Abstract
Purpose
Hippocampal neural stem-cell injury during whole-brain radiotherapy (WBRT) may play a role in memory decline. Intensity-modulated radiotherapy can be used to avoid conformally the hippocampal neural stem-cell compartment during WBRT (HA-WBRT). RTOG 0933 was a single-arm phase II study of HA-WBRT for brain metastases with prespecified comparison with a historical control of patients treated with WBRT without hippocampal avoidance.
Patients and Methods
Eligible adult patients with brain metastases received HA-WBRT to 30 Gy in 10 fractions. Standardized cognitive function and quality-of-life (QOL) assessments were performed at baseline and 2, 4, and 6 months. The primary end point was the Hopkins Verbal Learning Test–Revised Delayed Recall (HVLT-R DR) at 4 months. The historical control demonstrated a 30% mean relative decline in HVLT-R DR from baseline to 4 months. To detect a mean relative decline ≤ 15% in HVLT-R DR after HA-WBRT, 51 analyzable patients were required to ensure 80% statistical power with α = 0.05.
Results
Of 113 patients accrued from March 2011 through November 2012, 42 patients were analyzable at 4 months. Mean relative decline in HVLT-R DR from baseline to 4 months was 7.0% (95% CI, −4.7% to 18.7%), significantly lower in comparison with the historical control (P < .001). No decline in QOL scores was observed. Two grade 3 toxicities and no grade 4 to 5 toxicities were reported. Median survival was 6.8 months.
Conclusion
Conformal avoidance of the hippocampus during WBRT is associated with preservation of memory and QOL as compared with historical series.
INTRODUCTION
Formation of new memory has been associated with a lifelong mitotically active and radiosensitive compartment of neural stem cells located in the subgranular zone of the hippocampal dentate gyrus.1 Injury to this neural stem-cell compartment has been hypothesized to be central to the pathogenesis of radiation-induced early cognitive decline.2 Preclinical studies have demonstrated that relatively modest doses of radiation cause an early and significant decline in neurogenesis in the subgranular zone and that this loss in neurogenic capacity is associated with suppression of new memory formation and impaired recall.3 In addition, recent clinical studies have observed a dose-response relationship between radiation dose received by the hippocampus and risk of postradiotherapy decline in list-learning delayed recall.4
Evidence-based guidelines developed by multiple professional societies have established a role for whole-brain radiotherapy (WBRT) in the setting of multiple brain metastases.5,6 However, WBRT has been associated with 4- and 6-month decline in recall and delayed recall, assessed using the Hopkins Verbal Learning Test–Revised (HVLT-R),7 in addition to decline in patient-reported quality of life (QOL).8 Similar decline in HVLT-R and QOL has also been observed after prophylactic cranial irradiation for lung cancer.9,10 To prevent these adverse early cognitive effects of therapeutic or prophylactic cranial irradiation, modern intensity-modulated radiotherapy (IMRT) techniques have been developed to avoid conformally the hippocampal neural stem-cell niche during WBRT, often referred to as hippocampal-avoidance WBRT (HA-WBRT).11,12 The principle of HA-WBRT centers around preservation of the radiosensitive, memory-specific neural stem compartment, rather than any anatomic and/or physiologic components of the hippocampus. HA-WBRT techniques have demonstrated the ability to reduce mean dose to this neural stem-cell compartment by at least 80%, while providing acceptable coverage and dose homogeneity to the remaining whole-brain parenchyma.11
On the basis of these feasibility analyses, an international multi-institution single-arm phase II trial of HA-WBRT for brain metastases was conducted through the Radiation Therapy Oncology Group (RTOG; RTOG 0933). HVLT-R delayed recall (HVLT-R DR) was the primary end point, with a prespecified statistical comparison of HVLT-R DR outcomes with a historical control of patients treated with WBRT without hippocampal avoidance from a published phase III trial (PCI-P-120-9801).13
PATIENTS AND METHODS
Study Design and Patients
Patients with brain metastases outside a 5-mm margin around either hippocampus, pathologically proven diagnosis of nonhematopoetic malignancy other than small-cell lung cancer or germ cell malignancy, RTOG recursive partitioning analysis class I or II, and English proficiency were factors for inclusion. Patients age < 18 years and those with leptomeningeal metastases, radiographic evidence of hydrocephalus, prior radiation to the brain, planned upfront radiosurgery or surgical resection, contraindication to magnetic resonance imaging (MRI), serum creatinine > 1.4 mg/dL ≤ 30 days before study entry, or non–small-cell lung cancer–associated brain metastases with ≥ two organ sites of extracranial metastases were excluded.
All eligibility criteria matched eligibility criteria for the historical control, which comprised patients with brain metastases treated with standard WBRT to 30 Gy in 10 fractions without hippocampal avoidance in the control arm of the PCI-P-120-9801 phase III trial.13 Stable systemic disease was initially included as an eligibility criterion but was subsequently removed with the first protocol amendment (December 5, 2011), because it was not included in the historical control trial.
Pretreatment assessments consisted of medical history and physical examination, baseline cognitive assessment, and completion of baseline QOL questionnaires (for patients opting to participate in QOL portion of study). Before enrolling patients, all sites were required to meet specific technologic requirements and provide baseline physics information for the use of IMRT in this study. In addition, all treating physicians and sites were required to successfully complete a dry-run quality assurance test involving fusion of MRI and radiotherapy-planning computed tomography (CT), hippocampal contouring, and development of an IMRT plan for HA-WBRT for a sample patient chosen from a test group of five patients whose MRI and CT imaging were provided electronically. To train on the techniques of hippocampal contouring and IMRT planning for HA-WBRT, multiple didactic workshops were held by the principal investigators of the trial (V.G. and M.P.M.) during RTOG semiannual meetings, and a contouring atlas for hippocampal delineation was made available electronically on the RTOG Web site.14
All patients provided written informed consent. The study was approved by the National Cancer Institute and by the institutional review boards of the participating centers.
Procedures
For hippocampal contouring and HA-WBRT planning, all patients required a three-dimensional spoiled gradient echo, magnetization-prepared rapid gradient echo, or turbo field echo axial MRI scan of the brain with axial slice thickness ≤ 1.5 mm, fused to a radiotherapy-planning head CT scan with axial slice thickness ≤ 2.5 mm. Bilateral hippocampal contours were manually generated on the fused MRI-CT image set and expanded by 5 mm to generate the hippocampal avoidance regions. The clinical target volume was defined as the whole-brain parenchyma, and the planning target volume (PTV) was defined as the clinical target volume excluding the hippocampal avoidance regions. No set-up margin was included in the PTV. IMRT was delivered to a dose of 30 Gy in 10 fractions to cover the PTV while avoiding the hippocampus. This dose-fractionation scheme matched the treatment approach used for the historical control.13
Central rapid review of hippocampal contours and HA-WBRT planning was conducted in real time before initiation of treatment. Per protocol, dose to 100% of the hippocampus could not exceed 9 Gy, and maximal hippocampal dose could not exceed 16 Gy; dose to 100% of the hippocampus exceeding 10 Gy and maximal hippocampal dose exceeding 17 Gy were considered unacceptable deviations and required re-planning before treatment initiation. Treating physicians who enrolled three consecutive patients without unacceptable contouring or planning deviations were permitted to enroll additional patients without pretreatment central review. However, all of these treatment plans were reviewed post-treatment.
End Points
Standardized cognitive assessments and health-related QOL were completed at baseline and at 2-, 4-, and 6-month follow-up. Cognitive assessments included HVLT-R, which incorporates six different versions, helping to mitigate practice effects of repeated administrations. Each version includes 12 nouns (targets) with four words drawn from three semantic categories, which differ across the six versions. The test involves memorizing a list of 12 targets for three consecutive trials (total recall or HVLT-R TR), identifying the 12 targets from a list of semantically related or unrelated items (immediate recognition or HVLT-R IR), and recalling the 12 targets after a 20-minute delay (delayed recall or HVLT-R DR). The timing of HVLT-R IR immediately after HVLT-R TR, as opposed to after HVLT-R DR, represents a departure from HVLT-R used in more contemporary studies, but it was in keeping with the method of administration for the control cohort.13 This approach has been used in prior phase III cooperative group studies.9,15
QOL questionnaires included the Functional Assessment of Cancer Therapy–Brain subscale (FACT-BR) and the Barthel Index of Activities of Daily Living (ADLs). The FACT-BR is a multidimensional, self-reported QOL instrument specifically designed and validated for use in patients with brain malignancies. It measures QOL related to symptoms or problems across five scales: physical well being (seven items), social/family well being (seven items), emotional well being (six items), functional well being (seven items), and concerns relevant to patients with brain tumors (23 items). Scores on the FACT-BR range from 0 to 92, with lower scores indicating worse QOL. The Barthel Index of ADLs is a self-reported 10-item instrument designed to assess a patient's ability to carry out ADLs as reported by the patient or his or her family or caregiver. The Barthel Index score ranges from 0 to 20, with lower scores indicating worse functional status. Patients underwent MRI assessments at 2, 4, and 6 months from the start of HA-WBRT and then quarterly until death.
Statistical Analysis
Raw scores were derived from the cognitive assessments and QOL questionnaires. Each patient served as her or his own control, and the relative decline in HVLT-R score from baseline to prespecified post-treatment intervals was defined as follows: ΔHVLTi = (HVLTB − HVLTF) ÷ HVLTB, where B = baseline and F = follow-up. A positive change indicated a decline in function.
The primary end point was decline in HVLT-R DR score from baseline to 4 months after the start of HA-WBRT. Data from the WBRT-alone arm (n = 85) of the PCI-P-120-9801 phase III trial demonstrated 30% mean relative decline in HVLT-R DR score from baseline to 4 months, with a standard deviation of 41%. To detect a minimum relative 50% improvement in this end point, leading to an absolute ≤ 15% mean relative decline in HVLT-R DR after HA-WBRT, 51 analyzable patients were required to ensure 80% statistical power with α = 0.05. Assuming a death rate of 40% before 4 months (based on PCI-P-120-9801 trial) and a 10% nonevaluable rate, the target sample size was 102 registered patients.
Comparison of HVLT-R DR results between this trial and the historical control was tested using the one-sided Wilcoxon signed rank test with significance level of .05. Using a version of the reliable change index,16,17 significant deterioration was defined as a drop from baseline of at least 5 points for HVLT-R TR, 2 points for HVLT-R IR, and 3 points for HVLT-R DR.18 The generalized linear mixed-effects model with significance level of .05 was used to evaluate change in HVLT-R and QOL scores over time, as well as potential univariable associations of age (≥ 60 v < 60 years), baseline neurologic function status (no symptoms v at least minor symptoms), recursive partitioning analysis class (I v II), primary disease site (lung v other), maximum hippocampal dose, and dose delivered to 100% (D100%) of the hippocampus on the primary end point HVLT-R DR. These models assumed an unstructured covariance structure and a missing-at-random missing data mechanism and used maximum-likelihood estimation of parameters. The Kaplan-Meier estimator was used to determine median time to radiographic progression and median time to death. Alive patients were censored at their last follow-up date.
RESULTS
From March 31, 2011, to November 7, 2012, a total of 113 patients were accrued, 13 of whom were excluded from the analysis: seven received no protocol treatment, and six were ineligible (five did not meet eligibility criteria, and one did not complete baseline evaluation form). Table 1 lists the characteristics of the 100 analyzable patients.
Table 1.
Patient Demographic and Clinical Characteristics
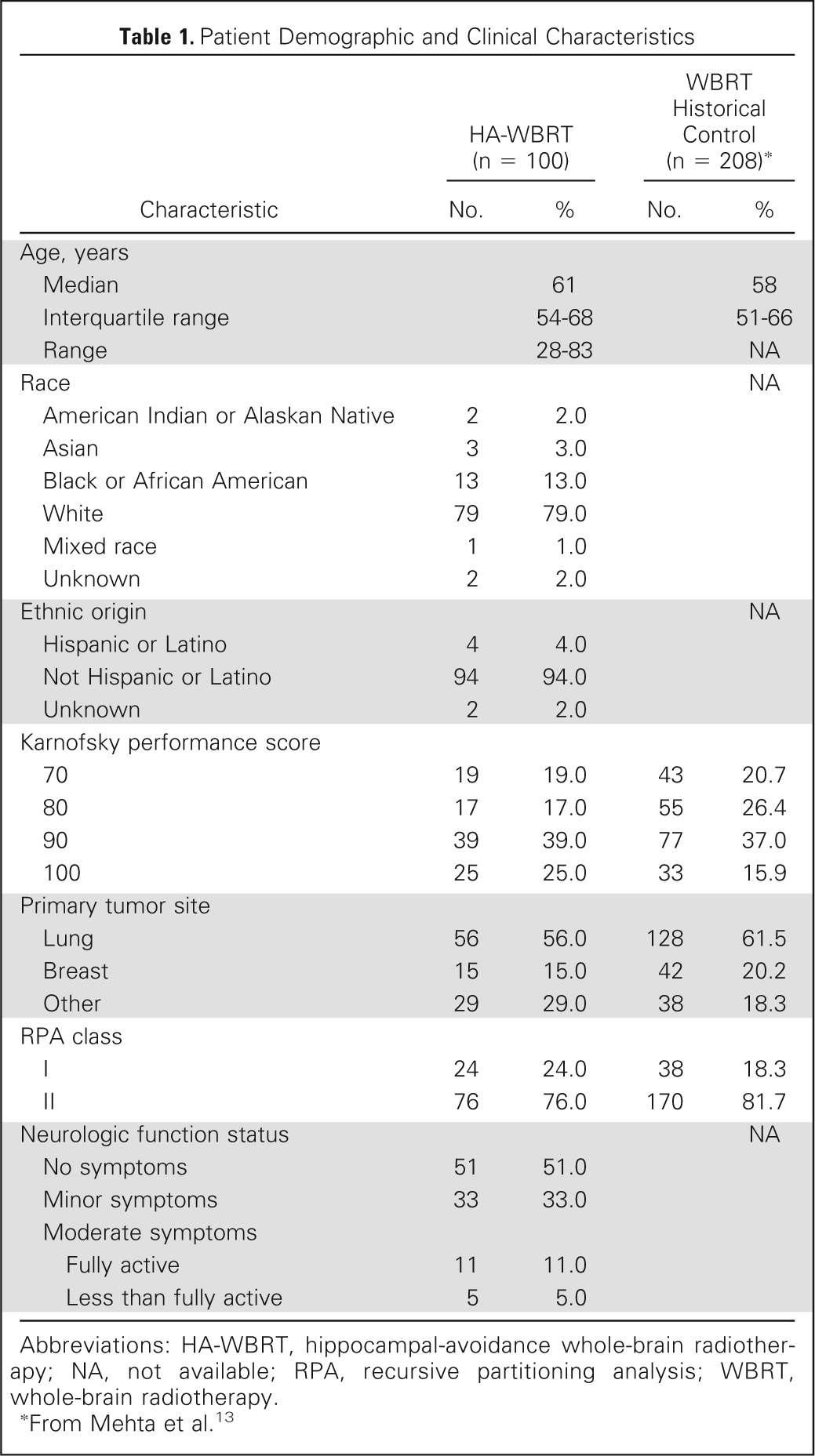
| Characteristic | HA-WBRT (n = 100) |
WBRT Historical Control (n = 208)* |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 61 | 58 | ||
| Interquartile range | 54-68 | 51-66 | ||
| Range | 28-83 | NA | ||
| Race | NA | |||
| American Indian or Alaskan Native | 2 | 2.0 | ||
| Asian | 3 | 3.0 | ||
| Black or African American | 13 | 13.0 | ||
| White | 79 | 79.0 | ||
| Mixed race | 1 | 1.0 | ||
| Unknown | 2 | 2.0 | ||
| Ethnic origin | NA | |||
| Hispanic or Latino | 4 | 4.0 | ||
| Not Hispanic or Latino | 94 | 94.0 | ||
| Unknown | 2 | 2.0 | ||
| Karnofsky performance score | ||||
| 70 | 19 | 19.0 | 43 | 20.7 |
| 80 | 17 | 17.0 | 55 | 26.4 |
| 90 | 39 | 39.0 | 77 | 37.0 |
| 100 | 25 | 25.0 | 33 | 15.9 |
| Primary tumor site | ||||
| Lung | 56 | 56.0 | 128 | 61.5 |
| Breast | 15 | 15.0 | 42 | 20.2 |
| Other | 29 | 29.0 | 38 | 18.3 |
| RPA class | ||||
| I | 24 | 24.0 | 38 | 18.3 |
| II | 76 | 76.0 | 170 | 81.7 |
| Neurologic function status | NA | |||
| No symptoms | 51 | 51.0 | ||
| Minor symptoms | 33 | 33.0 | ||
| Moderate symptoms | ||||
| Fully active | 11 | 11.0 | ||
| Less than fully active | 5 | 5.0 | ||
Abbreviations: HA-WBRT, hippocampal-avoidance whole-brain radiotherapy; NA, not available; RPA, recursive partitioning analysis; WBRT, whole-brain radiotherapy.
From Mehta et al.13
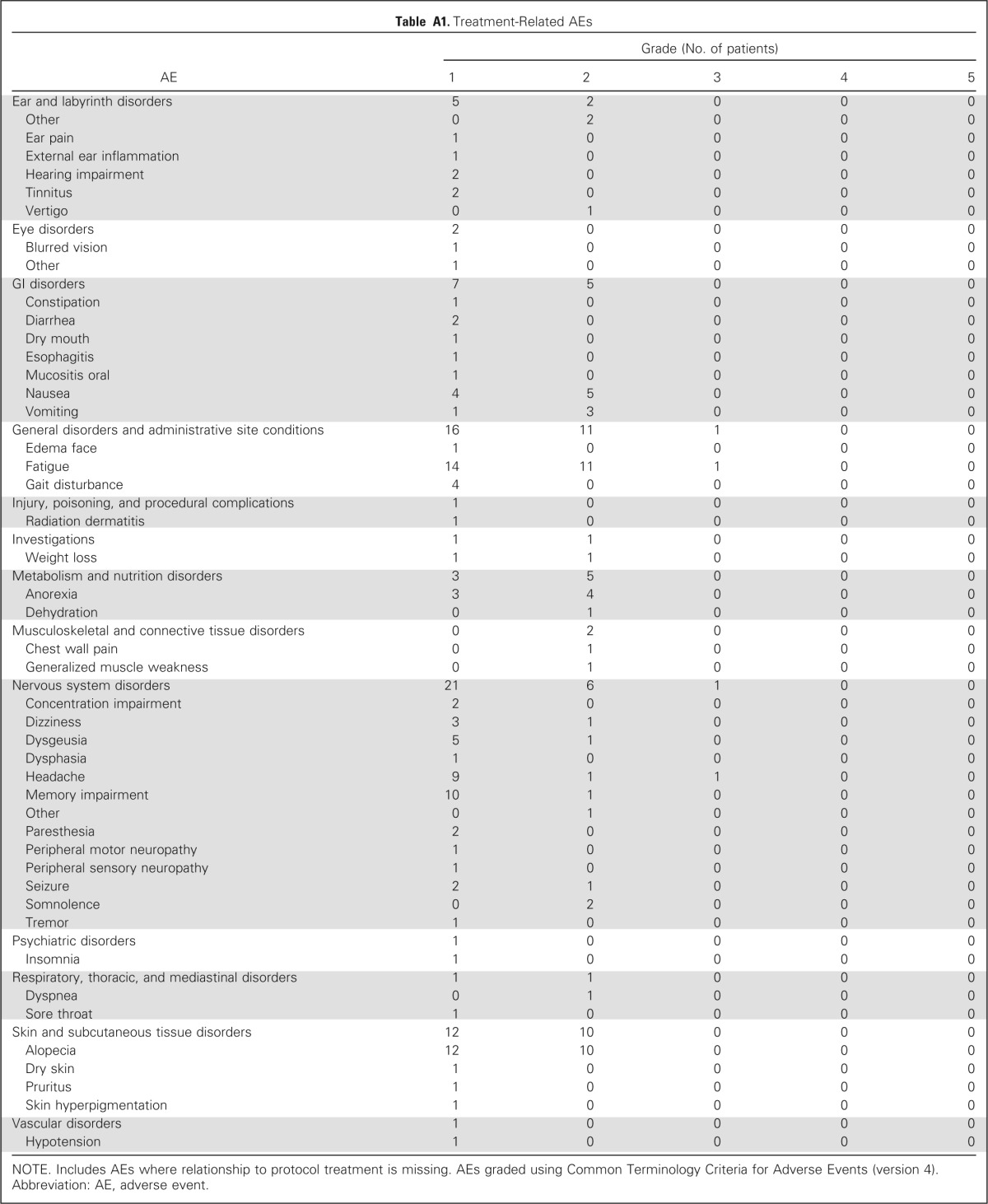
Two treatment-related grade 3 adverse events (fatigue and headache) were reported, and no treatment-related grade 4 to 5 adverse events were observed (Appendix Table A1, online only). Grade 1 to 2 treatment-related adverse events occurred in 42 patients, with fatigue (25 events) and alopecia (22 events) being the most common.
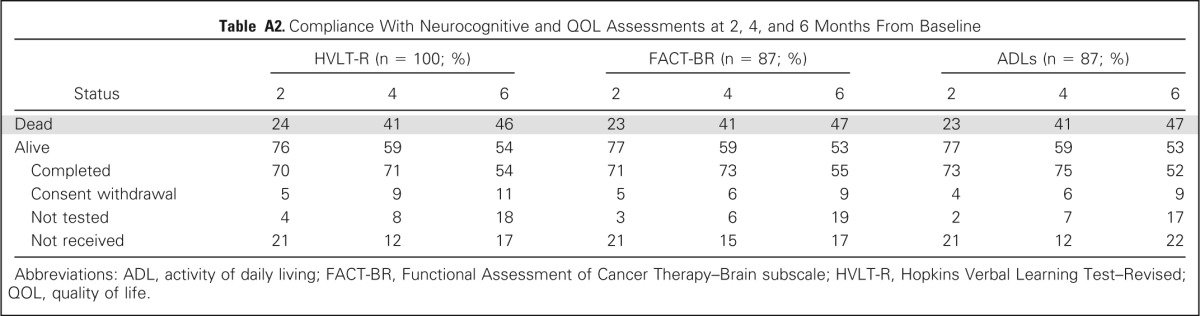
Compliance with HVLT-R, FACT-BR, and ADL assessments was > 70% among surviving patients at 2 and 4 months and > 50% among surviving patients at 6 months for all assessments (Appendix Table A2, online only). For the primary end point of HVLT-R DR decline at 4 months, 42 patients were analyzable, 17 patients were alive but not analyzable primarily because of noncompliance with HVLT-R testing, and 41 patients had died.
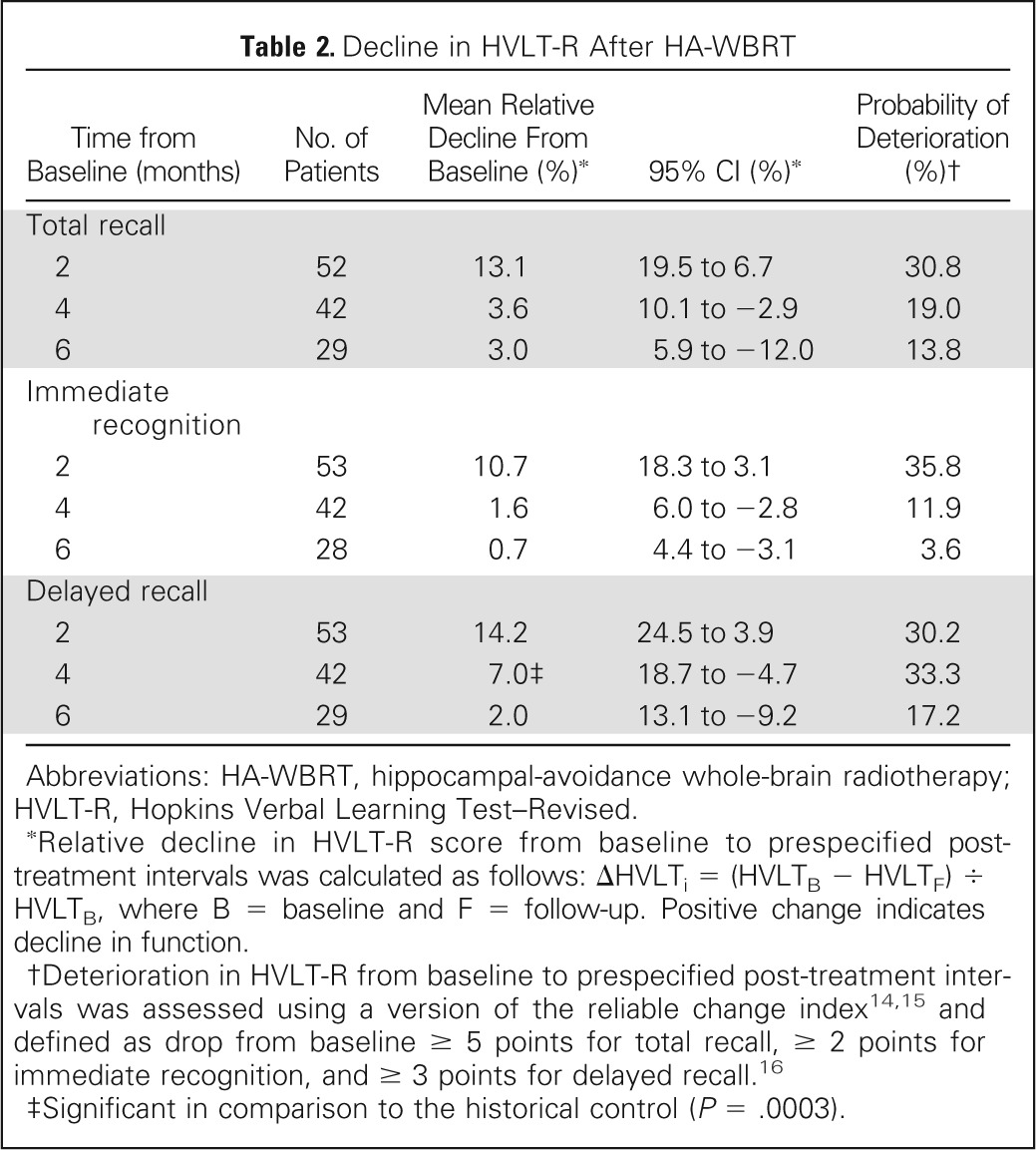
Table 2 lists mean relative decline and probability of deterioration in HVLT-R from baseline to each time point. Mean relative decline in HVLT-R DR from baseline to 4 months was 7.0% (95% CI, −4.7% to 18.7%), which was significantly lower in comparison with the 30% mean relative HVLT-R DR decline observed in the historical control (P < .001). In addition, the probability of HVLT-R TR deterioration, assessed using the reliable change index, was 19.0% at 4 months.
Table 2.
Decline in HVLT-R After HA-WBRT
| Time from Baseline (months) | No. of Patients | Mean Relative Decline From Baseline (%)* | 95% CI (%)* | Probability of Deterioration (%)† |
|---|---|---|---|---|
| Total recall | ||||
| 2 | 52 | 13.1 | 19.5 to 6.7 | 30.8 |
| 4 | 42 | 3.6 | 10.1 to −2.9 | 19.0 |
| 6 | 29 | 3.0 | 5.9 to −12.0 | 13.8 |
| Immediate recognition | ||||
| 2 | 53 | 10.7 | 18.3 to 3.1 | 35.8 |
| 4 | 42 | 1.6 | 6.0 to −2.8 | 11.9 |
| 6 | 28 | 0.7 | 4.4 to −3.1 | 3.6 |
| Delayed recall | ||||
| 2 | 53 | 14.2 | 24.5 to 3.9 | 30.2 |
| 4 | 42 | 7.0‡ | 18.7 to −4.7 | 33.3 |
| 6 | 29 | 2.0 | 13.1 to −9.2 | 17.2 |
Abbreviations: HA-WBRT, hippocampal-avoidance whole-brain radiotherapy; HVLT-R, Hopkins Verbal Learning Test–Revised.
Relative decline in HVLT-R score from baseline to prespecified post-treatment intervals was calculated as follows: ΔHVLTi = (HVLTB − HVLTF) ÷ HVLTB, where B = baseline and F = follow-up. Positive change indicates decline in function.
Deterioration in HVLT-R from baseline to prespecified post-treatment intervals was assessed using a version of the reliable change index14,15 and defined as drop from baseline ≥ 5 points for total recall, ≥ 2 points for immediate recognition, and ≥ 3 points for delayed recall.16
Significant in comparison to the historical control (P = .0003).
For the 50 patients who were alive at 6 months, including patients with missing HVLT-R data, Figure 1 shows HVLT-R scores over time. For the 46 patients who died by 6 months, Figure 2 shows HVLT-R scores over time. No follow-up information was available for four patients. On generalized linear mixed-effects modeling accounting for missing data from patients who had died by 6 months, HVLT-R DR significantly declined over time (P = .0083). HVLT-R TR (P = .180) and HVLT-R IR (P = .499) remained relatively stable. Age ≥ 60 years (P < .001), presence of at least minor neurologic symptoms at baseline (P = .0033), and higher hippocampal D100% (P = .0037) predicted greater decline over time in HVLT-R DR.
Fig 1.
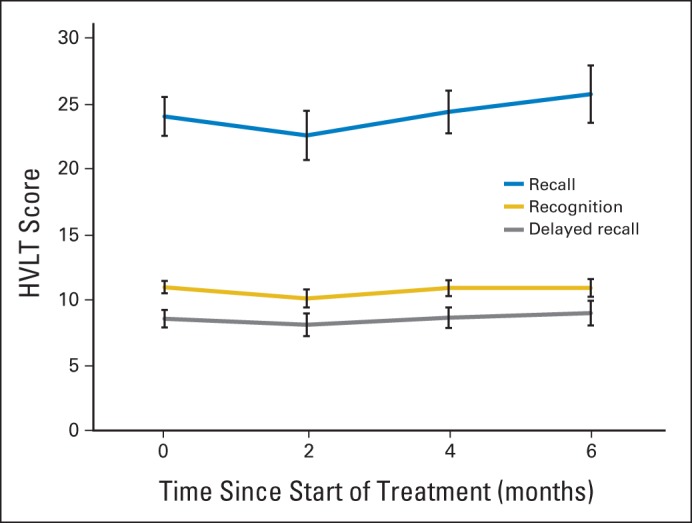
Hopkins Verbal Learning Test (HVLT) scores for 50 patients alive at 6 months.
Fig 2.
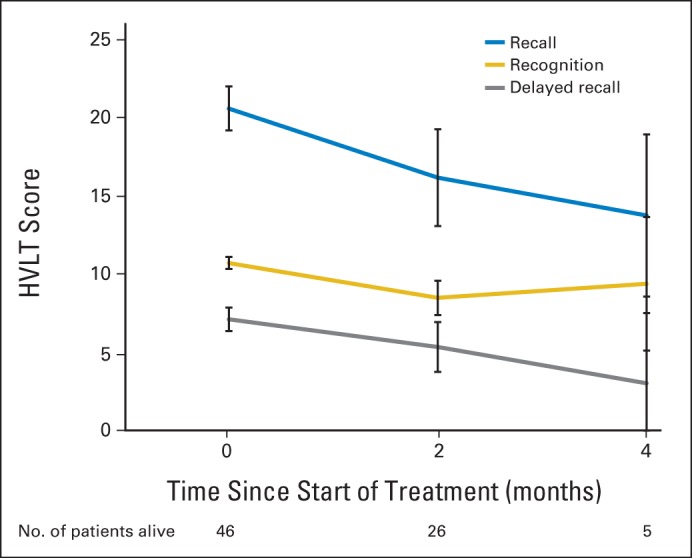
Hopkins Verbal Learning Test (HVLT) scores for 46 patients who had died by 6 months.
Figure 3 shows FACT-BR and ADL scores at baseline and 2, 4, and 6 months after HA-WBRT. FACT-BR emotional well being improved over time (P = .042), whereas other FACT-BR and ADL scores remained stable.
Fig 3.
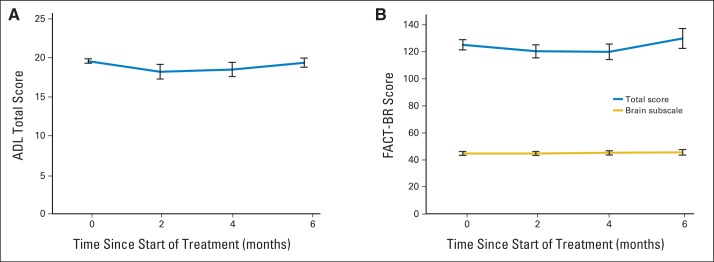
Quality of life assessed using (A) Barthel's Index of Activities of Daily Living (ADLs) and (B) Functional Assessment of Cancer Therapy–Brain subscale (FACT-BR).
Median survival was 6.8 months (95% CI, 4.8 to 10.9 months; Fig 4); 62% of patients died as a result of their primary disease, whereas 7.3% of patients died as a result of their brain metastases. Median progression-free survival was 5.9 months (95% CI, 4.7 to 8.4 months). Of the 67 patients who developed intracranial progression, three (4.5%) experienced progression in the hippocampal avoidance area.
Fig 4.
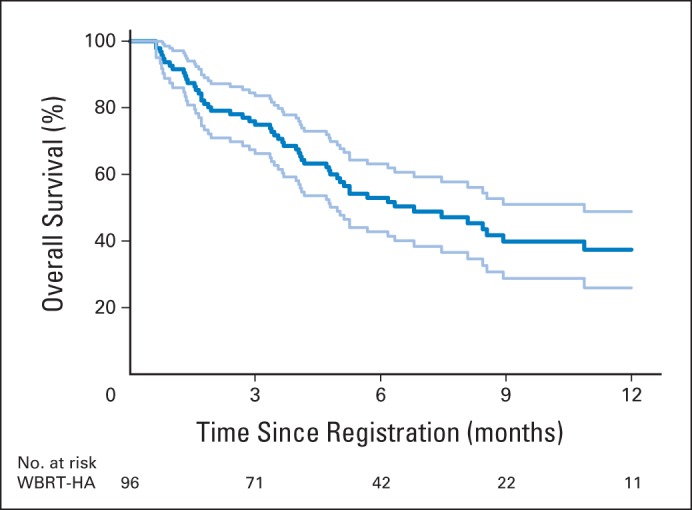
Overall survival. Light blue lines represent upper and lower limits of 95% CI. WBRT-HA, hippocampal-avoidance whole-brain radiotherapy.
DISCUSSION
Conformal avoidance of the hippocampal neural stem-cell compartment during WBRT using IMRT for patients with brain metastases is associated with significant memory preservation, as measured by prevention of HVLT-R DR decline, compared with a prespecified historical control of patients with brain metastases treated with WBRT without hippocampal avoidance.13 In addition, remaining HVLT-R domains demonstrated preservation up to 6-month follow-up. As a relative comparison, the MD Anderson Cancer Center phase III trial of radiosurgery with or without WBRT for one to three brain metastases used a reliable change index to assess HVLT-R TR deterioration at 4 months as its primary end point.7 That study observed a 24% rate of 4-month HVLT-R TR deterioration after radiosurgery alone and a 52% rate of 4-month HVLT-R TR deterioration after radiosurgery with WBRT. In RTOG 0933, HA-WBRT was associated with a 19% rate of HVLT-R TR deterioration at 4 months (as measured using the same reliable change index), comparing favorably with the MD Anderson series. Although prior studies have suggested a possible relationship between hippocampal irradiation and subsequent memory decline,4 this study provides the first direct clinical evidence, to our knowledge, that the hippocampal neural stem-cell niche is central to the pathophysiology of radiotherapy-induced acute and subacute memory decline.
In addition to prevention of memory loss, HA-WBRT is associated with preservation of QOL, assessed using FACT-BR and Barthel Index of ADLs. Li et al19 previously demonstrated a correlation between FACT-BR and ADL scores and HVLT-R outcomes, with decline in HVLT-R, and especially HVLT-R DR, predicting subsequent FACT-BR and ADL score decline. Thus, prevention of HVLT-R decline may represent one potential mechanism for the QOL preservation observed after HA-WBRT.
The effect of older age on declining HVLT-R TR and HVLT-R DR scores after HA-WBRT seems consistent with preclinical studies showing an age-dependent inflammatory response of the hippocampal dentate gyrus to WBRT.20 This finding suggests that patients age > 60 years may be a high-risk group of patients for whom neuroprotective interventions beyond HA-WBRT may be required. In addition, higher hippocampal D100% predicted for greater HVLT-R DR decline, suggesting that further lowering the dose to the entire hippocampal neural stem-cell compartment may affect list-learning recall outcomes. Evaluation of this potential dose-response relationship in terms of other dosimetric metrics (eg, dose to 40% of hippocampus4) and by subsegmentation of the hippocampal dentate gyrus, as well as radiographic and clinical evaluation of long-term survivors, is currently under investigation.
Conformal avoidance of the hippocampus poses the risk of attenuating the benefit of WBRT because of the emergence of new brain metastases within the hippocampal avoidance region. Prior estimates have approximated this risk at 8.6% (95% CI, 5.7% to 11.5%).21 However, these prior estimates were based on calculating the occurrence of brain metastases in the hippocampal avoidance region in patients presenting with brain metastases, which seems to have resulted in overestimation of the actual risk, because only three patients (4.5%) experienced progression in the hippocampal avoidance area in this phase II HA-WBRT study. Given the absence of any treatment-related grade 4 to 5 adverse events and only two treatment-related grade 3 events, the summation of these data suggests that HA-WBRT can be safely delivered to patients with brain metastases.
These promising results warrant further validation within the phase III setting, in part because of the expected limitations of such a single-arm phase II study. For instance, the median survival estimate (6.8 months) of this study exceeded the median survival estimates of the prespecified historical WBRT control (4.9 months)13 and the radiosurgery-plus-WBRT arm of the MD Anderson series (5.7 months)7 in a nonsignificant manner. As shown in Figure 2, significant HVLT-R decline could be seen at 2 and 4 months in patients who did not survive beyond 6 months. Thus, improved survivorship may in part play a role in the promising HVLT-R and QOL results of this phase II study. An additional limitation is the inability to statistically compare QOL results from RTOG 0933 with QOL results from the prespecified historical control, because these results have not been previously reported and were not accessible to the study team. A third limitation is HVLT-R data compliance, which was improved over prior cooperative group studies9,22 but lower than the prespecified historical control.13 This is primarily because the industry-sponsored historical control trial supported a team of dedicated clinical research specialists who proactively pursued and encouraged compliance. However, despite this limitation, the number of patients analyzable for the primary end point, although lower than statistically required, was sufficient to detect a benefit relative to the prespecified historical control, potentially because the effect size was larger than expected. Finally, the capacity of hippocampal avoidance to prevent longer-term (ie, beyond 6 months) effects of WBRT, such as white matter imaging changes23 and/or cognitive effects, could not be assessed given the limited sample size and high patient death rate in this phase II study. However, such an analysis is anticipated in planned phase III studies of hippocampal avoidance.
Building on results of RTOG 0614,22 NRG CC001 is a National Cancer Institute–approved phase III trial that will evaluate the potential combined neuroprotective effects of hippocampal avoidance in addition to prophylactic memantine during WBRT for brain metastases. NRG CC1432 is a National Cancer Institute–approved randomized phase II/III trial of hippocampal avoidance during prophylactic cranial irradiation for small-cell lung cancer. Given the increased cost of hippocampal avoidance, these studies will also perform a comparative-effectiveness analysis by including EQ5D as a secondary patient-reported outcome. These randomized trials of hippocampal avoidance will build on the robust quality assurance infrastructure established for RTOG 0933. This infrastructure included pre-enrollment credentialing of sites and treating physicians on the published HA-WBRT techniques and central review of all treatment plans, with most plans undergoing real-time pretreatment rapid review.
Glossary Terms
- intensity-modulated radiation therapy:
radiation treatment using beams with nonuniform fluence profiles that shape the dose distribution in the target volume and adjacent normal structures. Beam modulation is typically achieved via multileaf collimators or custom-milled compensators to achieve the appropriate fluence profiles calculated by inverse optimization algorithms. The radiation beam is divided into beamlets of varying intensity such that the sum from multiple beams via inverse planning results in improved tumor targeting and normal tissue sparing. A technique of radiation therapy delivery in which the intensity of each beamlet of radiation coming from a specific angle can be adjusted to provide a desired dose distribution when the doses delivered from all beamlets are added from a single angle and from all dose delivery angles. An advanced type of high-precision radiotherapy, which aims to improve the coverage of the radiotherapy target and/or minimize radiation dose to surrounding normal tissue.
Appendix
Table A1.
Treatment-Related AEs
| AE | Grade (No. of patients) |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Ear and labyrinth disorders | 5 | 2 | 0 | 0 | 0 |
| Other | 0 | 2 | 0 | 0 | 0 |
| Ear pain | 1 | 0 | 0 | 0 | 0 |
| External ear inflammation | 1 | 0 | 0 | 0 | 0 |
| Hearing impairment | 2 | 0 | 0 | 0 | 0 |
| Tinnitus | 2 | 0 | 0 | 0 | 0 |
| Vertigo | 0 | 1 | 0 | 0 | 0 |
| Eye disorders | 2 | 0 | 0 | 0 | 0 |
| Blurred vision | 1 | 0 | 0 | 0 | 0 |
| Other | 1 | 0 | 0 | 0 | 0 |
| GI disorders | 7 | 5 | 0 | 0 | 0 |
| Constipation | 1 | 0 | 0 | 0 | 0 |
| Diarrhea | 2 | 0 | 0 | 0 | 0 |
| Dry mouth | 1 | 0 | 0 | 0 | 0 |
| Esophagitis | 1 | 0 | 0 | 0 | 0 |
| Mucositis oral | 1 | 0 | 0 | 0 | 0 |
| Nausea | 4 | 5 | 0 | 0 | 0 |
| Vomiting | 1 | 3 | 0 | 0 | 0 |
| General disorders and administrative site conditions | 16 | 11 | 1 | 0 | 0 |
| Edema face | 1 | 0 | 0 | 0 | 0 |
| Fatigue | 14 | 11 | 1 | 0 | 0 |
| Gait disturbance | 4 | 0 | 0 | 0 | 0 |
| Injury, poisoning, and procedural complications | 1 | 0 | 0 | 0 | 0 |
| Radiation dermatitis | 1 | 0 | 0 | 0 | 0 |
| Investigations | 1 | 1 | 0 | 0 | 0 |
| Weight loss | 1 | 1 | 0 | 0 | 0 |
| Metabolism and nutrition disorders | 3 | 5 | 0 | 0 | 0 |
| Anorexia | 3 | 4 | 0 | 0 | 0 |
| Dehydration | 0 | 1 | 0 | 0 | 0 |
| Musculoskeletal and connective tissue disorders | 0 | 2 | 0 | 0 | 0 |
| Chest wall pain | 0 | 1 | 0 | 0 | 0 |
| Generalized muscle weakness | 0 | 1 | 0 | 0 | 0 |
| Nervous system disorders | 21 | 6 | 1 | 0 | 0 |
| Concentration impairment | 2 | 0 | 0 | 0 | 0 |
| Dizziness | 3 | 1 | 0 | 0 | 0 |
| Dysgeusia | 5 | 1 | 0 | 0 | 0 |
| Dysphasia | 1 | 0 | 0 | 0 | 0 |
| Headache | 9 | 1 | 1 | 0 | 0 |
| Memory impairment | 10 | 1 | 0 | 0 | 0 |
| Other | 0 | 1 | 0 | 0 | 0 |
| Paresthesia | 2 | 0 | 0 | 0 | 0 |
| Peripheral motor neuropathy | 1 | 0 | 0 | 0 | 0 |
| Peripheral sensory neuropathy | 1 | 0 | 0 | 0 | 0 |
| Seizure | 2 | 1 | 0 | 0 | 0 |
| Somnolence | 0 | 2 | 0 | 0 | 0 |
| Tremor | 1 | 0 | 0 | 0 | 0 |
| Psychiatric disorders | 1 | 0 | 0 | 0 | 0 |
| Insomnia | 1 | 0 | 0 | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | 1 | 1 | 0 | 0 | 0 |
| Dyspnea | 0 | 1 | 0 | 0 | 0 |
| Sore throat | 1 | 0 | 0 | 0 | 0 |
| Skin and subcutaneous tissue disorders | 12 | 10 | 0 | 0 | 0 |
| Alopecia | 12 | 10 | 0 | 0 | 0 |
| Dry skin | 1 | 0 | 0 | 0 | 0 |
| Pruritus | 1 | 0 | 0 | 0 | 0 |
| Skin hyperpigmentation | 1 | 0 | 0 | 0 | 0 |
| Vascular disorders | 1 | 0 | 0 | 0 | 0 |
| Hypotension | 1 | 0 | 0 | 0 | 0 |
NOTE. Includes AEs where relationship to protocol treatment is missing. AEs graded using Common Terminology Criteria for Adverse Events (version 4).
Abbreviation: AE, adverse event.
Table A2.
Compliance With Neurocognitive and QOL Assessments at 2, 4, and 6 Months From Baseline
| Status | HVLT-R (n = 100; %) |
FACT-BR (n = 87; %) |
ADLs (n = 87; %) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 2 | 4 | 6 | 2 | 4 | 6 | |
| Dead | 24 | 41 | 46 | 23 | 41 | 47 | 23 | 41 | 47 |
| Alive | 76 | 59 | 54 | 77 | 59 | 53 | 77 | 59 | 53 |
| Completed | 70 | 71 | 54 | 71 | 73 | 55 | 73 | 75 | 52 |
| Consent withdrawal | 5 | 9 | 11 | 5 | 6 | 9 | 4 | 6 | 9 |
| Not tested | 4 | 8 | 18 | 3 | 6 | 19 | 2 | 7 | 17 |
| Not received | 21 | 12 | 17 | 21 | 15 | 17 | 21 | 12 | 22 |
Abbreviations: ADL, activity of daily living; FACT-BR, Functional Assessment of Cancer Therapy–Brain subscale; HVLT-R, Hopkins Verbal Learning Test–Revised; QOL, quality of life.
Footnotes
See accompanying editorial on page 3789
Supported by Radiation Therapy Oncology Group Grant No. U10 CA21661 and Community Clinical Oncology Program Grant No. U10 CA37422.
Presented in abstract form at the 55th Annual Meeting of the American Society of Radiation Oncology, Atlanta, GA, September 22-25, 2013, 15th World Conference on Lung Cancer for the International Association for the Study of Lung Cancer, Sydney, Australia, October 27-31, 2013, and 18th Annual Meeting of the Society for Neuro-Oncology, San Francisco, CA, November 21-24, 2013.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The contents of this article are the sole responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Clinical trial information: NCT01227954.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Vinai Gondi, Wolfgang A. Tome, Chip Caine, Ben Corn, Andrew Kanner, Howard Rowley, Minesh P. Mehta
Financial support: Vinai Gondi, Chip Caine, Lisa Kachnic, Minesh P. Mehta
Provision of study materials or patients: Vijayananda Kundapur, Albert DeNittis, Jeffrey N. Greenspoon, Andre A. Konski, Glenn S. Bauman, Sunjay Shah, Wenyin Shi, Merideth Wendland, Lisa Kachnic
Collection and assembly of data: Vinai Gondi, Stephanie L. Pugh, Chip Caine
Data analysis and interpretation: Vinai Gondi, Stephanie L. Pugh, Wolfgang A. Tome, Ben Corn, Andrew Kanner, Howard Rowley, Vijayananda Kundapur, Albert DeNittis, Jeffrey N. Greenspoon, Andre A. Konski, Glenn S. Bauman, Sunjay Shah, Wenyin Shi, Merideth Wendland, Lisa Kachnic, Minesh P. Mehta
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Preservation of Memory With Conformal Avoidance of the Hippocampal Neural Stem-Cell Compartment During Whole-Brain Radiotherapy for Brain Metastases (RTOG 0933): A Phase II Multi-Institutional Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Vinai Gondi
No relationship to disclose
Stephanie L. Pugh
Travel, Accommodations, Expenses: Genentech/Roche
Wolfgang A. Tome
Consulting or Advisory Role: View Ray
Research Funding: Philips Medical System, Accuray
Patents, Royalties, Other Intellectual Property: Wisconsin Alumni Research Foundation
Chip Caine
No relationship to disclose
Ben Corn
No relationship to disclose
Andrew Kanner
Research Funding: Novocure (Inst)
Howard Rowley
Honoraria: Bracco Diagnostics
Consulting or Advisory Role: Bracco Diagnostics, Guerbet, Genentech, Eli Lilly, Gore, Lundbeck
Research Funding: GE Healthcare (Inst), Bracco Diagnostics (Inst)
Patents, Royalties, Other Intellectual Property: GE Healthcare
Travel, Accommodations, Expenses: Bracco Diagnostics, Guerbet
Vijayananda Kundapur
No relationship to disclose
Albert DeNittis
No relationship to disclose
Jeffrey N. Greenspoon
No relationship to disclose
Andre A. Konski
No relationship to disclose
Glenn S. Bauman
No relationship to disclose
Sunjay Shah
No relationship to disclose
Wenyin Shi
Honoraria: Varian Medical Systems
Consulting or Advisory Role: Elekta
Research Funding: Bristol-Myers Squibb, Roche, Millennium Pharmaceuticals
Merideth Wendland
Employment: US Oncology
Lisa Kachnic
Honoraria: Leidos Biomed Research
Patents, Royalties, Other Intellectual Property: UpToDate
Expert Testimony: Bennett, Bigelow & Leedom, Swedish Inst./Health Services Asset Management, Hollingsworth
Travel, Accommodations, Expenses: Leidos Biomed Research
Minesh P. Mehta
Leadership: Pharmacyclics
Stock or Other Ownership: Accuray
Honoraria: Merck
Consulting or Advisory Role: Abbott Laboratories, Elekta, Genentech, Merck, Novocure
Research Funding: Novocure
REFERENCES
- 1.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 2.Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010;97:370–376. doi: 10.1016/j.radonc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monje ML, Mizumatsu S, Fike JR. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 4.Gondi V, Hermann BP, Mehta MP, et al. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int J Radiat Oncol Biol Phys. 2013;85:348–354. doi: 10.1016/j.ijrobp.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 5.Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaspar LE, Mehta MP, Patchell RA, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:17–32. doi: 10.1007/s11060-009-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 8.Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: Quality-of-life results. J Clin Oncol. 2013;31:65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]
- 9.Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non–small-cell lung cancer: Neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gondi V, Paulus R, Bruner DW, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: Pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013;86:656–664. doi: 10.1016/j.ijrobp.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: A “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu F, Carolan H, Nichol A, et al. Whole brain radiotherapy with hippocampal avoidance and simultaneous integrated boost for 1-3 brain metastases: A feasibility study using volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2010;76:1480–1485. doi: 10.1016/j.ijrobp.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 14.Gondi V, Tome WA, Rowley H, et al. Hippocampal contouring: A contouring atlas for RTOG 0933. http://www.rtog.org/CoreLab/ContouringAtlases/HippocampalSparing.aspx.
- 15.Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:77–84. doi: 10.1016/j.ijrobp.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 17.Chelune GJ, Naugle RI, Luders H, et al. Individual change after epilepsy surgery: Practice effects and base-rate information. Neuropsychology. 1993;7:41–52. [Google Scholar]
- 18.Benedict RHB, Schretlen D, Groninger L, et al. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 19.Li J, Bentzen SM, Li J, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71:64–70. doi: 10.1016/j.ijrobp.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 20.Schindler MK, Forbes ME, Robbins ME, et al. Aging-dependent changes in the radiation response of the adult rat brain. Int J Radiat Oncol Biol Phys. 2008;70:826–834. doi: 10.1016/j.ijrobp.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gondi V, Tome WA, Marsh J, et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: Safety profile for RTOG 0933. Radiother Oncol. 2010;95:327–331. doi: 10.1016/j.radonc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monaco EA, 3rd, Faraji AH, Berkowitz O, et al. Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer. 2013;119:226–232. doi: 10.1002/cncr.27504. [DOI] [PubMed] [Google Scholar]


