MIBC) in the United States, bladder-preserving treatment strategies have evolved over the past 20 years with continued refinements in radiation therapy (RT), chemotherapy for radiation sensitization, and patient selection that can provide selected patients with an excellent chance for long-term survival with an intact, functioning bladder. The hallmarks of modern bladder-preserving therapy include combined-modality therapy (CMT) with maximal transurethral resection of bladder tumor (TURBT), RT, and concurrent chemotherapy; cystoscopic assessment of response to therapy with prompt salvage RC for nonresponders; and careful follow-up with cystoscopic surveillance and prompt RC for invasive recurrence. Successive Radiation Therapy Oncology Group (RTOG) studies have demonstrated that this bladder-preserving CMT approach in patients presenting with MIBC can achieve high rates of complete tumor response, bladder preservation in the majority of patients, and survival rates similar to those seen in contemporary RC series.1–8 Contemporary bladder-preserving approaches in patients with clinically staged MIBC can achieve complete response (CR) rates of 60% to 80%, 5-year disease-specific survival (DSS) rates of 60% to 70%, and bladder intact survival rates of 40% to 45%.9,10
The long-term, 10-year results of CMT have been reported from several single-institution series including from the Massachusetts General Hospital (MGH; Boston, MA) and University of Erlangen (Erlangen, Germany).9–12 Long-term follow-up of 348 patients with MIBC treated with chemotherapy and RT in 1986 to 2002 at MGH showed 10- and 15-year DSS rates of 59% and 57%, respectively, and 10- and 15-year OS rates of 35% and 22%, respectively. The University of Erlangen reported on 415 patients treated from 1982 to 2000 and demonstrated a 10-year DSS rate of 42%, with 80% of surviving patients preserving their bladders. Long-term follow-up of CMT in a pooled analysis of patients from RTOG bladder-preservation studies demonstrated a low incidence of late pelvic toxicity in patients retaining their bladder.13
However, the long-term outcomes of bladder-preserving CMT for MIBC, including OS, DSS, and long-term bladder-preservation rates, have not been examined in the multi-institutional setting. Here, we report a pooled secondary analysis of the long-term outcomes of patients who received bladder-preserving CMT in six RTOG studies.
PATIENTS AND METHODS
Data and outcomes of 468 patients with MIBC enrolled onto six RTOG bladder-preservation studies were pooled, including five phase II studies (RTOG 8802, 9506, 9706, 9906, and 0233) and one phase III study (RTOG 8903; Appendix Table A1, online only). Eligibility criteria for these trials have been previously described but included clinical T2-4a stage and excluded patients with biopsy-proven nodal disease or metastatic disease. Trials subsequent to RTOG 8802 and RTOG 8903 excluded patients with hydronephrosis.
RTOG Trials in the Pooled Analysis
RTOG 8802.
This phase II trial enrolled 90 eligible patients from 1988 to 1990 who received two cycles of neoadjuvant methotrexate (30 mg/m2), cisplatin (70 mg/m2), and vinblastine (3 mg/m2; MCV) followed by once-daily RT to 39.6 Gy with concurrent cisplatin (70 mg/m2 every 3 weeks).14 The RT consisted of a small pelvic four-field technique (similar for all protocols), encompassing the whole bladder, bladder tumor, prostate (in men), and adjacent pelvic lymph nodes.
RTOG 8903.
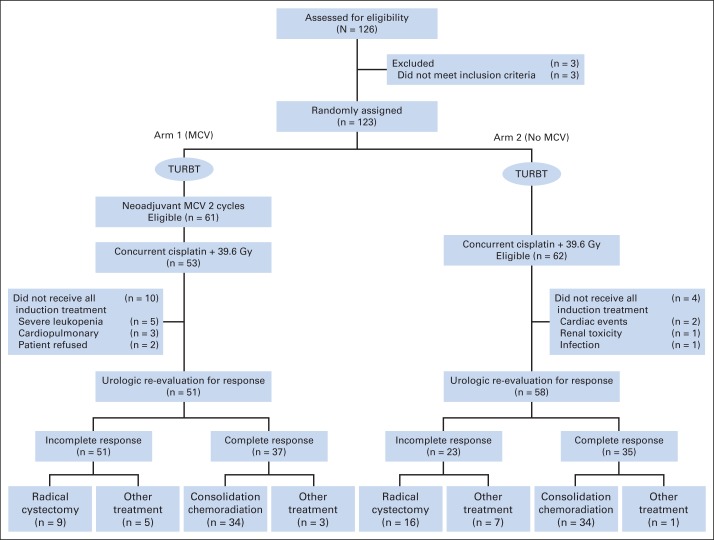
This phase III trial randomly assigned 123 eligible patients from 1990 to 1993 at 37 centers to two cycles of neoadjuvant MCV versus no MCV, followed by once-daily pelvic RT to 39.6 Gy and two cycles of concurrent cisplatin (100 mg/m2 every 3 weeks; Fig 1).15 Patients with a CR on rebiopsy after 39.6 Gy or RT received consolidation therapy with 25.2 Gy of RT (total dose, 64.8 Gy) and one additional cycle of cisplatin.
Fig 1.
CONSORT diagram of Radiation Therapy Oncology Group protocol 8903. MCV, methotrexate, cisplatin, and vinblastine; TURBT, transurethral resection of bladder tumor. Data adapted.15
RTOG 9506.
This phase I/II trial enrolled 34 eligible patients from 1995 to 1997 at 11 institutions. After TURBT, patients received induction accelerated hypofractionated (3 Gy per fraction) twice-daily RT to the pelvis (24 Gy) with concurrent cisplatin (15 mg/m2 on days 1 through 3 and 15 through 17) and fluorouracil (FU; 400 mg/m2 on days 1 through 3 and 15 through 17). Patients with a CR received twice-daily RT (2.5 Gy per fraction) to the whole bladder and the bladder tumor (total dose, 44 Gy to bladder and tumor and 24 Gy to pelvic lymph nodes) with concurrent FU/cisplatin.16
RTOG 9706.
This phase I/II study enrolled 47 eligible patients from 1997 to 1999 at 17 institutions. After TURBT, patients received induction twice-daily RT with 1.8 Gy to the pelvis in the morning and 1.6 Gy to the bladder tumor in the afternoon for 13 days (40.8 Gy to bladder tumor and 21.6 Gy to the pelvis) with concurrent weekly cisplatin (20 mg/m2 first 2 days per week). Patients with a CR on rebiopsy after 41.6 Gy of RT received twice-daily RT (1.5 Gy per fraction) given to each site for 8 days (total dose, 45.6 Gy to pelvis and bladder and 64.8 Gy to bladder tumor) with weekly cisplatin.17
RTOG 9906.
This phase I/II trial enrolled 81 eligible patients from 1999 to 2002 at 26 institutions. After TURBT, patients underwent induction twice-daily accelerated RT over 13 days, including 1.6 Gy to the pelvis in the morning, and 1.5 Gy to the bladder for the first 5 days (7.5 Gy) and then 1.5 Gy to the tumor for the next 8 days (12.0 Gy) in the afternoons (total dose, 20.8 Gy to pelvis, 28.3 Gy to whole bladder, and 40.3 Gy to bladder tumor) with concurrent weekly cisplatin (20 mg/m2 2 days a week) and paclitaxel (50 mg/m2 per week). Patients with a CR on rebiopsy after 40.3 Gy of RT received 1.5-Gy twice-daily pelvic RT to a dose of 24 Gy (total dose, 64.3 Gy to the bladder tumor and 44.8 Gy to the pelvis) with concurrent weekly cisplatin/paclitaxel. Patients then received four cycles of adjuvant cisplatin (70 mg/m2) and gemcitabine (1,000 mg/m2).18
RTOG 0233.
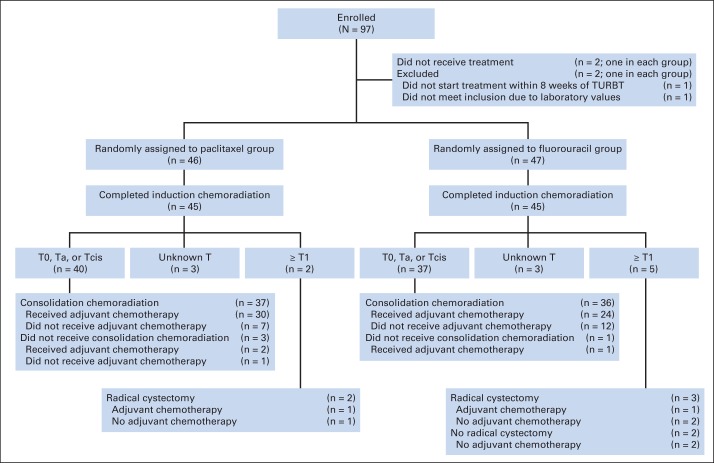
This phase II randomized trial enrolled 93 eligible patients from 2003 to 2007 at 24 institutions (Fig 2).19 After TURBT, patients received twice-daily RT, as in RTOG 9906, and were randomly assigned to either concurrent paclitaxel (50 mg/m2 per week) plus cisplatin (15 mg/m2 3 days per week; n = 46) or FU (500 mg/m2 2 days per alternate week) plus cisplatin (n = 47) during the induction and consolidation phases followed by adjuvant gemcitabine/paclitaxel/cisplatin.
Fig 2.
CONSORT diagram of Radiation Therapy Oncology Group protocol 0233. MCV, methotrexate, cisplatin, and vinblastine; TURBT, transurethral resection of bladder tumor. Data adapted.19
Criteria for CR and Follow-Up
Clinical CR was defined as no tumor palpable on bimanual examination under anesthesia, no tumor visible on cystoscopy, negative tumor site biopsy, and negative urine cytology. Patients with preserved bladders underwent routine active surveillance including cystoscopy, tumor site biopsy, bimanual examination under anesthesia, and urine cytology every 3 months for the first year, and then cystoscopy and cytology every 3 to 4 months during the second year, every 6 months for 3 years, and then annually. Patients with non–muscle-invasive local failure (LF) were promptly considered for intravesical therapy, and patients with a muscle-invasive LF underwent salvage RC.
End Points
All end points were measured from the date of study entry (phase II studies) or random assignment (phase III study) to the date of first documented event. For overall survival (OS) and DSS, survival time was measured to date of death (as a result of any cause) or death from disease, respectively. Time to LF was measured to the date of documented tumor recurrence after CR or to the date of 1 day after study entry/random assignment in patients who did not achieve CR. LF after a CR to induction CMT was examined in the following two subcategories: muscle-invasive and non–muscle-invasive LF. Nodal recurrence was defined as documented presence or progression of regional (pelvic) nodes. Time to distant metastasis (DM) was defined by the date of first documented DM. Time to bladder-intact disease-free survival was defined as time to the earliest of muscle-invasive local recurrence in the bladder, regional pelvic recurrence, DM, bladder cancer–related death, or cystectomy.
Statistical Analyses
OS and bladder-intact disease-free survival were estimated using the Kaplan-Meier method.20 LF, nodal recurrences, DM, and DSS were estimated using cumulative incidence methodology.21 Fine and Gray's proportional hazards regression model was performed to identify clinical variables associated with DSS. The following covariates were included in the multiple regression model: age, sex, T stage (T2 v T3/T4a), histology (urothelial carcinoma v other), tumor grade (low grade v high grade), presence of hydronephrosis, and whether the TURBT was visibly complete or not. All statistical comparisons were two-sided, and P < .05 was considered statistically significant. SAS software (SAS Institute, Cary, NC) was used for all analyses, except for the Fine and Gray's modeling, which was done in R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patients
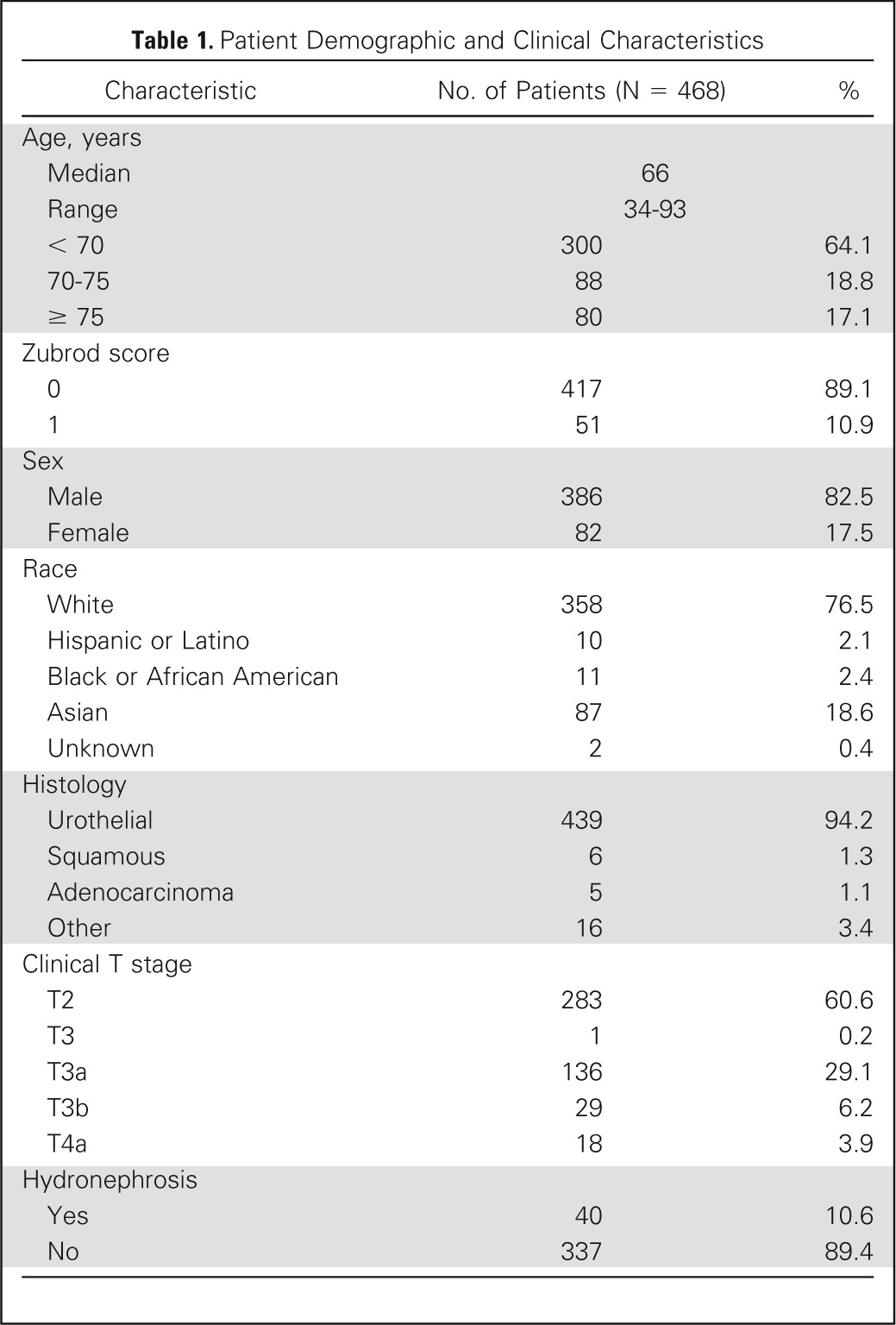
From 1988 to 2007, 468 eligible patients were enrolled onto these successive RTOG protocols. Patient and tumor characteristics are listed in Table 1.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | No. of Patients (N = 468) | % |
|---|---|---|
| Age, years | ||
| Median | 66 | |
| Range | 34-93 | |
| < 70 | 300 | 64.1 |
| 70-75 | 88 | 18.8 |
| ≥ 75 | 80 | 17.1 |
| Zubrod score | ||
| 0 | 417 | 89.1 |
| 1 | 51 | 10.9 |
| Sex | ||
| Male | 386 | 82.5 |
| Female | 82 | 17.5 |
| Race | ||
| White | 358 | 76.5 |
| Hispanic or Latino | 10 | 2.1 |
| Black or African American | 11 | 2.4 |
| Asian | 87 | 18.6 |
| Unknown | 2 | 0.4 |
| Histology | ||
| Urothelial | 439 | 94.2 |
| Squamous | 6 | 1.3 |
| Adenocarcinoma | 5 | 1.1 |
| Other | 16 | 3.4 |
| Clinical T stage | ||
| T2 | 283 | 60.6 |
| T3 | 1 | 0.2 |
| T3a | 136 | 29.1 |
| T3b | 29 | 6.2 |
| T4a | 18 | 3.9 |
| Hydronephrosis | ||
| Yes | 40 | 10.6 |
| No | 337 | 89.4 |
Long-Term Outcomes
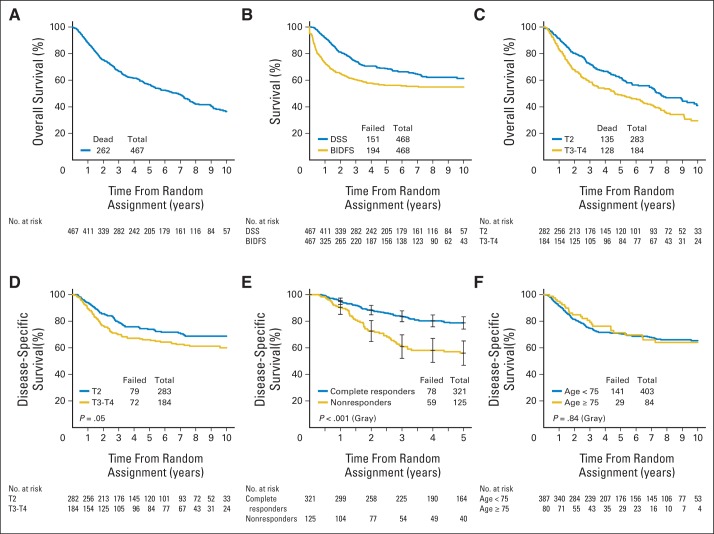
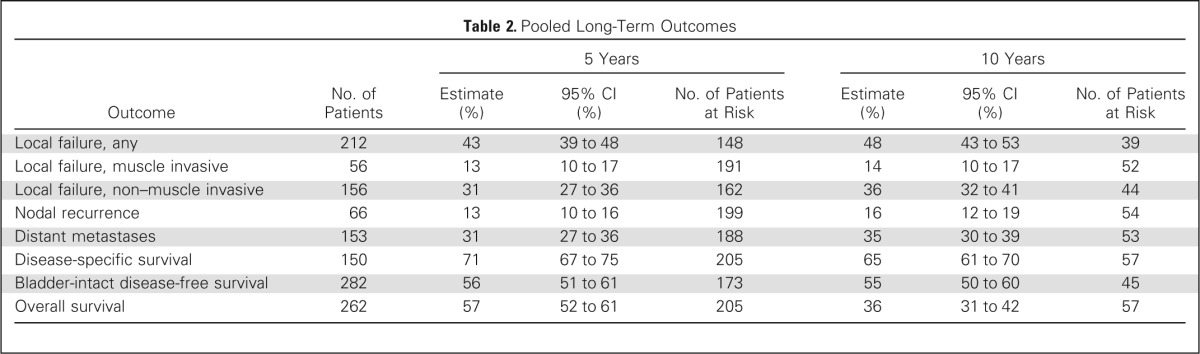
A CR to induction chemotherapy and RT occurred in 69% of patients (321 of 468 patients). With a median follow-up of 4.3 years among all patients and 7.8 years among patients alive at the time of this analysis (n = 205), the 5- and 10-year OS rates were 57% and 36%, respectively (Fig 3A). The 5- and 10-year DSS rates were 71% and 65%, respectively (Fig 3B; Table 2). Bladder cancer was the cause of death in 24% of patients who had died by 5 years (n = 191). Of the 205 patients alive at 5 years, 80% had an intact bladder.
Fig 3.
(A) Overall survival in all patients. (B) Disease-specific survival (DSS) and bladder-intact disease-free survival (BIDFS) in all patients. (C) Overall survival in patients with clinical T2 versus T3/4 disease. (D) DSS in patients with clinical T2 versus T3/4 disease. (E) DSS in patients with a complete response after combined-modality therapy compared with patients who were nonresponders. (F) DSS in patients age 75 years or older versus patients younger than age 75 years.
Table 2.
Pooled Long-Term Outcomes
| Outcome | No. of Patients | 5 Years |
10 Years |
||||
|---|---|---|---|---|---|---|---|
| Estimate (%) | 95% CI (%) | No. of Patients at Risk | Estimate (%) | 95% CI (%) | No. of Patients at Risk | ||
| Local failure, any | 212 | 43 | 39 to 48 | 148 | 48 | 43 to 53 | 39 |
| Local failure, muscle invasive | 56 | 13 | 10 to 17 | 191 | 14 | 10 to 17 | 52 |
| Local failure, non–muscle invasive | 156 | 31 | 27 to 36 | 162 | 36 | 32 to 41 | 44 |
| Nodal recurrence | 66 | 13 | 10 to 16 | 199 | 16 | 12 to 19 | 54 |
| Distant metastases | 153 | 31 | 27 to 36 | 188 | 35 | 30 to 39 | 53 |
| Disease-specific survival | 150 | 71 | 67 to 75 | 205 | 65 | 61 to 70 | 57 |
| Bladder-intact disease-free survival | 282 | 56 | 51 to 61 | 173 | 55 | 50 to 60 | 45 |
| Overall survival | 262 | 57 | 52 to 61 | 205 | 36 | 31 to 42 | 57 |
The 5- and 10-year LF estimates were 43% and 48%, respectively, and the majority of LFs were non–muscle-invasive LFs instead of muscle-invasive LF (Table 2). For all patients, including those with and without salvage RC, the 5- and 10-year estimates of nodal recurrence were 13% and 16%, respectively. The 5- and 10-year DM estimates were 31% and 35%, respectively. The long-term outcomes by trial are listed in Appendix Table A2 (online only).
Outcomes by Response
In patients with a CR after induction CMT, DSS was significantly higher than in patients who did not have a CR (5-year DSS, 79% v 56%, respectively; 10-year DSS, 74% v 47%, respectively; P < .001; Fig 3E). The 5- and 10-year OS rates for patients with a CR were 65% and 43%, respectively, compared with 44% and 25%, respectively, in patients who did not have a CR.
Outcomes in Patients Undergoing Cystectomy
One hundred patients (21%) enrolled onto the six trials ultimately underwent cystectomy; 62% underwent immediate cystectomy for incomplete response to induction chemotherapy and RT, 36% underwent salvage cystectomy after CMT for recurrences detected in follow-up, and 2% underwent cystectomy for other causes. The absolute incidence of nodal recurrence was 22% after any cystectomy (n = 22), 27% after immediate cystectomy (n = 17), and 14% after salvage cystectomy (n = 5). Among patients undergoing cystectomy, the 5- and 10-year OS rates were 45% and 18%, respectively, whereas the 5- and 10-year DSS rates were 60% and 47%, respectively.
Subgroup and Multivariable Analyses
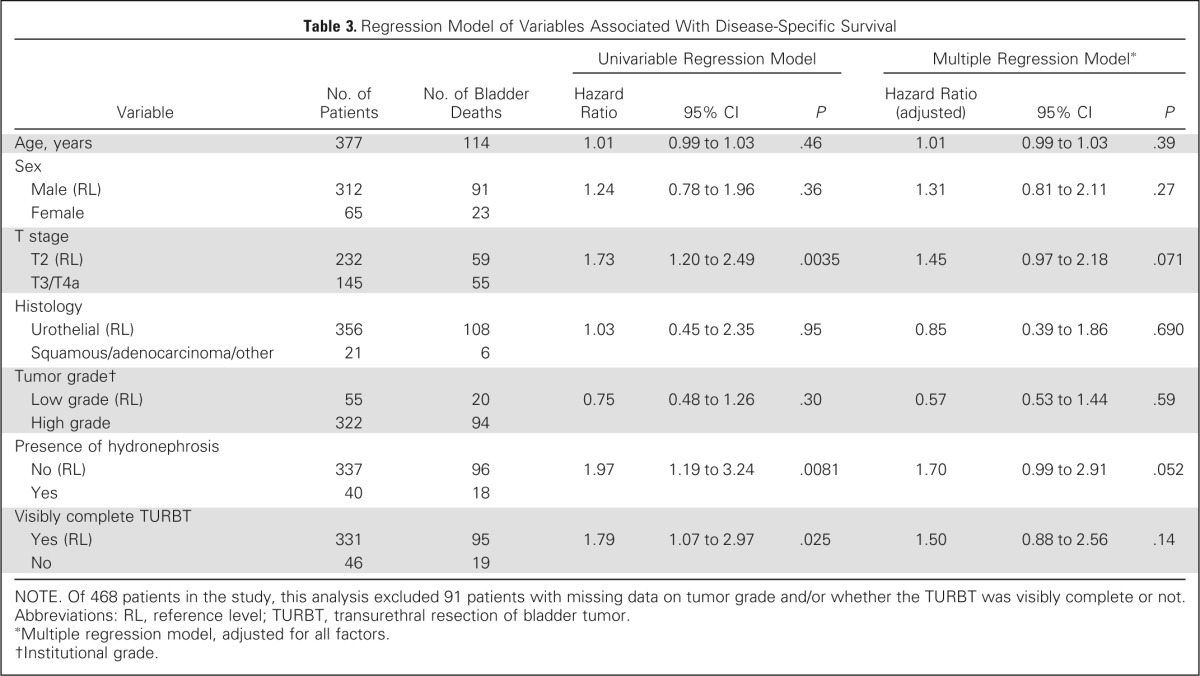
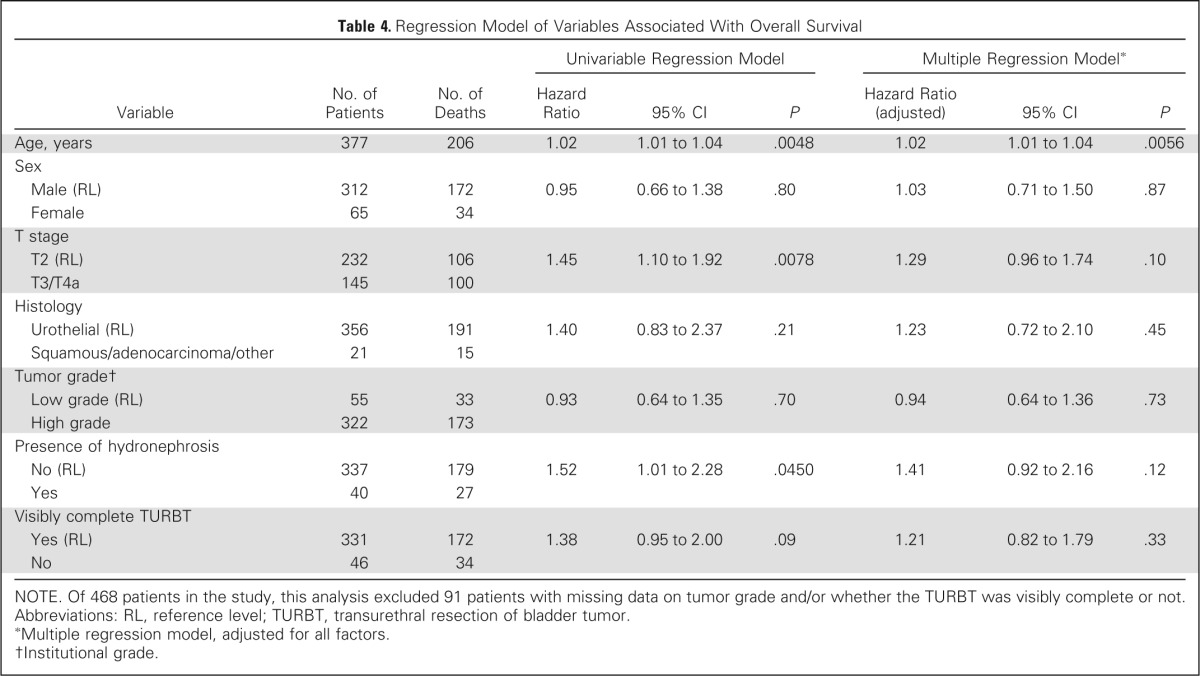
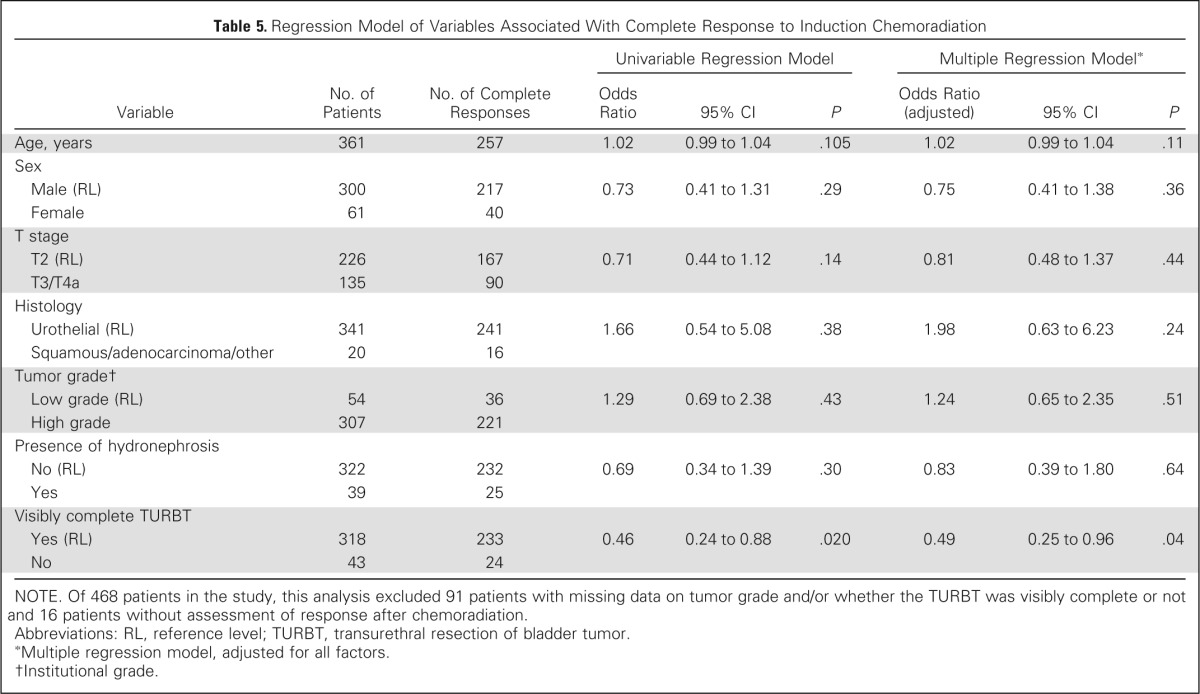
On univariable analysis, higher T stage and presence of hydronephrosis were associated with decreased DSS, and a visibly complete TURBT was associated with increased DSS, but not on multivariable analysis (Table 3). On univariable analysis, higher T stage, hydronephrosis, older age, and less than visibly complete TURBT were associated with decreased OS, but only age was associated with OS on multivariable analysis (Table 4). Visibly complete TURBT was associated with a higher CR rate to treatment in both univariable and multivariable analysis (Table 5).
Table 3.
Regression Model of Variables Associated With Disease-Specific Survival
| Variable | No. of Patients | No. of Bladder Deaths | Univariable Regression Model |
Multiple Regression Model* |
||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio (adjusted) | 95% CI | P | |||
| Age, years | 377 | 114 | 1.01 | 0.99 to 1.03 | .46 | 1.01 | 0.99 to 1.03 | .39 |
| Sex | ||||||||
| Male (RL) | 312 | 91 | 1.24 | 0.78 to 1.96 | .36 | 1.31 | 0.81 to 2.11 | .27 |
| Female | 65 | 23 | ||||||
| T stage | ||||||||
| T2 (RL) | 232 | 59 | 1.73 | 1.20 to 2.49 | .0035 | 1.45 | 0.97 to 2.18 | .071 |
| T3/T4a | 145 | 55 | ||||||
| Histology | ||||||||
| Urothelial (RL) | 356 | 108 | 1.03 | 0.45 to 2.35 | .95 | 0.85 | 0.39 to 1.86 | .690 |
| Squamous/adenocarcinoma/other | 21 | 6 | ||||||
| Tumor grade† | ||||||||
| Low grade (RL) | 55 | 20 | 0.75 | 0.48 to 1.26 | .30 | 0.57 | 0.53 to 1.44 | .59 |
| High grade | 322 | 94 | ||||||
| Presence of hydronephrosis | ||||||||
| No (RL) | 337 | 96 | 1.97 | 1.19 to 3.24 | .0081 | 1.70 | 0.99 to 2.91 | .052 |
| Yes | 40 | 18 | ||||||
| Visibly complete TURBT | ||||||||
| Yes (RL) | 331 | 95 | 1.79 | 1.07 to 2.97 | .025 | 1.50 | 0.88 to 2.56 | .14 |
| No | 46 | 19 | ||||||
NOTE. Of 468 patients in the study, this analysis excluded 91 patients with missing data on tumor grade and/or whether the TURBT was visibly complete or not.
Abbreviations: RL, reference level; TURBT, transurethral resection of bladder tumor.
Multiple regression model, adjusted for all factors.
Institutional grade.
Table 4.
Regression Model of Variables Associated With Overall Survival
| Variable | No. of Patients | No. of Deaths | Univariable Regression Model |
Multiple Regression Model* |
||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio (adjusted) | 95% CI | P | |||
| Age, years | 377 | 206 | 1.02 | 1.01 to 1.04 | .0048 | 1.02 | 1.01 to 1.04 | .0056 |
| Sex | ||||||||
| Male (RL) | 312 | 172 | 0.95 | 0.66 to 1.38 | .80 | 1.03 | 0.71 to 1.50 | .87 |
| Female | 65 | 34 | ||||||
| T stage | ||||||||
| T2 (RL) | 232 | 106 | 1.45 | 1.10 to 1.92 | .0078 | 1.29 | 0.96 to 1.74 | .10 |
| T3/T4a | 145 | 100 | ||||||
| Histology | ||||||||
| Urothelial (RL) | 356 | 191 | 1.40 | 0.83 to 2.37 | .21 | 1.23 | 0.72 to 2.10 | .45 |
| Squamous/adenocarcinoma/other | 21 | 15 | ||||||
| Tumor grade† | ||||||||
| Low grade (RL) | 55 | 33 | 0.93 | 0.64 to 1.35 | .70 | 0.94 | 0.64 to 1.36 | .73 |
| High grade | 322 | 173 | ||||||
| Presence of hydronephrosis | ||||||||
| No (RL) | 337 | 179 | 1.52 | 1.01 to 2.28 | .0450 | 1.41 | 0.92 to 2.16 | .12 |
| Yes | 40 | 27 | ||||||
| Visibly complete TURBT | ||||||||
| Yes (RL) | 331 | 172 | 1.38 | 0.95 to 2.00 | .09 | 1.21 | 0.82 to 1.79 | .33 |
| No | 46 | 34 | ||||||
NOTE. Of 468 patients in the study, this analysis excluded 91 patients with missing data on tumor grade and/or whether the TURBT was visibly complete or not.
Abbreviations: RL, reference level; TURBT, transurethral resection of bladder tumor.
Multiple regression model, adjusted for all factors.
Institutional grade.
Table 5.
Regression Model of Variables Associated With Complete Response to Induction Chemoradiation
| Variable | No. of Patients | No. of Complete Responses | Univariable Regression Model |
Multiple Regression Model* |
||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P | Odds Ratio (adjusted) | 95% CI | P | |||
| Age, years | 361 | 257 | 1.02 | 0.99 to 1.04 | .105 | 1.02 | 0.99 to 1.04 | .11 |
| Sex | ||||||||
| Male (RL) | 300 | 217 | 0.73 | 0.41 to 1.31 | .29 | 0.75 | 0.41 to 1.38 | .36 |
| Female | 61 | 40 | ||||||
| T stage | ||||||||
| T2 (RL) | 226 | 167 | 0.71 | 0.44 to 1.12 | .14 | 0.81 | 0.48 to 1.37 | .44 |
| T3/T4a | 135 | 90 | ||||||
| Histology | ||||||||
| Urothelial (RL) | 341 | 241 | 1.66 | 0.54 to 5.08 | .38 | 1.98 | 0.63 to 6.23 | .24 |
| Squamous/adenocarcinoma/other | 20 | 16 | ||||||
| Tumor grade† | ||||||||
| Low grade (RL) | 54 | 36 | 1.29 | 0.69 to 2.38 | .43 | 1.24 | 0.65 to 2.35 | .51 |
| High grade | 307 | 221 | ||||||
| Presence of hydronephrosis | ||||||||
| No (RL) | 322 | 232 | 0.69 | 0.34 to 1.39 | .30 | 0.83 | 0.39 to 1.80 | .64 |
| Yes | 39 | 25 | ||||||
| Visibly complete TURBT | ||||||||
| Yes (RL) | 318 | 233 | 0.46 | 0.24 to 0.88 | .020 | 0.49 | 0.25 to 0.96 | .04 |
| No | 43 | 24 | ||||||
NOTE. Of 468 patients in the study, this analysis excluded 91 patients with missing data on tumor grade and/or whether the TURBT was visibly complete or not and 16 patients without assessment of response after chemoradiation.
Abbreviations: RL, reference level; TURBT, transurethral resection of bladder tumor.
Multiple regression model, adjusted for all factors.
Institutional grade.
Examining outcomes by subgroups, higher clinical T stage (T2 v T3/T4) was associated with decreased OS (5-year OS, 62% v 49%, respectively; 10-year OS, 41% v 30%, respectively; P = .002; Fig 3C) and DSS (5-year DSS, 74% v 66%, respectively; 10-year DSS, 69% v 60%, respectively; P = .05; Fig 3D). Analyzing outcomes by age, elderly patients (age ≥ 75 years) completed induction chemotherapy and RT less frequently than younger patients (age < 75 years; 78% v 88%, respectively; P = .028), but there was no difference in receiving more than 60 Gy of RT by age group (67% v 71%, respectively; P = .42). Between elderly and younger patients, there was no difference in CR rates (72% v 73%, respectively; P = .78) and DSS rates (5-year DSS, 71% v 70%, respectively; 10-year DSS, 64% v 65%, respectively; P = .84; Fig 3F). Among elderly survivors at 5 years (n = 22), 76% had an intact bladder, which was not significantly different than younger patients (81%; P = .55). Bladder cancer was the cause of death at 5 years in 16% of elderly patients compared with 26% of younger patients (P = .29).
DISCUSSION
In this RTOG pooled analysis of long-term outcomes of selective bladder-preserving CMT in the multi-institutional setting, we demonstrate that this treatment approach results in low rates of invasive tumor recurrence (10-year invasive LF, 14%) and high long-term DSS (5- and 10-year DSS, 71% and 65%, respectively) and OS (5- and 10-year OS, 57% and 36%, respectively), with 80% of patients retaining an intact bladder at 5 years. This study provides a unique insight into the outcomes of the bladder-preserving CMT approach over two decades in the multi-institutional setting with one of the largest cohorts of patients reported to date.
Our findings build on decades of experience with bladder-preservation approaches involving more than 1,000 patients treated in single institutions and cooperative groups in North America and Europe. The MGH and Erlangen series demonstrated 5-year OS rates of 52% and 45%, 10-year OS rates of 35% and 29%,9–12 5-year DSS rates of 63% and 56%, and 10-year DSS rates of 59% and 42%, respectively, which are comparable to the results observed in this pooled analysis.
Although our study demonstrates excellent long-term cancer control, bladder-preserving CMT has not been routinely adopted because of concerns including long-term toxicity from radiation to the bladder and the feasibility and curability of salvage cystectomy in patients with local recurrences. First, although we do not report toxicity outcomes in this study, previously reported long-term toxicity data from patients enrolled onto RTOG studies13 and studies from other centers22–25 have demonstrated a low risk of toxicity and good quality-of-life outcomes with preservation of a functional bladder. Second, we found in this study that patients who ultimately required salvage cystectomy for nonresponse to CMT or recurrent disease still had a 5-year DSS of 60% and 10-year DSS of 47%. Combined with recent data showing that the risk of complications from RC after CMT (16% incidence of major complications within 90 days) is acceptable compared with upfront RC,26 bladder-preserving CMT is a reasonable alternative treatment in selected patients.
Although there are no randomized studies comparing RC with bladder-preserving CMT and any direct comparison is difficult because of selection bias and confounding from discordance between clinical and pathologic staging,27 the 5- and 10-year OS and DSS rates in contemporary RC series of clinically staged patients with T2-4a MIBC are comparable to those seen in this study and other bladder-preserving studies.1–8 Although organ-conserving approaches with CMT have become adopted for anal cancer and head and neck cancers, the general acceptance and adoption of bladder-preserving therapy for MIBC have been met with resistance. It is unlikely that a randomized trial comparing RC versus bladder-preserving CMT will be completed, given the recent failure of the United Kingdom Selective Bladder Preservation Against Radical Excision trial to accrue.28 Thus, the results of this pooled analysis provide a unique insight into the efficacy of the RTOG bladder-preserving CMT approach and serve as a useful benchmark for future bladder-preserving approaches.
Regarding patterns of failure observed in this pooled analysis, the majority of LFs were non–muscle invasive. With more than 7 years of follow-up in survivors, the majority of muscle-invasive and metastatic failures occurred within 5 years, and such recurrences beyond 5 years were uncommon. However, the 10-year non–muscle-invasive recurrence rate was 36%, underscoring the importance of close surveillance with routine cystoscopy and treatment if indicated after CMT. Such long-term outcome data from these RTOG studies are important in establishing selective bladder-preserving CMT as a safe and effective alternative to cystectomy.29 Although concurrent chemotherapy was used in all of these RTOG studies and many of the studies included neoadjuvant or adjuvant cisplatin-based chemotherapy, DM remained a substantial problem (10-year estimate, 35%), which underscores the need for continued refinement of the CMT approach by incorporating new, efficacious systemic therapies with lower toxicity profiles.
This pooled analysis allowed the comparison of outcomes by subgroups, which is of interest in a relatively rare disease. Elderly patients (age ≥ 75 years) had similar incidences of completion of RT greater than 60 Gy, bladder preservation, and DSS compared with younger patients, demonstrating that this potentially curative CMT should be considered for elderly patients who may not be eligible for surgery and have historically had limited treatment options. In addition, higher clinical T stage (T2 v T3/4) and presence of hydronephrosis were associated with decreased DSS, whereas visibly complete TURBT was associated with increased DSS on univariable analysis; these are associations that have been observed in other large series.9–11 A visibly complete TURBT was associated with higher CR rate on both univariable and multivariable analysis, and thus, we recommend an aggressive, visibly complete TURBT when feasible. However, the inability to perform a complete TURBT should not preclude an attempt at bladder preservation, because our study demonstrates that more than 50% of patients with an incomplete TURBT can still have a postinduction CR. Finally, our study again demonstrated that hydronephrosis is associated with worse outcomes with CMT, as it is with RC, and remains a relative contraindication to bladder-preserving CMT in RTOG studies.
As a pooled analysis, this study is not powered to compare the different CMT approaches in each trial. Given the difficulties in accruing large numbers of patients to bladder-preservation trials, randomized trials to compare CMT regimens will likely be difficult to complete. Thus, the current approach of the RTOG to systematically refine the bladder-preserving CMT approach with successive phase II trials testing new regimens will lead to the continued evolution of CMT.30 Although the optimal bladder-preserving regimen continues to evolve, common contemporary approaches include the RTOG approaches of concurrent chemotherapy with cisplatin/FU or RT-sensitizing low-dose gemcitabine. Recent results from a United Kingdom bladder-preservation trial demonstrated that a regimen of concurrent FU (500 mg/m2 continuous infusion, days 1 through 5 and 16 through 20) and mitomycin (12 mg/m2, day 1 only) also resulted in high response rates, bladder preservation, and OS31 and provides an additional chemotherapy option for patients who are unable to tolerate platinum or gemcitabine.
In conclusion, over several decades, the RTOG has refined the CMT approach by improving patient selection, RT techniques, and chemotherapeutics. This pooled analysis of RTOG trials of bladder-preserving CMT for MIBC demonstrates long-term outcomes similar to cystectomy. Thus, bladder-preserving CMT has become a safe, tested, efficacious alternative to RC in selected patients with MIBC who desire to keep their bladders. For patients with MIBC who are noncystectomy candidates or for select patients who are motivated to keep their native bladders, bladder-preserving CMT has been recognized recently in the guidelines by the International Consultation on Urological Diseases–European Association of Urology29 and by the National Comprehensive Cancer Center Network32 as an effective alternative to RC and should be considered for these patients with MIBC. Future work will continue within the RTOG and other groups to refine the bladder-preserving approach by developing new RT sensitizers33 and identifying predictive biomarkers34 to further improve outcomes.
Supplementary Material
Appendix
Table A1.
Overview of RTOG Bladder-Preserving Combined-Modality Therapy Trials for Muscle-Invasive Bladder Cancer
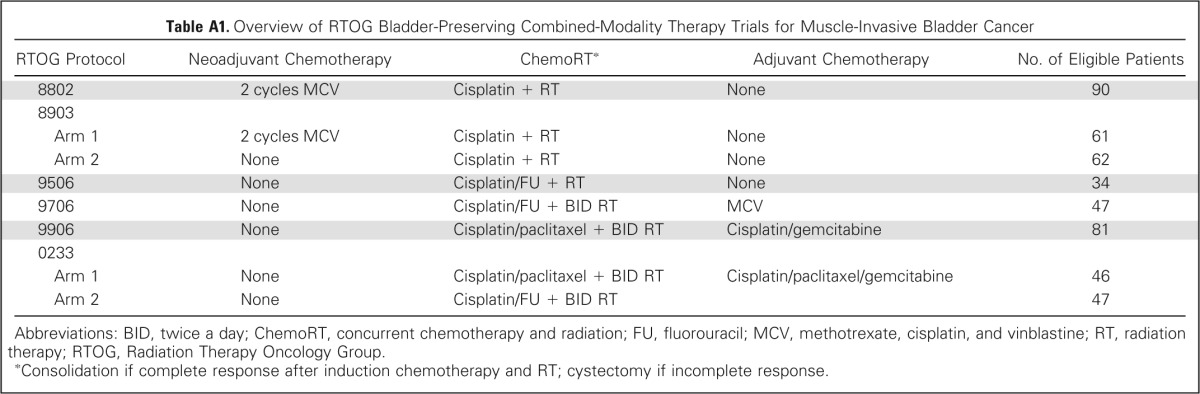
| RTOG Protocol | Neoadjuvant Chemotherapy | ChemoRT* | Adjuvant Chemotherapy | No. of Eligible Patients |
|---|---|---|---|---|
| 8802 | 2 cycles MCV | Cisplatin + RT | None | 90 |
| 8903 | ||||
| Arm 1 | 2 cycles MCV | Cisplatin + RT | None | 61 |
| Arm 2 | None | Cisplatin + RT | None | 62 |
| 9506 | None | Cisplatin/FU + RT | None | 34 |
| 9706 | None | Cisplatin/FU + BID RT | MCV | 47 |
| 9906 | None | Cisplatin/paclitaxel + BID RT | Cisplatin/gemcitabine | 81 |
| 0233 | ||||
| Arm 1 | None | Cisplatin/paclitaxel + BID RT | Cisplatin/paclitaxel/gemcitabine | 46 |
| Arm 2 | None | Cisplatin/FU + BID RT | 47 |
Abbreviations: BID, twice a day; ChemoRT, concurrent chemotherapy and radiation; FU, fluorouracil; MCV, methotrexate, cisplatin, and vinblastine; RT, radiation therapy; RTOG, Radiation Therapy Oncology Group.
Consolidation if complete response after induction chemotherapy and RT; cystectomy if incomplete response.
Table A2.
Long-Term Outcomes by Trial
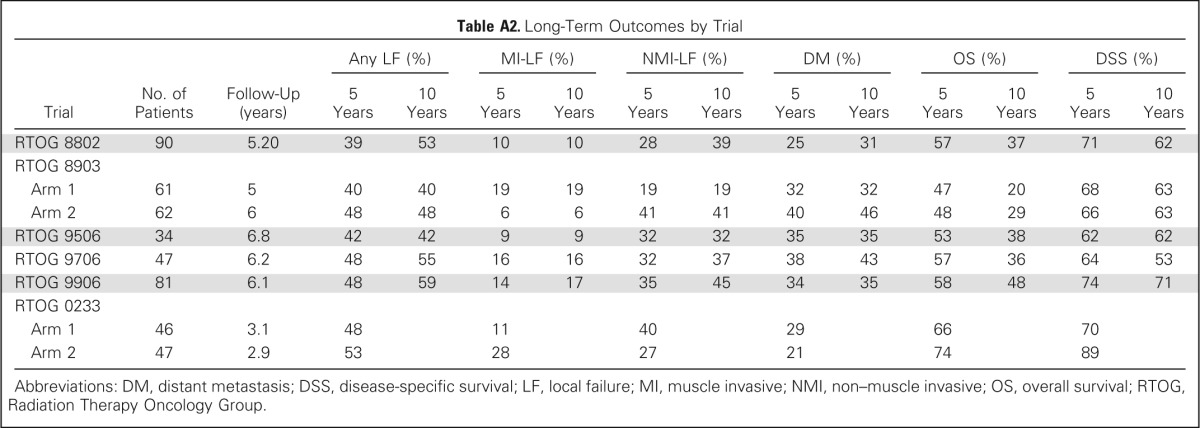
| Trial | No. of Patients | Follow-Up (years) | Any LF (%) |
MI-LF (%) |
NMI-LF (%) |
DM (%) |
OS (%) |
DSS (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 Years | 10 Years | 5 Years | 10 Years | 5 Years | 10 Years | 5 Years | 10 Years | 5 Years | 10 Years | 5 Years | 10 Years | |||
| RTOG 8802 | 90 | 5.20 | 39 | 53 | 10 | 10 | 28 | 39 | 25 | 31 | 57 | 37 | 71 | 62 |
| RTOG 8903 | ||||||||||||||
| Arm 1 | 61 | 5 | 40 | 40 | 19 | 19 | 19 | 19 | 32 | 32 | 47 | 20 | 68 | 63 |
| Arm 2 | 62 | 6 | 48 | 48 | 6 | 6 | 41 | 41 | 40 | 46 | 48 | 29 | 66 | 63 |
| RTOG 9506 | 34 | 6.8 | 42 | 42 | 9 | 9 | 32 | 32 | 35 | 35 | 53 | 38 | 62 | 62 |
| RTOG 9706 | 47 | 6.2 | 48 | 55 | 16 | 16 | 32 | 37 | 38 | 43 | 57 | 36 | 64 | 53 |
| RTOG 9906 | 81 | 6.1 | 48 | 59 | 14 | 17 | 35 | 45 | 34 | 35 | 58 | 48 | 74 | 71 |
| RTOG 0233 | ||||||||||||||
| Arm 1 | 46 | 3.1 | 48 | 11 | 40 | 29 | 66 | 70 | ||||||
| Arm 2 | 47 | 2.9 | 53 | 28 | 27 | 21 | 74 | 89 | ||||||
Abbreviations: DM, distant metastasis; DSS, disease-specific survival; LF, local failure; MI, muscle invasive; NMI, non–muscle invasive; OS, overall survival; RTOG, Radiation Therapy Oncology Group.
Footnotes
See accompanying editorial on page 3787
Supported by Radiation Therapy Oncology Group Grant No. U10 CA21661 and Community Clinical Oncology Program Grant No. U10 CA37422 from the National Cancer Institute.
Presented in part at the 2012 Genitourinary Cancers Symposium, San Francisco, CA, February 2-4, 2012, and the 54th Annual Meeting of the American Society for Radiation Oncology, Boston, MA, October 28-31, 2012.
Clinical trial information: NCT00055601, NCT00003930.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Raymond H. Mak, Daniel Hunt, William U. Shipley, Jason A. Efstathiou, William J. Tester, Anthony L. Zietman
Provision of study materials or patients: William J. Tester
Collection and assembly of data: Raymond H. Mak, Daniel Hunt, William J. Tester, Michael P. Hagan, Donald S. Kaufman
Data analysis and interpretation: Raymond H. Mak, Daniel Hunt, William U. Shipley, Jason A. Efstathiou, Niall M. Heney, Anthony L. Zietman
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Outcomes in Patients With Muscle-Invasive Bladder Cancer After Selective Bladder-Preserving Combined-Modality Therapy: A Pooled Analysis of Radiation Therapy Oncology Group Protocols 8802, 8903, 9506, 9706, 9906, and 0233
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Raymond H. Mak
Consulting or Advisory Role: Celgene
Stock or Other Ownership: Boehringer Ingelheim
Daniel Hunt
Employment: Puma
Stock or Other Ownership: Puma
Research Funding: Puma
Travel, Accommodations, Expenses: Puma
William U. Shipley
Stock or Other Ownership: Pfizer
Jason A. Efstathiou
Consulting or Advisory Role: Medivation/Astellas, Bayer
William J. Tester
No relationship to disclose
Michael P. Hagan
No relationship to disclose
Donald S. Kaufman
No relationship to disclose
Niall M. Heney
No relationship to disclose
Anthony L. Zietman
No relationship to disclose
REFERENCES
- 1.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Dalbagni G, Genega E, Hashibe M, et al. Cystectomy for bladder cancer: A contemporary series. J Urol. 2001;165:1111–1116. [PubMed] [Google Scholar]
- 3.Zehnder P, Studer UE, Skinner EC, et al. Super extended versus extended pelvic lymph node dissection in patients undergoing radical cystectomy for bladder cancer: A comparative study. J Urol. 2011;186:1261–1268. doi: 10.1016/j.juro.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Madersbacher S, Hochreiter W, Burkhard F, et al. Radical cystectomy for bladder cancer today: A homogeneous series without neoadjuvant therapy. J Clin Oncol. 2003;21:690–696. doi: 10.1200/JCO.2003.05.101. [DOI] [PubMed] [Google Scholar]
- 5.Hautmann RE, Gschwend JE, de Petriconi RC, et al. Cystectomy for transitional cell carcinoma of the bladder: Results of a surgery only series in the neobladder era. J Urol. 2006;176:486–492. doi: 10.1016/j.juro.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 6.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 7.Munro NP, Sundaram SK, Weston PM, et al. A 10-year retrospective review of a nonrandomized cohort of 458 patients undergoing radical radiotherapy or cystectomy in Yorkshire, UK. Int J Radiat Oncol Biol Phys. 2010;77:119–124. doi: 10.1016/j.ijrobp.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 8.Kotwal S, Choudhury A, Johnston C, et al. Similar treatment outcomes for radical cystectomy and radical radiotherapy in invasive bladder cancer treated at a United Kingdom specialist treatment center. Int J Radiat Oncol Biol Phys. 2008;70:456–463. doi: 10.1016/j.ijrobp.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Shipley WU, Kaufman DS, Zehr E, et al. Selective bladder preservation by combined modality protocol treatment: Long-term outcomes of 190 patients with invasive bladder cancer. Urology. 2002;60:62–67. doi: 10.1016/s0090-4295(02)01650-3. [DOI] [PubMed] [Google Scholar]
- 10.Rödel C, Grabenbauer GG, Kühn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: Long-term results. J Clin Oncol. 2002;20:3061–3071. doi: 10.1200/JCO.2002.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Efstathiou JA, Spiegel DY, Shipley WU, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. Eur Urol. 2012;61:705–711. doi: 10.1016/j.eururo.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Weiss C, Engehausen DG, Krause FS, et al. Radiochemotherapy with cisplatin and 5-fluorouracil after transurethral surgery in patients with bladder cancer. Int J Radiat Oncol Biol Phys. 2007;68:1072–1080. doi: 10.1016/j.ijrobp.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Efstathiou JA, Bae K, Shipley WU, et al. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer: RTOG 89-03, 95-06, 97-06, 99-06. J Clin Oncol. 2009;27:4055–4061. doi: 10.1200/JCO.2008.19.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tester W, Caplan R, Heaney J, et al. Neoadjuvant combined modality program with selective organ preservation for invasive bladder cancer: Results of Radiation Therapy Oncology Group phase II trial 8802. J Clin Oncol. 1996;14:119–126. doi: 10.1200/JCO.1996.14.1.119. [DOI] [PubMed] [Google Scholar]
- 15.Shipley WU, Winter KA, Kaufman DS, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: Initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol. 1998;16:3576–3583. doi: 10.1200/JCO.1998.16.11.3576. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman DS, Winter KA, Shipley WU, et al. The initial results in muscle-invading bladder cancer of RTOG 95-06: Phase I/II trial of transurethral surgery plus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservation or cystectomy depending on the initial response. Oncologist. 2000;5:471–476. doi: 10.1634/theoncologist.5-6-471. [DOI] [PubMed] [Google Scholar]
- 17.Hagan MP, Winter KA, Kaufman DS, et al. RTOG 97-06: Initial report of a phase I-II trial of selective bladder conservation using TURBT, twice-daily accelerated irradiation sensitized with cisplatin, and adjuvant MCV combination chemotherapy. Int J Radiat Oncol Biol Phys. 2003;57:665–672. doi: 10.1016/s0360-3016(03)00718-1. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman DS, Winter KA, Shipley WU, et al. Phase I-II RTOG study (99-06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology. 2009;73:833–837. doi: 10.1016/j.urology.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 19.Mitin T, Hunt D, Shipley WU, et al. Transurethral surgery and twice-daily radiation plus paclitaxel-cisplatin or fluorouracil-cisplatin with selective bladder preservation and adjuvant chemotherapy for patients with muscle invasive bladder cancer (RTOG 0233): A randomised multicentre phase 2 trial. Lancet Oncol. 2013;14:863–872. doi: 10.1016/S1470-2045(13)70255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:447–457. [Google Scholar]
- 21.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1143. [Google Scholar]
- 22.Zietman AL, Sacco D, Skowronski U, et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: Results of a urodynamic and quality of life study on long-term survivors. J Urol. 2003;170:1772–1776. doi: 10.1097/01.ju.0000093721.23249.c3. [DOI] [PubMed] [Google Scholar]
- 23.Henningsohn L, Steven K, Kallestrup EB, et al. Distressful symptoms and well-being after radical cystectomy and orthotopic bladder substitution compared with a matched control population. J Urol. 2002;168:168–174. doi: 10.1016/s0022-5347(05)64854-9. [DOI] [PubMed] [Google Scholar]
- 24.Lagrange JL, Bascoul-Mollevi C, Geoffrois L, et al. Quality of life assessment after concurrent chemoradiation for invasive bladder cancer: Results of a multicenter prospective study (GETUG 97-015) Int J Radiat Oncol Biol Phys. 2011;79:172–178. doi: 10.1016/j.ijrobp.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 25.Caffo O, Fellin G, Graffer U, et al. Assessment of quality of life after cystectomy or conservative therapy for patients with infiltrating bladder carcinoma: A survey by a self-administered questionnaire. Cancer. 1996;78:1089–1097. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1089::AID-CNCR20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 26.Eswara JR, Efstathiou JA, Heney NM, et al. Complications and long-term results of salvage cystectomy after failed bladder sparing therapy for muscle invasive bladder cancer. J Urol. 2012;187:463–468. doi: 10.1016/j.juro.2011.09.159. [DOI] [PubMed] [Google Scholar]
- 27.Gray PJ, Fedewa SA, Shipley WU, et al. Clinical-pathologic stage discrepancy in patients with bladder cancer treated with radical cystectomy: Associations with clinical variables and survival. J Clin Oncol. 2013;(suppl 6):31. abstr 248. [Google Scholar]
- 28.Huddart RA, Hall E, Lewis R, et al. Life and death of SPARE (selective bladder preservation against radical excision): Reflections on why the SPARE trial closed. BJU Int. 2010;106:753–755. doi: 10.1111/j.1464-410X.2010.09537.x. [DOI] [PubMed] [Google Scholar]
- 29.Gakis G, Efstathiou J, Lerner SP, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Shipley WU, Efstathiou JA. Radiation-based bladder preserving strategies: Radiation alone or combined with other modalities. In: Soloway M, Khoury S, editors. Bladder Cancer: International Consultation on Bladder Cancer–Vienna (ed 2) Paris, France: International Consultation on Urological Diseases–European Association of Urology; 2012. pp. 316–326. [Google Scholar]
- 31.James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 32.Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw. 2013;11:446–475. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 33.Hoskin PJ, Rojas AM, Bentzen SM, et al. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol. 2010;28:4912–4918. doi: 10.1200/JCO.2010.28.4950. [DOI] [PubMed] [Google Scholar]
- 34.Choudhury A, Nelson LD, Teo MT, et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010;70:7017–7026. doi: 10.1158/0008-5472.CAN-10-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.