Abstract
Purpose
We tested the efficacy and toxicity of cisplatin plus accelerated fractionation with a concomitant boost (AFX-C) versus standard fractionation (SFX) in locally advanced head and neck carcinoma (LA-HNC).
Patients and Methods
Patients had stage III to IV carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx. Radiation therapy schedules were 70 Gy in 35 fractions over 7 weeks (SFX) or 72 Gy in 42 fractions over 6 weeks (AFX-C). Cisplatin doses were 100 mg/m2 once every 3 weeks for two (AFX-C) or three (SFX) cycles. Toxicities were scored by using National Cancer Institute Common Toxicity Criteria 2.0 and the Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer criteria. Overall survival (OS) and progression-free survival (PFS) rates were estimated by using the Kaplan-Meier method and were compared by using the one-sided log-rank test. Locoregional failure (LRF) and distant metastasis (DM) rates were estimated by using the cumulative incidence method and Gray's test.
Results
In all, 721 of 743 patients were analyzable (361, SFX; 360, AFX-C). At a median follow-up of 7.9 years (range, 0.3 to 10.1 years) for 355 surviving patients, no differences were observed in OS (hazard ratio [HR], 0.96; 95% CI, 0.79 to 1.18; P = .37; 8-year survival, 48% v 48%), PFS (HR, 1.02; 95% CI, 0.84 to 1.24; P = .52; 8-year estimate, 42% v 41%), LRF (HR, 1.08; 95% CI, 0.84 to 1.38; P = .78; 8-year estimate, 37% v 39%), or DM (HR, 0.83; 95% CI, 0.56 to 1.24; P = .16; 8-year estimate, 15% v 13%). For oropharyngeal cancer, p16-positive patients had better OS than p16-negative patients (HR, 0.30; 95% CI, 0.21 to 0.42; P < .001; 8-year survival, 70.9% v 30.2%). There were no statistically significant differences in the grade 3 to 5 acute or late toxicities between the two arms and p-16 status.
Conclusion
When combined with cisplatin, AFX-C neither improved outcome nor increased late toxicity in patients with LA-HNC. Long-term high survival rates in p16-positive patients with oropharyngeal cancer support the ongoing efforts to explore deintensification.
INTRODUCTION
Concurrent chemoradiotherapy has become the standard of care for locally advanced head and neck squamous cell carcinoma (LA-HNC). Meta-analyses revealed the benefit of biologically sound modified-fractionation radiation therapy regimens relative to standard fractionation (SFX)1 and the superiority of adding chemotherapy to radiation versus radiation therapy alone.2,3 Whether modified fractionation could yield incremental advantages over SFX when combined with cisplatin remained an open question.
The Radiation Therapy Oncology Group (RTOG) completed a phase III trial comparing the efficacy of three modified-fractionation regimens: hyperfractionation (HFX) or accelerated fractionation (AFX) with either concomitant boost (AFX-C) or with split course (AFX-S) against SFX in LA-HNC.4 Two regimens, HFX and AFX-C, were found to significantly improve tumor control rates. Because of the lower overall workload favoring AFX-C over HFX, RTOG chose AFX-C for further study; this decision predated the meta-analyses, which found a significant survival benefit only when HFX with dose escalation was used.1 After establishing the safety of combining AFX-C with cisplatin,5 a phase III trial, RTOG 0129 (Phase III Trial of Concurrent Radiation and Chemotherapy [Followed by Surgery for Residual Primary/N2-3 Nodal Disease] for Advanced Head and Neck Carcinomas), was launched to assess whether AFX-C would yield better survival than SFX when combined with cisplatin. We previously reported survival results (hazard ratio [HR], 0.90; 95% CI, 0.72 to 1.13 for overall survival [OS]) along with the high prognostic significance of tumor human papillomavirus (HPV) status in oropharyngeal carcinoma (OPC).6,7 This article presents the updated overall outcomes.
PATIENTS AND METHODS
Patients
Patients with newly diagnosed stage III to IV (excluding T1N+ or T2N1) carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx; good performance status (Zubrod score 0 to 1); age ≥ 18 years; and who had adequate bone marrow, hepatic, and renal function were eligible for RTOG 0129, which is registered with the National Cancer Institute and approved by institutional review boards. All patients provided written informed consent.
Randomization and Assessment
After stratification by tumor site (larynx v other), nodal status (N0 v N1-N2b v N2c-N3), and Zubrod score (0 v 1), patients were assigned to receive the experimental AFX-C or the control SFX. AFX-C consisted of 72 Gy in 42 fractions over 6 weeks with twice-per-day irradiation for the last 12 treatment days,8 whereas SFX consisted of 70 Gy in 35 fractions over 7 weeks. Cisplatin dose was 100 mg/m2 given once every 3 weeks for two cycles to patients on the AFX-C arm and for three cycles to patients on the SFX arm. Radiation technique consisted of two opposed lateral fields for the primary and upper nodes matched to an anterior field for the low neck. The initial target volume required a 2- to 3-cm margin around the gross disease and involved nodes, and the boost volume required a 1- to 1.5-cm margin. Intensity modulated radiation therapy was not allowed.
Acute toxicity was evaluated each week during therapy by using National Cancer Institute Common Toxicity Criteria Version 2.0. Physical examinations, laboratory testing, and imaging studies were performed once per quarter for 2 years, semiannually through year 5, then annually to assess tumor status and late toxicity by the RTOG late effects criteria.9
Laboratory Studies
The analysis of tumor HPV was restricted to patients with OPC because of the low prevalence of HPV among nonoropharyngeal sites. This subgroup analysis was not part of the initial design of the study. Determination of HPV status and tumor p16 expression has been described previously.6
Statistical Analysis
The trial was designed with a sample size of 720 to detect a 25% reduction in the death rate with 80% power, assuming a 2-year overall survival (OS) rate of 45% in the control arm,10,11 with a one-sided log-rank test at the .05 level.
OS was defined as time from random assignment until death as a result of any cause. Secondary end points included progression-free survival (PFS), defined as time from random assignment to death or first documented relapse, categorized as either locoregional failure (LRF; primary site or regional nodes) or distant metastasis (DM) or death. Neck dissection more than 15 weeks after radiation therapy or salvage surgery for the primary site (unless pathology showed no disease) and death as a result of index cancer without a documented site of recurrence or unknown cause were considered LRF. PFS and its components (LRF and DM) were reported instead of protocol-specified secondary end points (eg, disease-free-survival) to facilitate comparison with published meta-analyses.12 We further analyzed LRF and DM as site of first failure. Rates of OS and PFS were estimated by the Kaplan-Meier method13 and were compared by one-sided log-rank tests.14 The cumulative incidence method15 and one-sided Gray's test16 were used to estimate and compare rates of LRF and DM. HRs were estimated with Cox proportional hazards regression models and expressed as experimental over control.17 Other end points for this study included acute and late toxicities and compliance with protocol-defined treatment delivery. Toxicity and feeding tube rates were compared by Fisher's exact test, and outcomes between p16-positive and p16-negative groups were compared by two-sided log-rank or Gray's test. Eligible patients were analyzed per intent-to-treat.
RESULTS
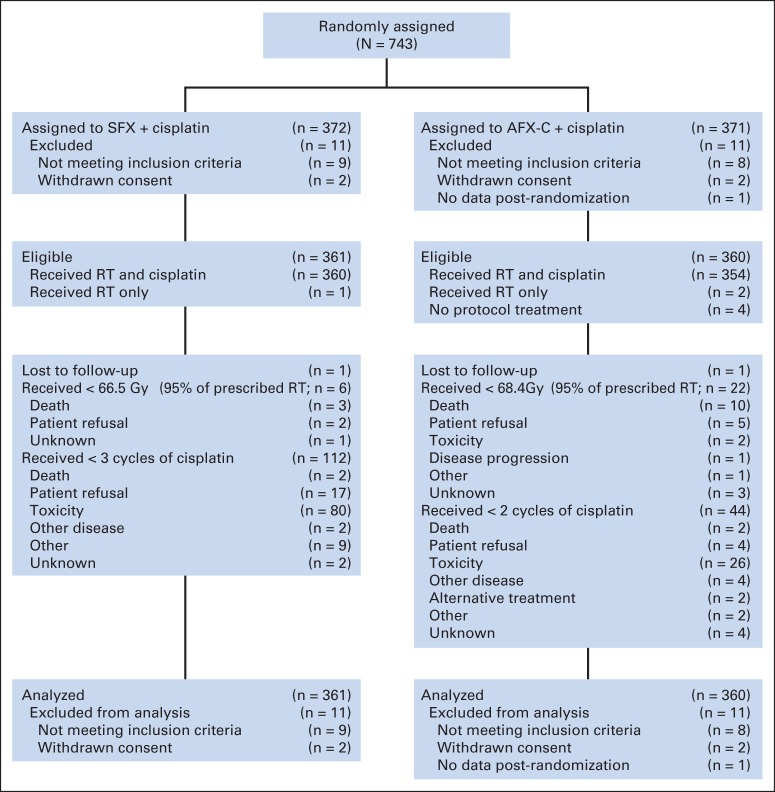
In all, 743 patients were accrued from July 2002 to June 2005, and 721 were analyzable (Fig 1). Table 1 provides the demographics of the analyzable patients, 361 on the SFX arm and 360 on the AFX-C arm. Briefly, 597 (82.8%) were male, 563 (78.1%) had stage IV disease, 433 (60.1%) had OPC, and HPV status was determined in 323 (74.6%) of the 433 patients with OPC. Of these 323 patients, 106 (64.2%) on the SFX arm were HPV-positive and 100 (63.3%) on the AFX-C arm were HPV-positive. The p16 status was known for 316 patients (73%) with OPC. On the SFX arm, 114 (70.4%) of 162 were p16-positive, and on the AFX-C arm, 101 (65.6%) of 154 were p16-positive. When compared with p16-negative patients, those who were p16-positive had several favorable prognostic factors, including younger age, white race, absence of anemia, lower number of cumulative tobacco smoking pack-years, and smaller primary lesions.
Fig 1.
CONSORT diagram. AFX-C, accelerated fractionation with a concomitant boost; RT, radiation therapy; SFX, standard fractionation.
Table 1.
Baseline Characteristics of the Study Patients and Their Tumors, According to Patient Group
| Characteristic | All Patients |
Oropharynx Only With p16 Determination |
P* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SFX + Cisplatin (n = 361) |
AFX-C + Cisplatin (n = 360) |
p16 Positive (n = 215) |
p16 Negative (n = 101) |
||||||
| No. | % | No. | % | No. | % | No. | % | ||
| Treatment assignment | .36† | ||||||||
| SFX + cisplatin | 361 | 100.0 | 0 | 0.0 | 114 | 53.0 | 48 | 47.5 | |
| AFX-C + cisplatin | 0 | 0.0 | 360 | 100.0 | 101 | 47.0 | 53 | 52.5 | |
| Age, years | .02‡ | ||||||||
| Median | 56 | 55 | 53 | 57 | |||||
| Range | 34-82 | 26-82 | 31-78 | 37-82 | |||||
| Sex | .15† | ||||||||
| Male | 309 | 85.6 | 288 | 80.0 | 184 | 85.6 | 80 | 79.2 | |
| Female | 52 | 14.4 | 72 | 20.0 | 31 | 14.4 | 21 | 20.8 | |
| Race | .002† | ||||||||
| White | 290 | 80.3 | 299 | 83.1 | 194 | 90.2 | 78 | 77.2 | |
| Nonwhite | 71 | 19.7 | 61 | 16.9 | 21 | 9.8 | 23 | 22.8 | |
| Zubrod performance status | .12† | ||||||||
| 0 | 206 | 57.1 | 211 | 58.6 | 145 | 67.4 | 59 | 58.4 | |
| 1 | 155 | 42.9 | 149 | 41.4 | 70 | 32.6 | 42 | 41.6 | |
| Anemia§ | .001† | ||||||||
| No | 250 | 69.3 | 247 | 68.6 | 169 | 78.6 | 62 | 61.4 | |
| Yes | 111 | 30.7 | 113 | 31.4 | 46 | 21.4 | 39 | 38.6 | |
| Primary site | |||||||||
| Oral cavity | 24 | 6.6 | 18 | 5.0 | 0 | 0.0 | 0 | 0.0 | |
| Oropharynx | 216 | 59.8 | 217 | 60.3 | 215 | 100.0 | 101 | 100.0 | |
| Hypopharynx | 31 | 8.6 | 27 | 7.5 | 0 | 0.0 | 0 | 0.0 | |
| Larynx | 90 | 24.9 | 98 | 27.2 | 0 | 0.0 | 0 | 0.0 | |
| T stage | .004‖ | ||||||||
| T2 | 69 | 19.1 | 99 | 27.5 | 73 | 34.0 | 22 | 21.8 | |
| T3 | 169 | 46.8 | 159 | 44.2 | 87 | 40.5 | 38 | 37.6 | |
| T4 | 123 | 34.1 | 102 | 28.3 | 55 | 25.6 | 41 | 40.6 | |
| N stage | .65‖ | ||||||||
| N0 | 67 | 18.6 | 69 | 19.2 | 15 | 7.0 | 8 | 7.9 | |
| N1 | 54 | 15.0 | 53 | 14.7 | 27 | 12.6 | 19 | 18.8 | |
| N2a | 28 | 7.8 | 32 | 8.9 | 25 | 11.6 | 11 | 10.9 | |
| N2b | 94 | 26.0 | 95 | 26.4 | 80 | 37.2 | 26 | 25.7 | |
| N2c | 89 | 24.7 | 84 | 23.3 | 45 | 20.9 | 29 | 28.7 | |
| N3 | 29 | 8.0 | 27 | 7.5 | 23 | 10.7 | 8 | 7.9 | |
| AJCC stage | .25† | ||||||||
| III | 77 | 21.3 | 81 | 22.5 | 26 | 12.1 | 17 | 16.8 | |
| IV | 284 | 78.7 | 279 | 77.5 | 189 | 87.9 | 84 | 83.2 | |
| Smoking history | < .001† | ||||||||
| Never-smoker | 44 | 12.2 | 69 | 19.2 | 65 | 30.2 | 9 | 8.9 | |
| Former smoker | 191 | 52.9 | 183 | 50.8 | 115 | 53.5 | 44 | 43.6 | |
| Current smoker | 83 | 23.0 | 68 | 18.9 | 23 | 10.7 | 31 | 30.7 | |
| Unknown | 43 | 11.9 | 40 | 11.1 | 12 | 5.6 | 17 | 16.8 | |
| Pack-years¶ | < .001‡ | ||||||||
| Median | 33 | 24 | 10 | 40 | |||||
| Range | 0-137.5 | 0-152 | 0-152 | 0-100 | |||||
| HPV status, oropharynx primaries only | < .001† | ||||||||
| Positive | 106 | 49.1 | 100 | 46.1 | 192 | 89.3 | 7 | 6.9 | |
| Negative | 59 | 27.3 | 58 | 26.7 | 22 | 10.2 | 94 | 93.1 | |
| Unknown | 51 | 23.6 | 59 | 27.2 | 1 | 0.5 | 0 | 0.0 | |
| p16 status, oropharynx primaries only | |||||||||
| Positive | 114 | 52.8 | 101 | 46.5 | 215 | 100.0 | 0 | 0.0 | |
| Negative | 48 | 22.2 | 53 | 24.4 | 0 | 0.0 | 101 | 100.0 | |
| Unknown | 54 | 25.0 | 63 | 29.0 | 0 | 0.0 | 0 | 0.0 | |
Abbreviations: AFX-C, accelerated fractionation with concomitant boost radiotherapy; AJCC, American Joint Committee on Cancer; HPV, human papillomavirus; SFX, standard fractionation radiotherapy.
Comparing p16 positive with p16 negative..
Pearson χ2 test.
Kolmogorov-Smirnov test.
Anemia is defined as hemoglobin ≤ 13.5 g/dLfor males and ≤ 12.5 g/dL for females.
Kruskal-Wallis test.
A pack-year is defined as the equivalent of smoking one pack of cigarettes per day for 1 year. Pack-years are missing for 28 patients (13.0%) with p16-positive tumors and 28 (27.7%) with p16-negative tumors.
Table 2 describes treatment compliance. Radiation therapy was delivered per protocol or with acceptable variation in 96.4% of patients on the SFX arm and in 92.5% of those on the AFX-C arm. On the AFX-C arm, 91.1% of the patients received at least 10 of the 12 planned twice-per-day fractionation days. On the SFX arm, 92.8% of patients received two or more cycles of cisplatin, whereas on the AFX-C arm, 87.8% of patients received two cycles of cisplatin.
Table 2.
Protocol Treatment Delivered by Assigned Arm
| Treatment | SFX + Cisplatin (n = 361) |
AFX-C + Cisplatin (n = 360) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Total dose of radiation, Gy | ||||
| Mean | 69.8 | 70.0 | ||
| Standard deviation | 5.5 | 10.6 | ||
| Median | 70 | 72 | ||
| Range | 4-76.4 | 0-76.02 | ||
| Q1-Q3 | 70-70.4 | 72-72 | ||
| Total fractions of radiation | ||||
| Mean | 34.7 | 40.7 | ||
| Standard deviation | 2.7 | 6.2 | ||
| Median | 35 | 42 | ||
| Range | 2-42 | 0-51 | ||
| Q1-Q3 | 35-35 | 42-42 | ||
| No. of days of RT* | ||||
| Mean | 52.3 | 43.6 | ||
| Standard deviation | 8.9 | 9.9 | ||
| Median | 51 | 43 | ||
| Range | 2-135 | 0-173 | ||
| Q1-Q3 | 50-53 | 42-45 | ||
| Less than per protocol | 14 | 3.9 | 24 | 6.7 |
| Per protocol + 0-7 | 291 | 80.6 | 300 | 83.3 |
| Per protocol + 8-14 | 35 | 9.7 | 28 | 7.8 |
| Per protocol + 15 or more | 21 | 5.8 | 8 | 2.2 |
| RT schedule | ||||
| RT dose twice per day | 1 | 0.3 | 348 | 96.7 |
| RT did not include twice-per-day dose | 357 | 98.9 | 8 | 2.2 |
| No RT given | 0 | 0.0 | 4 | 1.1 |
| RT not evaluable | 3 | 0.8 | 0 | 0.0 |
| No. of days missed of twice-per-day dosage | ||||
| Not applicable | 361 | 100.0 | 0 | 0.0 |
| 0 | 0 | 0.0 | 266 | 73.9 |
| 1-6 | 0 | 0.0 | 73 | 20.3† |
| 7-12 | 0 | 0.0 | 17 | 4.7‡ |
| No RT given | 0 | 0.0 | 4 | 1.1 |
| Cisplatin total dose, mg/m2 | ||||
| Mean | 254.8 | 183.1 | ||
| Standard deviation | 63.5 | 39.9 | ||
| Median | 298.4 | 200.0 | ||
| Range | 0-309.4 | 0-207.1 | ||
| Q1-Q3 | 200.0-300.0 | 195.8-200.0 | ||
| 0 | 1 | 0.3 | 6 | 1.7 |
| > 0, < 100 | 3 | 0.8 | 9 | 2.5 |
| ≥ 100, < 200 | 53 | 14.7 | 120 | 33.3 |
| ≥ 200 | 304 | 84.2 | 225 | 62.5 |
| Cisplatin cycles delivered | ||||
| 0 | 1 | 0.3 | 6 | 1.7 |
| 1 | 25 | 6.9 | 38 | 10.6 |
| 2 | 86 | 23.8 | 316 | 87.8 |
| 3 | 249 | 69.0 | 0 | 0.0 |
| Reason cisplatin discontinued after second cycle§ | ||||
| Toxicity | 39 | 47.6 | ||
| Patient condition (not toxicity/progression) | 7 | 8.5 | ||
| Physician refusal | 17 | 20.7 | ||
| Patient refusal | 15 | 18.3 | ||
| Other | 4 | 4.9 | ||
Abbreviations: AFX-C, accelerated fractionation with concomitant boost radiotherapy; Q1, first quartile; Q3, third quartile; RT, radiation therapy; SFX, standard fractionation radiotherapy.
SFX, per protocol is 47 days if starting on a Monday, 49 days otherwise; AFX-C, per protocol is 40 days if starting on a Monday, 42 days otherwise.
Missing 1-6 days of twice-per-day dosage: 12 of 73 had fewer than 42 fractions.
Missing 7-12 days of twice-per-day dosage: 15 of 17 had fewer than 42 fractions.
For patients who received first and second cycles but not a third cycle.
For p16-positive patients, radiation therapy was delivered per protocol or with acceptable variation for 97.4% of the patients on the SFX arm and for 95% of the patients on the AFX-C arm. On the AFX-C arm, 94.1% received at least 10 of the 12 planned twice-per-day fractionation days. At least two cycles of cisplatin were given per protocol in 87.7% of the patients on the SFX arm and in 94.1% of the patients on the AFX-C arm.
For p16-negative patients, radiation therapy was delivered per protocol or with acceptable deviation for 100% of the patients on the SFX arm and for 92.5% of the patients on the AFX-C arm. On the AFX-C arm, 92.5% received at least 10 of the 12 planned twice-per-day fractionation days. At least two cycles of cisplatin were given per protocol for 81.3% of the patients on the SFX arm and for 90.6% of the patients on the AFX-C arm.
On the SFX and AFX-C arms, 87 patients (24.1%) and 85 patients (23.6%) had neck dissection within 15 weeks of radiation therapy completion, of which 60 (69%) and 57 (67.1%), respectively, had no tumor in the specimen. On the SFX and AFX-C arms, 35 patients (9.7%) and 37 patients (10.3%) had neck dissection beyond 15 weeks of radiation therapy completion, of which 20 (57.1%) and 23 (62.2%), respectively, had no tumor in the specimen.
Outcome and Toxicity
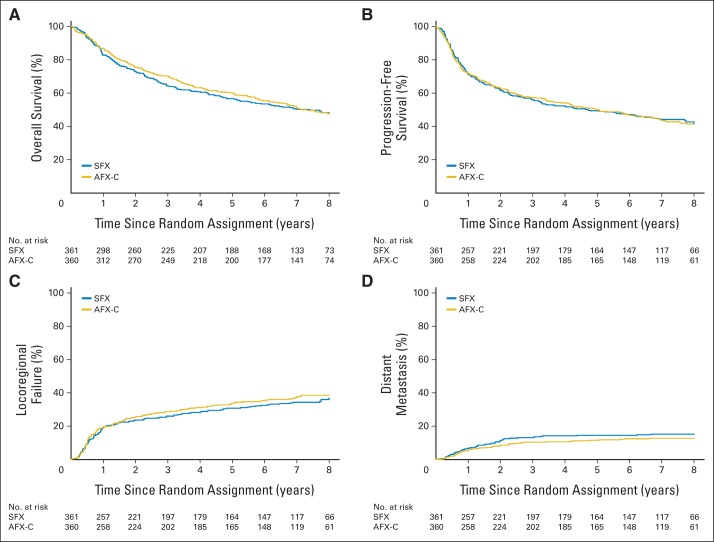
At the time of analysis (January 2013), 355 patients were alive, with a median follow-up of 7.9 years (range, 0.3 to 10.1 years). Only six patients (0.8%) were censored with less than 2 years of follow-up. Overall, 52.2% of deaths were attributed to the treated cancer, 9.3% to a secondary malignancy, and 1.9% to treatment complications (Data Supplement); there was no difference in the cause of death between the two arms. The most common first site of failure was locoregional, accounting for 21.1% of all patients and 37.2% of all failures; DM as the sole site of first failure accounted for only 13.7% of all patients and 24.2% of all failures. No difference in the relapse pattern was noted between the two arms (Data Supplement). No significant differences were found between the two arms in OS (HR, 0.96; 95% CI, 0.79 to 1.18; P = .37), PFS (HR, 1.02; 95% CI, 0.84 to 1.24; P = .58), LRF (HR, 1.08; 95% CI, 0.84 to 1.38; P = .78), or DM (HR, 0.83; 95% CI, 0.56 to 1.24; P = .16) as shown in Figures 2A to 2D). The 8-year rates for OS were 47.6% versus 47.7%; PFS, 42.1% versus 41.4%; LRF, 36.7% versus 38.5%; and DM, 15.2% versus 12.8% for the SFX and AFX-C arms, respectively. Treatment effect by subgroup is shown in the Data Supplement.
Fig 2.
Kaplan-Meier estimates of overall survival and progression-free survival and cumulative incidence estimates of locoregional failure and distant metastasis by assigned treatment. There were no statistically significant differences in (A) overall survival (hazard ratio [HR], 0.96; 95% CI, 0.79 to 1.18; P = .37), (B) progression-free survival (HR, 1.02; 95% CI, 0.84 to 1.24; P = .58), (C) locoregional failure (HR, 1.08; 95% CI, 0.84 to 1.38; P = .78), or (D) distant metastasis (HR, 0.83; 95% CI, 0.56 to 1.24; P = .16). Five-year rates for overall survival were 56.6% (95% CI, 51.5% to 61.8%) for the standard fractionation (SFX) arm and 60.0% (95% CI, 54.9% to 65.1%) for the accelerated fractionation with a concomitant boost (AFX-C) arm; for progression-free survival, 49.4% (95% CI, 44.2% to 54.6%) and 50.0% (95% CI, 44.8% to 55.2%); for locoregional failure, 30.8% (95% CI, 25.9% to 35.7%) and 33.7% (95% CI, 28.7% to 38.8%); and for distant metastasis, 14.5% (95% CI, 10.9% to 18.2%) and 11.5% (95% CI, 8.2% to 14.8%), respectively.
The 8-year rates for OS were 70.9% versus 30.2%; PFS, 64.0% versus 23.3%; LRF, 19.5% versus 52.4%; and DM, 10.3% versus 16.1% for p16-positive and p16-negative patients, respectively (Data Supplement). After adjustment for prognostic covariates, p16-positive patients had significantly better OS (HR, 0.34; 95% CI, 0.22 to 0.52), PFS (HR, 0.43; 95% CI, 0.29 to 0.64), and LRF (HR, 0.29; 95% CI, 0.17 to 0.48) than p16-negative patients, but not better DM (HR, 0.59; 95% CI, 0.26 to 1.35). The 8-year rates for good-risk patients with p16-positive tumors (T1-2N1-2b or T3N0-2b, and ≤ 10 pack-years of smoking history) were 81.4% for OS, 78.3% for PFS, 13.5% for LRF, and 8.3% for DM. Both arms had similar percentages of deaths as a result of the study cancer (40.6% v 43.3%) whereas deaths as a result of second malignancy were more common in p16-negative patients, but unrelated cause of death was more common in p16-positive patients (Data Supplement).
Figure 3 shows the OS by fractionation and cisplatin cycles delivered. Patients receiving one cisplatin cycle had significantly worse OS compared with patients receiving two or three cycles, regardless of the radiation therapy regimen (HR, 4.17; 95% CI, 2.66 to 6.53 for SFX + 1 v SFX + 3; HR, 2.29; 95% CI, 1.50 to 3.51 for AFX-C + 1 v SFX + 3; HR, 1.15; 95% CI, 0.81 to 1.62 for SFX + 2 v SFX + 3; and HR, 1.01; 95% CI, 0.79 to 1.29 for AFX-C + 2 v SFX + 3). However, patients who received only one cycle of cisplatin tended to be older, have more advanced stage and poorer performance status, and be more likely to be p16-negative (Data Supplement).
Fig 3.
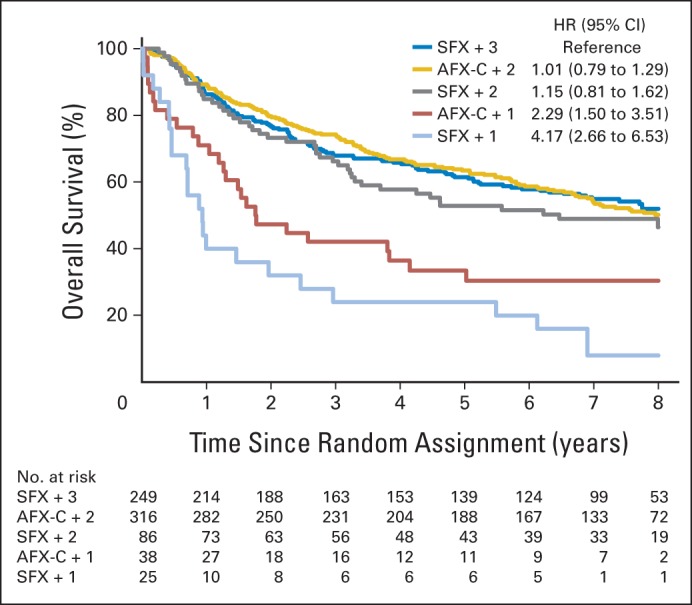
Overall survival by fractionation and number of cisplatin cycles delivered. AFX-C, accelerated fractionation with a concomitant boost; HR, hazard ratio; SFX, standard fractionation.
Table 3 summarizes the distribution of worst grade 3 to 5 toxicity. Regardless of scoring system (Common Toxicity Criteria or Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer), no differences were noted between the two arms. Grade 5 toxicities occurred in two patients on the SFX arm and six patients on the AFX-C arm (Data Supplement). All reported toxicities are listed in the Data Supplement. Death rates within 30 days of treatment completion were similar between the two arms: 1.9% on the SFX arm and 3.3% on the AFX-C arm (P = .26). Before treatment, 24.7% of patients on the SFX arm and 21.9% on the AFX-C arm (P = .43) had a feeding tube. By treatment completion, this rose to 68.7% versus 67.1% (P = .80) and then declined to 28.6% versus 26.1% at 1 year (P = .53) and to 5.9% versus 13.2% at 5 years (P = .08) for the SFX and AFX-C arms, respectively.
Table 3.
Grade 3 to 5 Toxicity Summary
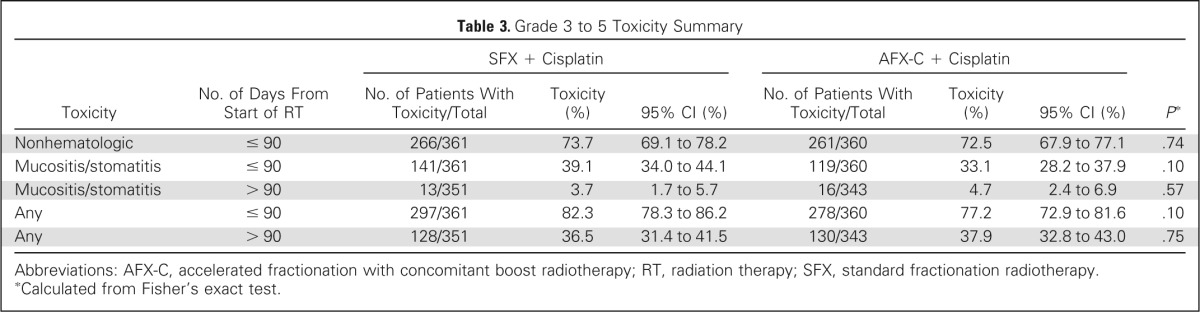
| Toxicity | No. of Days From Start of RT | SFX + Cisplatin |
AFX-C + Cisplatin |
P* | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients With Toxicity/Total | Toxicity (%) | 95% CI (%) | No. of Patients With Toxicity/Total | Toxicity (%) | 95% CI (%) | |||
| Nonhematologic | ≤ 90 | 266/361 | 73.7 | 69.1 to 78.2 | 261/360 | 72.5 | 67.9 to 77.1 | .74 |
| Mucositis/stomatitis | ≤ 90 | 141/361 | 39.1 | 34.0 to 44.1 | 119/360 | 33.1 | 28.2 to 37.9 | .10 |
| Mucositis/stomatitis | > 90 | 13/351 | 3.7 | 1.7 to 5.7 | 16/343 | 4.7 | 2.4 to 6.9 | .57 |
| Any | ≤ 90 | 297/361 | 82.3 | 78.3 to 86.2 | 278/360 | 77.2 | 72.9 to 81.6 | .10 |
| Any | > 90 | 128/351 | 36.5 | 31.4 to 41.5 | 130/343 | 37.9 | 32.8 to 43.0 | .75 |
Abbreviations: AFX-C, accelerated fractionation with concomitant boost radiotherapy; RT, radiation therapy; SFX, standard fractionation radiotherapy.
Calculated from Fisher's exact test.
The distribution of worst grade 3 to 5 toxicity showed no differences by p16 status within the two arms, regardless of scoring system (Data Supplement). Death rates within 30 days of treatment completion were similar for p16-positive patients (1.8% on the SFX arm v 3% on the AFX-C arm; P = .67) and p16-negative patients (0% on the SFX arm v 1.9% on the AFX-C arm; P = 1.0). Before treatment, the feeding tube rates were 15.3% and 28.7% (P = .006); this increased to 64.5% and 74% (P = .12) at treatment completion and then declined to 16.7% and 40% (P < .001) at 1 year and to 5.4% and 23.1% (P = .01) at 5 years for p16-positive and p16-negative patients, respectively. We did not observe any differences in feeding tube rates between treatment arms for each known p16 status (Data Supplement).
DISCUSSION
Several randomized trials11,18–20 have shown that radiation therapy with concurrent cisplatin was better than radiation therapy alone for the nonsurgical treatment of LA-HNC. Because the fractionation regimens and cisplatin dosing schedules varied among trials, the relative impact of modified fractionation and the cumulative cisplatin dose on outcome remain unclear. RTOG 0129 was specifically designed to address whether AFX-C provided an incremental benefit over SFX when combined with high-dose cisplatin given once every third week. It was determined that only two cycles of cisplatin could be given concurrently with the 6-week AFX-C regimen as opposed to three cycles with the 7-week SFX regimen. Because two variables were modified on the AFX arm, the overall treatment time and the number of chemotherapy cycles, the results of this study should be interpreted on the basis of the study design including changes in both variables.
This randomized phase III trial showed no difference between the two arms on OS, PFS, LRF, and DM. Patients on the AFX-C arm, despite having one less cycle of cisplatin, did not show more DM, probably reflecting the true value of concomitant cisplatin as a radiosensitizer. The lack of benefit of accelerating radiation therapy suggests that three cycles of cisplatin virtually offset the effect of tumor clonogen repopulation during the 7-week course of fractionated radiation therapy. The data may also suggest that the effect of 1 week of radiation therapy acceleration approximated that of the third cycle of cisplatin.
The Groupe d'Oncologie Radiothérapie Tête et Cou (GORTEC) reported a randomized phase III trial (GORTEC 99-02; Concomitant Chemoradiotherapy Versus Acceleration of Radiotherapy With or Without Concomitant Chemotherapy in LA-HNC: An Open-Label Phase 3 Randomised Trial) for LA-HNC composed of three arms21: arm 1 treatment consisted of three cycles of platinum-based chemotherapy with 70 Gy SFX, arm 2 used the same chemotherapy for two cycles with accelerated radiation therapy of 70 Gy in 6 weeks, and arm 3 used very accelerated radiation therapy alone of 64.8 Gy in 3.5 weeks. This study did not find any added benefit of accelerated radiation therapy with concomitant chemotherapy when compared with conventional chemoradiotherapy. Similarly, the European Organisation for Research and Treatment of Cancer conducted a randomized trial on larynx preservation that compared four sequential cycles of cisplatin and fluorouracil followed by radiation therapy versus alternating the same chemotherapy with three cycles of 2-week radiation therapy. Larynx preservation, PFS, OS, and toxicities were similar in both arms.22 These data support the hypothesis that concomitant cisplatin inhibits repopulation during radiation therapy and that SFX with three cycles of concurrent cisplatin should remain a standard-of-care treatment.
It is difficult to administer the third cycle of chemotherapy with SFX in many patients because of toxicity. This is one of the reasons why alternative chemoradiotherapy regimens have been explored. But there have not yet been any large randomized trials that compare SFX with three cycles of high-dose cisplatin to two cycles of high-dose cisplatin or to a once-per-week cisplatin regimen. The cumulative dose of cisplatin needed and the type of delivery to achieve the best therapeutic ratio is not clear. We performed an exploratory analysis based on chemotherapy compliance and observed a worse survival rate for patients who received only one cycle of cisplatin regardless of the radiation therapy regimen. For patients who received two or more cycles of chemotherapy on the SFX arm, OS was similar to that of patients receiving two cycles of chemotherapy on the AFX-C arm. These data should be interpreted with caution. The number of patients receiving SFX plus two cycles of cisplatin was small (n = 86) compared with the number of patients receiving three cycles (n = 249), and the numbers of patients who received only one cisplatin cycle (n = 38 for AFX-C + 1 and n = 25 for SFX + 1) were even smaller. These patients tended to have poorer prognostic features, precluding them from withstanding treatment as per protocol. These data suggest that it is critical to provide supportive care to patients with acute toxicities to allow them to receive at least two cycles of chemotherapy to optimize outcome.
RTOG 0129 did not demonstrate any significant differences in acute or late toxicities between the two arms. There was a trend toward more dependence on feeding tubes at 5 years for the AFX-C arm compared with the SFX arm (13.2% v 5.9%; P = .08). The GORTEC 99-02 trial reported a different pattern of feeding tube dependency at 5 years: 13% for the conventional fractionated radiotherapy plus chemotherapy arm, 6% for the AFX plus chemotherapy arm, and 25% for the very accelerated radiation therapy arm. However, more than one third of patients, regardless of treatment, experienced grade 3 to 5 toxicities more than 90 days after treatment initiation. This is consistent with reported data that severe late toxicity is common after concurrent chemoradiotherapy.23 It is possible that we have reached a plateau in terms of outcome and toxicities with current platinum-based chemoradiotherapy regimens, and we need to define better strategies to address LA-HNC.
The better outcome for patients with HPV-positive OPC is still maintained after a longer median follow-up (4.8 v 7.9 years in initial and current reports, respectively).6 Yet there was no difference in the distribution of worst grade 3 to 5 toxicity for patients with known p16 status. We did, however, observe a higher rate of feeding tube dependency at 5 years for p16-negative versus p16-positive patients (23.1% v 5.4%; P = .01); this was not influenced by the treatment arms. This is probably due to more T4 tumors in p16-negative patients, leading to a higher incidence of pretreatment feeding tube use in this group (22.9% on the SFX arm and 34% on AFX-C arm). In addition, a larger volume of tumor and adjacent pharyngeal constrictor muscles receiving high radiation dose in the era before intensity modulated radiation therapy could partially account for the persistent swallowing dysfunction in these patients, leading to long-term dependence on feeding tubes. We also observed more death related to second malignancy in p16-negative patients, a finding consistent with their higher exposure to tobacco.
The recognition that despite their excellent prognosis, patients with p16-positive OPC experienced toxicity similar to that of p16-negative patients when treated with current standard therapy is an incentive to explore treatment de-escalation to reduce toxicity without compromising tumor control. For example, the RTOG along with the Eastern Cooperative Oncology Group (ECOG) is conducting a phase III trial (RTOG-1016; Radiation Therapy Plus Cetuximab Versus Chemoradiotherapy in HPV-Associated Oropharyngeal Cancer) comparing radiation therapy plus cisplatin to radiation therapy plus cetuximab, an antibody against the epidermal growth factor receptor. ECOG, in contrast, explored reduction of the radiation dose to 54 Gy given with cetuximab in patients with p16-positive OPC who achieved a complete response after induction chemotherapy in a phase II trial (ECOG-E1308; Paclitaxel, Cisplatin, and Cetuximab Followed By Cetuximab and Intensity-Modulated Radiation Therapy in Treating Patients With HPV-Associated Stage III or Stage IV Cancer of the Oropharynx That Can Be Removed By Surgery).24
HPV-unrelated LA-HNC, which responds poorly to current therapies, presents a therapeutic challenge. Treatment toxicity will likely limit further intensification of radiation and traditional chemotherapy. The addition of cetuximab to concomitant cisplatin-based chemoradiotherapy is not superior to cisplatin-based chemoradiotherapy alone.25 Novel targeted agents are needed for these patients.26–31 Limiting toxicity while introducing new therapeutic targets to traditional regimens is the challenge of ongoing and future trials.
Supplementary Material
Acknowledgment
The authors thank Andy Trotti, MD, for his additional effort in reviewing this article.
Glossary Terms
- accelerated fractionation:
radiation dose fractionation schedule with an effective rate of dose accumulation exceeding the traditional 10 Gy delivered in five fractions per week.
- Cox proportional hazards regression model:
a statistical model for regression analysis of censored survival data, examining the relationship of censored survival distribution to one or more covariates. This model produces a baseline survival curve, covariate coefficient estimates with their standard errors, risk ratios, 95% CIs, and significance levels.
- locoregional failure:
failure at the primary site or the regional lymphatics.
- overall survival:
the duration between random assignment and death.
- p16:
molecule that binds to cyclin-dependent kinase 4 and 6, thereby preventing their interaction with cyclin D. p16 (also known as p16INK4) behaves as a negative regulator of proliferation and arrests cells in the G0/G1 phase of the cell cycle.
Footnotes
Supported by Grant No. U10 CA21661 from the Radiation Therapy Oncology Group and by Community Clinical Oncology Program Grant No. U10 CA37422 from the National Cancer Institute.
Clinical trial information: NCT00047008.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
This article's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Maura L. Gillison, GlaxoSmithKline (U), Pfizer (U), Bristol-Myers Squibb (U) Stock Ownership: None Honoraria: Maura L. Gillison, Merck Serono Research Funding: None Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: Maura L. Gillison, Merck Serono
AUTHOR CONTRIBUTIONS
Conception and design: K. Kian Ang, David I. Rosenthal, Maura L. Gillison, Marcie List
Provision of study materials or patients: Phuc Felix Nguyen-Tan, André Fortin, Elizabeth Gore, Maura L. Gillison
Collection and assembly of data: Phuc Felix Nguyen-Tan, K. Kian Ang, Denis Soulieres, Harold Kim, Craig Silverman, Adam Raben, André Fortin, Elizabeth Gore, Richard C. Jordan, Maura L. Gillison
Data analysis and interpretation: Phuc Felix Nguyen-Tan, Qiang Zhang, K. Kian Ang, Randal S. Weber, David I. Rosenthal, Thomas J. Galloway, William H. Westra, Christine H. Chung, Richard C. Jordan, Maura L. Gillison, Quynh-Thu Le
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 2.Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data—MACH-NC Collaborative Group: Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 3.Pignon JP, le Maître A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Garden AS, et al. Concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: Radiation Therapy Oncology Group phase II trial 99-14. J Clin Oncol. 2005;23:3008–3015. doi: 10.1200/JCO.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 6.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ang K, Zhang Q, Wheeler RH, et al. A phase III trial (RTOG 0129) of two radiation-cisplatin regimens for head and neck carcinomas (HNC): Impact of radiation and cisplatin intensity on outcome. J Clin Oncol. 2010;28(suppl):422s. abstr 5507. [Google Scholar]
- 8.Ang KK, Peters LJ, Weber RS, et al. Concomitant boost radiotherapy schedules in the treatment of carcinoma of the oropharynx and nasopharynx. Int J Radiat Oncol Biol Phys. 1990;19:1339–1345. doi: 10.1016/0360-3016(90)90341-g. [DOI] [PubMed] [Google Scholar]
- 9.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 10.Marcial VA, Pajak TF, Mohiuddin M, et al. Concomitant cisplatin chemotherapy and radiotherapy in advanced mucosal squamous cell carcinoma of the head and neck: Long-term results of the Radiation Therapy Oncology Group study 81-17. Cancer. 1990;66:1861–1868. doi: 10.1002/1097-0142(19901101)66:9<1861::aid-cncr2820660902>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Michiels S, Le Maître A, Buyse M, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: Meta-analyses of individual patient data. Lancet Oncol. 2009;10:341–350. doi: 10.1016/S1470-2045(09)70023-3. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 15.Kalbfleish JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons; 1980. [Google Scholar]
- 16.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 17.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–229. [Google Scholar]
- 18.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798–1804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 19.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 20.Huguenin P, Beer KT, Allal A, et al. Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol. 2004;22:4665–4673. doi: 10.1200/JCO.2004.12.193. [DOI] [PubMed] [Google Scholar]
- 21.Bourhis J, Sire C, Graff P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): An open-label phase 3 randomised trial. Lancet Oncol. 2012;13:145–153. doi: 10.1016/S1470-2045(11)70346-1. [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre JL, Rolland F, Tesselaar M, et al. Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst. 2009;101:142–152. doi: 10.1093/jnci/djn460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marur S, Li S, Cmelak A, et al. E 1308: A phase II trial of induction chemotherapy (IC) followed by cetuximab with low dose versus standard dose IMRT in patients with human papilloma virus (HPV)-associated resectable squamous cell carcinoma of the oropharynx (OPSCC) J Clin Oncol. 2013;(suppl 15s):31. abstr 6005. [Google Scholar]
- 25.Ang KK, Zhang QE, Rosenthal DI, et al. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas (HNC) J Clin Oncol. 2011;29(suppl):360s. doi: 10.1200/JCO.2013.53.5633. abstr 5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin Grandis J, Melhem MF, Gooding WE, et al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 27.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 28.Pfister DG, Su YB, Kraus DH, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: A pilot phase II study of a new combined-modality paradigm. J Clin Oncol. 2006;24:1072–1078. doi: 10.1200/JCO.2004.00.1792. [DOI] [PubMed] [Google Scholar]
- 29.Kies MS, Holsinger FC, Lee JJ, et al. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: Results from a phase II prospective trial. J Clin Oncol. 2010;28:8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Argiris A, Heron DE, Smith RP, et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. J Clin Oncol. 2010;28:5294–5300. doi: 10.1200/JCO.2010.30.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merlano M, Russi E, Benasso M, et al. Cisplatin-based chemoradiation plus cetuximab in locally advanced head and neck cancer: A phase II clinical study. Ann Oncol. 2011;22:712–717. doi: 10.1093/annonc/mdq412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.