Abstract
Objective
Ischemic stroke is primarily attributable to thrombotic vascular occlusion. Elevated α2-antiplasmin levels correlate with increased stroke risk, but whether α2-antiplasmin contributes to the pathogenesis of stroke is unknown. We examined how α2-antiplasmin affects thrombosis, ischemic brain injury and survival following experimental cerebral thromboembolism.
Approach and Results
We evaluated the effects of α2-antiplasmin on stroke outcomes in mice with increased, normal or no circulating α2-antiplasmin, as well as in mice given an α2-antiplasmin-inactivating antibody. Higher α2-antiplasmin levels were correlated with greater ischemic brain injury (rs=0.88, p<0.001), brain swelling (rs=0.82, p<0.001) and reduced middle cerebral artery thrombus dissolution (rs=−0.93, p<0.001). In contrast, α2-antiplasmin deficiency enhanced thrombus dissolution, increased cerebral blood flow, reduced brain infarction and decreased brain swelling. By comparison to tissue plasminogen activator, α2-antiplasmin inactivation hours after thromboembolism still reduced brain infarction (p<0.001) and hemorrhage (p<0.05). Microvascular thrombosis, a process that enhances brain ischemia, was markedly reduced in α2-antiplasmin-deficient or α2-antiplasmininactivated mice compared with tissue plasminogen activator-treated mice or mice with increased α2-antiplasmin levels (all p<0.001) Matrix metalloproteinase-9 expression, which contributes to acute brain injury, was profoundly decreased in α2-antiplasmin-deficient or α2-antiplasmin-inactivated mice vs. tissue plasminogen activator-treated mice or mice with increased α2-antiplasmin levels (all p<0.001). Alpha-2-antiplasmin inactivation markedly reduced stroke mortality vs. tissue plasminogen activator (p<0.0001).
Conclusions
Alpha-2-antiplasmin has profound, dose-related effects on ischemic brain injury, swelling, hemorrhage, and survival following cerebral thromboembolism. By comparison to tissue plasminogen activator, the protective effects of α2-antiplasmin deficiency or inactivation appear to be mediated through reductions in microvascular thrombosis and matrix metalloproteinase-9 expression.
INTRODUCTION
Ischemic stroke affects 16.9 million people a year worldwide; it kills more than 5.4 million and disables millions of others.1 Thrombotic obstruction of a cerebral artery may be triggered by thromboembolism, plaque rupture, atrial fibrillation and other vascular events.2, 3 Thrombotic occlusion initiates a cascade of events that cause neuronal cell death, matrix metalloproteinase expression, breakdown of the blood brain barrier with brain swelling, hemorrhage, morbidity and mortality.2-6 Downstream from a thrombotic occlusion, ischemia may trigger the formation of microvascular thrombi that cause further reductions in blood flow thereby enhancing blood brain barrier breakdown and ischemic tissue injury.2, 3, 7 Clinical and experimental studies have demonstrated that tissue plasminogen activator (TPA), which initiates the conversion of plasminogen to plasmin, dissolves occlusive thrombi to improve outcomes after ischemic stroke.4 However, there is little known in ischemic stroke about the role of molecules that regulate fibrinolysis downstream of TPA.
Alpha 2-antiplasmin (a2AP) is a serine protease inhibitor that rapidly inhibits plasmin.8 Most a2AP circulates in the blood but a portion is crosslinked to fibrin by activated factor XIII during thrombus formation.9 Recent studies have emphasized the role of thrombus-bound a2AP in regulating fibrinolysis or dissolution of pathologic thrombi. 8, 9 Circulating a2AP inhibits circulating plasmin 100-1000-fold more efficiently than it inhibits fibrin or thrombus-bound plasmin; this has led some to propose that a primary role of circulating a2AP is to prevent bleeding by preventing the degradation of coagulation factors.10,11 However, recent studies indicate that high levels of circulating a2AP contribute to the failure of TPA therapy to dissolve thrombi and restore blood flow during ischemic stroke.12-14 Moreover, genetic deletion of a2AP protects against ischemic brain injury induced by non-thrombotic permanent surgical ligation of the middle cerebral artery.15, 16 Yet, within the neuronal and vascular compartments, a2AP and serpins that block TPA-initiated proteolytic pathways, such as the activation of matrix metalloproteinase-9 (MMP-9), may protect the brain by reducing cell death or neurotoxicity and may prevent bleeding complications.17-20
In this report we investigated how circulating and thrombus-bound a2AP affect endogenous fibrinolysis, microvascular thrombosis, hemorrhage, brain injury and other outcomes in an experimental thromboembolic model with translational relevance to human ischemic stroke. We find that thrombus-bound a2AP modulates dissolution of the culprit thromboembolus, while circulating a2AP activity also has dynamic, deleterious effects on the development of microvascular thrombosis, MMP-9 expression, brain injury, hemorrhage, disability and death following cerebral thromboembolism.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Dose-related effects of circulating a2AP on cerebral thromboembolism
If a2AP activity directly contributes to the pathogenesis of stroke after thromboembolism, there should be a dose-relationship between circulating levels of a2AP levels and outcomes. Three different experimental groups were examined: mice with increased levels of a2AP (achieved by intravenous supplementation), normal physiologic a2AP levels (controls) or no circulating a2AP (a2AP−/− mice).14 Intravenous supplementation increased blood a2AP levels by a median of 87.1 ug/ml (mean 79.3 ± 14.3 ug/ml) in mice measured at the end of the experiment which approximately doubled the a2AP levels found in in normal mice.21 Laser Doppler monitoring showed that MCA thromboembolism was associated with a marked drop in hemispheric blood flow to 20% or less of the initial baseline in all three groups as expected (Fig. 1A). During the period of observation there was a partial restoration of blood flow in a2AP−/− mice without circulating a2AP (Fig. 1A), while hemispheric blood flow remained suppressed in mice with normal or increased levels of a2AP. Pathologically there was evidence of proximal MCA occlusion (Fig. 1B) after thromboembolism and the extent of brain infarction differed among the experimental groups as shown in TTC-stained representative images of brain slices (Fig. 1B).
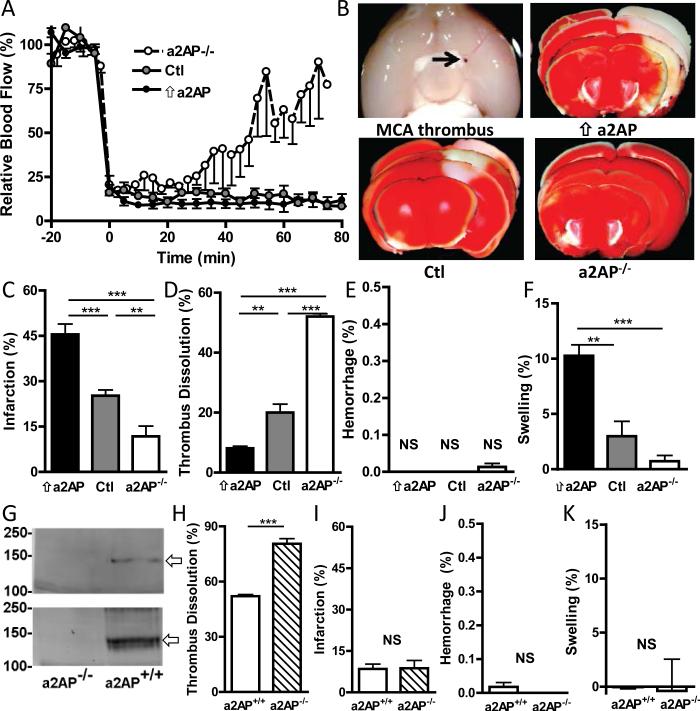
Figure 1.
The effects of circulating and thrombus-bound a2AP on brain infarction, swelling and dissolution of the culprit MCA thrombus. A) Changes in relative cerebral blood flow (mean ± SE) in the ischemic hemisphere in mice with elevated circulating levels of a2AP (⇑a2AP), normal a2AP levels (Ctl) and absent a2AP (a2AP−/−). B) Representative images of a proximal middle cerebral artery thrombus (arrow), and of TTC-stained brain slices showing viable (red) and infarcted tissue (white) 6 h after thromboembolism. Percent C) infarction, D) dissolution of the MCA thrombus, E) hemorrhage and F) swelling of the ischemic hemisphere 6 h after placement of an 125I-fibrin labeled MCA thrombus (containing normal levels of a2AP). G) Immunoblot analysis of a2AP in thromboemboli formed from a2AP+/+ and a2AP−/− mice. Immuno-stained bands consistent with thrombus-bound a2AP (fibrin-cross linked a2AP, arrows) in a2AP+/+ but not a2AP−/− clots. Top panel, immunostaining with polyclonal anti-a2AP antibody; bottom panel, immunostaining with pooled monoclonal a2AP antibodies. H-K) Effects of thrombus-bound a2AP in vivo on the percent H) MCA thrombus dissolution, I) infarction, J) hemorrhage and K) swelling in brains from a2AP−/− mice with MCA thromboemboli containing normal a2AP (a2AP+/+) or no a2AP (a2AP−/−), n = 7 per group, see Methods for additional details. Data in C-F, H-K represent the means ± SE. **p≤0.01, ***p≤0.001, NS not significant.
When compared to mice with normal a2AP levels, mice with increased circulating levels of a2AP had larger areas of brain infarction (Fig. 1B, C, p<0.001). In contrast, mice without circulating a2AP had significant reductions in brain infarction by comparison to mice with normal (Fig. 1B, C, p<0.01) or increased levels of a2AP (Fig. 1C, p<0.001). There was a significant positive dose-related correlation between a2AP levels and brain infarction (Spearman's r = 0.88, p<0.001).
In these experiments we monitored the dissolution of 125I-fibrin-labeled MCA thromboemboli (formed with pooled normal a2AP+/+ plasma) at 6 h to assess brain injury during a time when thrombus dissolution was still dynamic, as suggested by our pilot studies. By comparison to mice with normal physiologic levels of a2AP, mice with increased blood levels of a2AP had markedly decreased dissolution of the MCA thrombus (Fig. 1D p<0.01). In contrast, the dissolution of the MCA thrombus was accelerated in a2AP-deficient vs. mice with normal a2AP levels (Fig. 1D, p<0.001) and or vs. mice with increased levels of circulating a2AP (Fig. 1D, p<0.001). There was a significant negative correlation between the level of circulating a2AP and dissolution of the MCA thromboembolus (Spearman's r = -0.93, p<0.001). There was no significant relationship between a2AP levels and hemorrhage (Fig, 1E).
Brain swelling is one of the more serious complications of proximal MCA thrombosis. Brain swelling was significantly greater in mice with high circulating levels of a2AP than with normal a2AP levels (Fig. 1F, p<0.01) or a2AP-deficient mice (Fig. 1F, p<0.001). There was a significant positive correlation between circulating a2AP levels and brain swelling (Spearman's r = 0.82, p<0.001).
Effects of thrombus-bound a2AP on outcomes in a2AP-deficient mice
The previous experiments had examined the effect of different levels of circulating a2AP on outcomes in mice with MCA thromboemboli made from plasma containing physiologic levels of a2AP. In order to examine the potential additional contribution of thrombus-bound a2AP, we compared outcomes in a2AP-deficient mice with MCA thromboemboli made from plasma containing normal a2AP levels vs. a2AP-deficient plasma. As expected, thromboemboli made from normal (a2AP+/+) plasma showed evidence of fibrin-crosslinked a2AP by immunoblotting using specific anti-peptide or monoclonal antibodies (Fig. 1G) while no crosslinked a2AP was seen in thromboemboli made from a2AP-deficient (a2AP−/−) plasma (Fig. 1G). In a2AP-deficient mice, MCA thromboemboli containing normal levels of a2AP were more resistant to dissolution than a2AP-deficient thromboemboli, indicating that thrombus-bound a2AP affects endogenous fibrinolysis (Fig. 1h, p<0.001). However, despite the differences in the extent of thrombus dissolution, there was no difference in brain infarction in mice with normal or a2AP-deficient thrombi (Fig. 1I). In addition, the presence or absence of a2AP in the thromboembolus did not affect hemorrhage or brain swelling (Figs. 1J, K).
Effects of a2AP during prolonged ischemia after cerebral thromboembolism
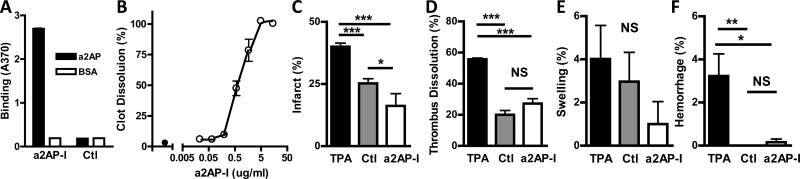
Taken together, these studies indicate that at the time of thromboembolism, levels of circulating a2AP have profound effects on stroke outcome. To examine whether a2AP continues to exert dynamic effects on the brain for hours after cerebral thromboembolism, mice were treated with a monoclonal antibody that inhibits a2AP (a2AP-I) and prevents the neutralization of plasmin. This a2AP-I bound specifically to mouse a2AP (Fig. 2A) and accelerated the dissolution of mouse plasma clots in vitro (Fig. 2B).22 Following MCA thromboembolism, laser Doppler monitoring showed a marked drop in hemispheric blood flow to 20% or less of the initial baseline in all three groups as expected (Fig. 1, online-only Data Supplement). Two and one-half hours after cerebral thromboembolism, mice were treated with a standard dose of TPA23 or with the a2AP-I. Plasma samples from mice receiving the a2AP-I confirmed that it significantly accelerated the dissolution of ex vivo clots (Fig. 2, online-only Data Supplement) and blood levels of the a2AP-I were 149 ± 23 ug/ml (mean ± standard error). By comparison to TPA, a2AP-I significantly reduced infarct size (Fig. 2C, p<0.001). Bederson neurological scores were better (i.e., lower) in mice treated with a2AP-I vs. TPA (median, 1 vs. 3, p<,0.02) indicating that a2AP-I treatment was associated with less neurological impairment. However, thrombus dissolution was significantly greater in TPA-treated mice than in those receiving a2AP-I (Fig. 2D, p<0.0001). There was no significant differences were detected in brain swelling (Fig. 2E), but there was significantly less brain hemorrhage in mice after a2AP-I administration than in mice treated with TPA (Fig. 2F, p<0.05).
Figure 2.
Binding specificity and effects of an a2AP inactivating (a2AP-I) monoclonal antibody and TPA on thrombus dissolution, infarction, swelling and hemorrhage after thromboembolism. A) The a2AP-I monoclonal antibody binds specifically to mouse a2AP vs. BSA in an ELISA by comparison to a control (anti-digoxin) monoclonal antibody. See Methods for additional details. B) The a2AP-I (o) accelerates the dissolution of 125I-fibrin labeled mouse plasma clots in vitro by comparison to no monoclonal antibody (●). Clot dissolution was determined as described in Methods. Effect of a2AP-I (9 mg/kg) or TPA (10 mg/kg) treatment given 2.5 hour after thromboembolism on the percent C) brain infarction, D) thrombus dissolution, E) brain swelling and F) hemorrhage. Brains were examined 6 h after cerebral thromboembolism. Data represent the means ± SE. n = 7 mice per group (B-E). *p≤0.05, ***p≤0.001, NS not significant.
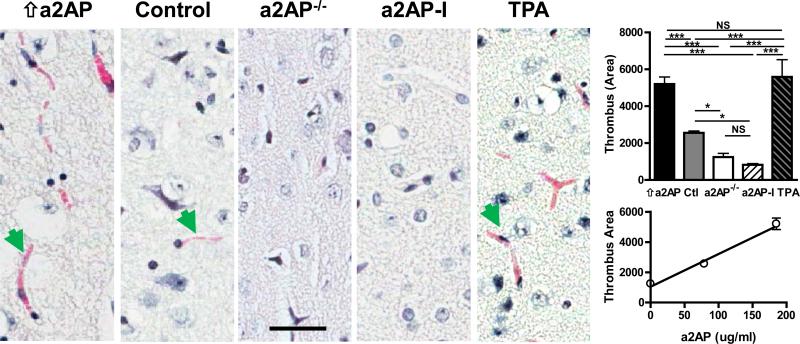
Effects of a2AP on the development of microvascular thrombosis
Previous studies have shown that brain ischemia triggers the development of downstream microvascular thrombosis that enhances vascular obstruction, aggravates tissue ischemia and contributes to breakdown of the blood brain barrier.24 Therefore we examined how circulating a2AP and TPA affected microvascular thrombosis. By comparison to control mice with normal levels of a2AP, microvascular thrombosis was more extensive in mice with high levels of circulating a2AP (Fig. 3, bar graph, p<0.001) or in mice treated with TPA (Fig. 3, bar graph, p<0.001). The extent of microvascular thrombosis was comparable in mice with high circulating levels of a2AP and in TPA-treated mice (Fig. 3, bar graph, p NS). In contrast, a2AP deficiency or a2AP-I markedly reduced microvascular thrombosis by comparison to mice with increased levels of circulating a2AP (Fig. 3, bar graph, p<0.001) and mice with treated with TPA (Fig.3, graph, p<0.001). Similarly, a2AP deficiency or a2AP-I reduced microvascular thrombosis by comparison to control mice with normal a2AP levels (Fig. 3, bar graph, p<0.05). Overall, there was a positive correlation between the levels of active a2AP and the amount of microvascular thrombosis (Fig. 3, line graph, Spearman's r = 0.94, p<0.001)
Figure 3.
Effects of a2AP and TPA on development of microvascular thrombosis after MCA thromboembolism. Representative images (40x) of microvascular thrombus (e.g., green arrows) in the ischemic brain detected by Martius-Scarlet-Blue staining in mice with increased a2AP levels (⇑aAP), normal aAP levels (control), a2AP deficiency (a2AP−/−), a2AP inactivation (a2AP-I) or TPA treatment. Bar graph, quantification of microvascular thrombus by digital imaging. The area of microvascular thrombus per 40x microscopic fields was quantitated by digital imaging in 12-15 random fields using Image-Pro Plus software. Line graph, relation between thrombus area and a2AP blood levels in mice supplemented with a2AP, normal and a2AP-deficient mice. Data represent means ± SE of n = 4-5 mice per group. Bar =20 μm. *p≤0.05, **p≤0.01, ***p≤0.001.
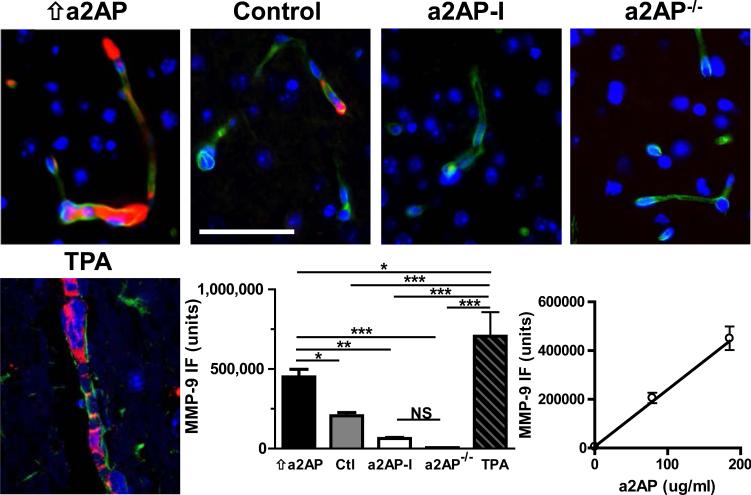
Circulating a2AP and MMP-9 expression
The close link between thrombosis and inflammation in ischemic stroke has suggested that ischemic stroke is a ‘thromboinflammatory’ disease.25 MMP-9 expression is linked to the inflammatory response to ischemic injury and is notably increased following cerebral thromboembolism 26. MMP-9 been identified as a key acute mediator of breakdown of the blood brain barrier, hemorrhage and brain edema in ischemic stroke.27-29 In control mice with normal a2AP levels, cerebral thromboembolism was associated with higher levels of expression of MMP-9 vs. shams (p<0.001, not shown). When compared to control mice with normal circulating levels of a2AP, mice with high levels of a2AP showed increased MMP-9 expression as assessed by quantitative immunofluorescence (Fig. 4, bar graph, p<0.05). MMP-9 expression was significantly lower in a2AP-deficient mice (p< 0.001) or a2AP-I treated mice (p< 0.01) by comparison to mice with elevated a2AP levels. Consistent with MMP-9 immunofluorescence and previous reports, in situ zymography activity appeared highest in the ischemic cortex of mice with increased a2AP levels, intermediate in mice with normal a2AP levels and lowest in mice with a2AP-deficiency. (Fig. 3, online-only Data Supplement). TPA treatment significantly increased MMP-9 expression by comparison to elevated levels of a2AP (Fig. 4, bar graph, p<0.05) or by comparison to control mice with normal levels of a2AP (Fig. 4, bar graph, p<0.001). By comparison, a2AP-I (Fig. 4 bar graph, p< 0.001) or a2AP deficiency (p<0.001) significantly reduced MMP-9 expression by comparison to TPA-treated mice. Overall, there was a positive correlation between levels of active a2AP and MMP-9 expression (Fig. 4 line graph, Spearman's r = 0.94, p<0.0001).
Figure 4.
MMP-9 expression after MCA thromboembolism. Representative images of MMP-9 immunofluorescence staining (red) in the ischemic hemispheres of mice with increased a2AP (⇑a2AP), normal a2AP levels (control), a2AP deficiency (a2AP−/−) as well as mice treated with a2AP inactivation (a2AP-I) or TPA. MMP-9 expression was assessed by double immunofluorescence staining for MMP-9 (DyLight549, red) and collagen IV (DyLight488, green). Representative micrographs with DAPI-stained nuclei (blue) were taken using a Zeiss LSM 710 confocal microscope at 40x magnification. Bar graph, quantitative digital analysis of the density of MMP-9 immunofluorescence (immunofluorescence units) of ten 40x fields from each stroke hemisphere. Data represent means ± SE of n = 4-5 mice per group. Line graph, relation between of MMP-9 immunofluorescence staining density (units) and a2AP blood levels in mice supplemented with a2AP, normal and a2AP-deficient mice. *p≤0.05, ** p≤0.001, ***p≤0.001. Bar =50 μm.
Dynamic effects of a2AP activity survival following cerebral thromboembolism
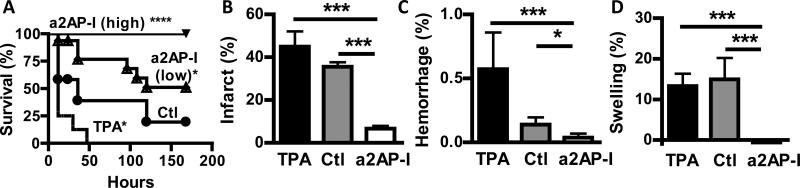
Proximal MCA stroke in humans is linked to extensive brain infarction and brain swelling that are associated with significant mortality. Since a2AP affected infarction and brain swelling after experimental thromboembolism, we examined whether a2AP-I after stroke onset may protect against mortality by comparison to control, untreated and TPA-treated mice. Control, untreated mice had a shortened survival following proximal MCA thromboembolism (Fig. 5A). Mice treated with TPA also had significant mortality following thromboembolism (Fig. 5A). Mice treated with a2AP-I, in the doses used in previous studies above, had significantly greater survival than control mice (p<0.05) or TPA-treated mice (p<0.001). To determine whether the dose or the molecular form of the a2AP-I affected outcomes we tested high dose a2AP in the form of an IgG (21.3 mg/kg) and as an Fab (9.5 mg/kg). All mice treated with high dose a2AP-I in IgG or Fab form survived (Fig. 5A). The survival of mice treated with high dose a2AP-I was significantly greater than mice treated with lower dose a2AP-I (9.3 mg/kg; p<0.01), TPA-treatment (p<0.0001) or control mice (p<0.0001).
Figure 5.
Effect of a2AP inactivation (a2AP-I) after cerebral thromboembolism on survival, brain infarction, hemorrhage and swelling. A) Survival in mice treated with no agent (control, Ctl), TPA (10 mg/kg), low dose a2AP-I (9 mg/kg) or high dose a2AP-I (as whole antibody (21.3 mg/kg) or Fab (9.3 mg/kg)) thirty minutes after stroke onset. Mice were observed for 7 days post stroke. Effects of treatment on B) brain infarction, C) brain hemorrhage and D) brain swelling after thromboembolism in surviving mice. Survival groups N= 12-16 in each cohort, data represent means ± SE. *p≤0.05, ***p≤0.001, ****p≤0.0001 vs. control or TPA.
Surviving mice were examined to determine how these treatments affected ischemic brain injury. By comparison to control mice, mice treated with all doses of a2AP inactivation had significantly smaller areas of brain infarction than control, untreated mice (Fig. 5B, p<0.001) or than TPA-treated mice (Fig. 5B, p<0.001). In a similar fashion, a2AP-I treated mice had smaller brain hemorrhage than control mice (Fig. 5C, p<0.05) and or than TPA-treated mice (Fig. 5C, p<0.001). Brain swelling was markedly reduced in mice treated with a2AP-I compared with control mice (Fig. 5D, p<0.001) or with mice treated with TPA (Fig. 5D, p<0.001).
Discussion
These data suggest that circulating a2AP has important effects on the dissolution of the culprit MCA thrombus as well as the development of brain microvascular thrombosis, infarction, blood brain barrier breakdown, hemorrhage, swelling, mortality and disability following cerebral thromboembolism. Several effects were dose-related as there were significant positive correlations between circulating a2AP levels or activity and the extent of brain infarction, brain swelling, microvascular thrombosis and MMP-9 expression. Conversely, there was a significant negative correlation between a2AP levels and dissolution of the culprit MCA thrombus. When compared to TPA-treated mice, a2AP-I given late after cerebral thromboembolism had a longer therapeutic window than TPA for protecting against brain injury and hemorrhage.
The finding that increased a2AP levels enhance brain infarction and the resistance of MCA thrombus to dissolution is consistent with clinical observations that a2AP may contribute to stroke in humans. In a case-cohort design, the Atherosclerosis Risk in Communities Study found that elevated a2AP levels were associated with an increased univariate risk of subsequent stroke.9 Recent studies have noted that genetic deficiency or plasmin-induced deficiency of a2AP reduce brain infarction in non-thrombotic models of focal ischemia induced by temporary or permanent surgical ligation of the MCA.15, 16, 30 The present findings are also consistent with recent reports that increased levels of a2AP may contribute to the failure of TPA treatment in humans and in mice.13, 14
In humans and in mice, thromboembolic occlusion of the MCA is associated with significant ischemic brain injury, edema, hemorrhage and reduced survival.31, 32 Consistent with these data, our study showed that proximal MCA thromboembolism had a high mortality. Treatment with an a2AP-I caused a marked reduction in mortality by comparison to TPA and to controls. Examination of surviving mice showed that a2AP-I treatment was associated with reduced hemorrhage and brain swelling, two key factors that contribute to mortality after MCA stroke.
In studies designed to examine the dynamics of MCA thrombus dissolution in relation to tissue injury, a2AP levels were inversely proportional to thrombus dissolution measured 6 h after thromboembolism, which is consistent with previous studies showing that circulating a2AP levels or activity regulate the extent of fibrinolysis.22, 33, 34 In addition, combined deficiency of thrombus-bound a2AP and circulating a2AP were associated with even greater dissolution of the MCA thromboembolus, than deficiency of circulating a2AP alone, indicating the important role played by thrombus-associated a2AP in this process.35, 36 Because of its role in fibrinolysis of the MCA thromboembolus, thrombus-bound a2AP may have been expected to affect tissue ischemia, but there was no detectable additional effect on brain infarction and swelling.
By comparison to controls or TPA-treated mice, the reduction of brain bleeding seen in mice treated with a2AP-I is unanticipated, as a2AP is often considered to protect against hemorrhage in fibrinolytic states.10 Since TPA and a2AP-I act in the same pathway to enhance plasmin activity, they may be expected to have similar effects on cerebral thromboembolism. It is notable that TPA therapy caused greater dissolution of the MCA thromboembolus than a2API, but it also caused greater ischemic brain injury, swelling and death. The mechanisms responsible for the different effects of TPA and a2AP-I are currently unknown, but may be attributable to singular effects of these agents on reperfusion injury, MMP-9 expression, acute inflammation, microvascular thrombosis, neuroprotection and/or other factors. A number of previous studies suggest that during prolonged ischemia, TPA has neurotoxic effects on the ischemic brain which include cleavage of the NMDA receptor, enhancement of excitotoxicity, blood brain barrier breakdown, promotion of apoptosis, etc.20, 37
Microvascular thrombosis plays an important role in ischemic stroke because microvascular thrombosis diminishes plasma flow, which accentuates brain injury and is associated with breakdown of the blood brain barrier, hemorrhage and other complications.24, 38-40 Microvascular thrombosis may be triggered initially by exposure of tissue factor, reflecting activation of endothelial cells, leukocytes or blood brain barrier breakdown.40-42 Inhibition of fibrin formation reduces ischemic injury arguing that it plays a key pathophysiologic role in ischemic stroke; still the role of fibrinolysis in microvascular thrombosis is poorly understood.40-43 These data provide the first evidence that levels of a2AP are positively correlated, in a dose-dependent fashion, with the extent of microvascular thrombosis in the ischemic hemisphere. These differences can't be explained by changes in blood cell populations as the complete blood counts of a2AP+/+ and a2AP−/− mice were similar (Table 1, online-only Data Supplement). Chopp and colleagues postulated that inhibition of fibrinolysis may accentuate microvascular thrombosis in stroke and studies have shown that microvascular thrombosis induced by endotoxemia is enhanced when fibrinolysis is inhibited.24, 44-46 Consistent with that hypothesis, our data shows that differences in a2AP levels, which affect dissolution of the MCA thrombus, also modulate the development of microvascular thrombosis. Still, it is important to note that, despite their common effects on promoting thrombus dissolution through plasmin, TPA accentuated, while a2AP-I diminished, microvascular thrombosis. The cause of this difference is unknown, though pharmacologic TPA has been shown to have pro-thrombotic or pro-coagulant effects after prolonged ischemia.47
Cerebrovascular ischemia triggers the release of endogenous TPA which generates plasmin and plasmin increases brain MMP-9 activity.20 MMP-9 enhances breakdown of the blood brain barrier, edema, hemorrhage and apoptosis.20 Viewed solely as a plasmin inhibitor, circulating a2AP may be expected to have a protective role by reducing MMP-9 activity; conversely inactivation or deficiency of circulating a2AP, may be predicted to be harmful by increasing MMP-9 activity. However, the data suggest the opposite: increased levels of a2AP were associated with significant elevations in MMP-9 expression in the brain and, inactivation of a2AP or a2AP deficiency was associated with reduced MMP-9 expression. Although we did not specifically quantitate MMP-9 activation, in situ gelatinase activity followed a similar pattern and previous studies have shown that MMP-9 expression and activity in ischemic stroke are closely related.48 Although the exact mechanism remains to be determined, a2AP may increase local MMP-9 levels through at least three mechanisms: 1) by enhancing microvascular thrombosis which increases accumulation of MMP-9-expressing leukocytes, 4926 2) by concentrating MMP-9 at the site through fibrin binding50 and, 3) by augmenting TPA secretion,51, 52 which in turn stimulates secretion of MMP-9. 53 Future studies will be necessary to determine the extent to which the harmful effects of a2AP are mediated through acute increases in MMP-9 activity.
In summary, these data suggest that circulating a2AP plays a critical, deleterious role in ischemic neurodegeneration and survival after cerebral thromboembolism by affecting microvascular thrombosis, hemorrhage, MMP-9 expression, swelling and breakdown of the blood brain barrier. These data add to a growing body of evidence from in vivo models that suggest that a2AP may have a role in modulating cellular injury and repair beyond its role fibrinolysis.15, 21, 33, 54, 55 In this translational model it is noteworthy that delayed a2AP inactivation after stroke onset reversed the deleterious effects of a2AP to reduce brain injury, bleeding, swelling, disability and death. By comparison to TPA, the protective effects of a2AP deficiency or inactivation appear to be mediated in part through reductions in microvascular thrombosis and MMP-9 expression. It will be important to better define the fibrinolytic and non-fibrinolytic mechanisms responsible for a2AP's effects and to further assess the extent to which therapeutic modulation of a2AP may protect ischemic tissue following thrombotic occlusion.
Supplementary Material
Significance.
Clinical observations suggest that alpha2-antiplasmin (a2AP) may increase the risk of ischemic stroke. We found that levels of a2AP were directly related to the severity of ischemic brain injury following cerebral thromboembolism. High levels of circulating a2AP increased brain infarction and swelling and, interfered with the dissolution of cerebral thromboemboli. Conversely, low levels of a2AP were neuroprotective. In a similar fashion, high levels of circulating a2AP worsened microvascular thrombosis and matrix metalloproteinase expression, two factors that contribute to ischemic brain injury. When compared to tissue plasminogen activator therapy, a2AP inactivation was less potent at dissolving thromboemboli. However, a2AP inactivation was significantly more effective than tissue plasminogen activator at reducing brain infarction, swelling, hemorrhage and mortality. Further studies are necessary to elucidate the mechanisms through which a2AP enhances microvascular thrombosis and ischemic injury during stroke, as well as to determine the potential protective value of a2AP inactivation.
Acknowledgements
Sources of Funding. This research was supported in part by NIH grants to G.R. (HL58496, HL92750 and NS73147).
Footnotes
Disclosures. GR is a founder of Translational Sciences. The other authors have no disclosures.
REFERENCES
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: Findings from the global burden of disease study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll G, Kleinschnitz C, Nieswandt B. Molecular mechanisms of thrombus formation in ischemic stroke: Novel insights and targets for treatment. Blood. 2008;112:3555–3562. doi: 10.1182/blood-2008-04-144758. [DOI] [PubMed] [Google Scholar]
- 3.Momi S, Tantucci M, Van Roy M, Ulrichts H, Ricci G, Gresele P. Reperfusion of cerebral artery thrombosis by the gpib-vwf blockade with the nanobody alx-0081 reduces brain infarct size in guinea pigs. Blood. 2013;121:5088–5097. doi: 10.1182/blood-2012-11-464545. [DOI] [PubMed] [Google Scholar]
- 4.Donnan GA, Davis SM, Parsons MW, Ma H, Dewey HM, Howells DW. How to make better use of thrombolytic therapy in acute ischemic stroke. Nat Rev Neurol. 2011;7:400–409. doi: 10.1038/nrneurol.2011.89. [DOI] [PubMed] [Google Scholar]
- 5.Kim EY, Lee SK, Kim DJ, Suh SH, Kim J, Heo JH, Kim DI. Detection of thrombus in acute ischemic stroke: Value of thin-section noncontrast-computed tomography. Stroke. 2005;36:2745–2747. doi: 10.1161/01.STR.0000185720.03803.41. [DOI] [PubMed] [Google Scholar]
- 6.Liebeskind DS, Sanossian N, Yong WH, et al. Ct and mri early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heye N, Paetzold C, Cervos-Navarro J. The role of microthrombi and microcirculatory factors in localization and evolution of focal cerebral ischemia. Neurosurg Rev. 1991;14:7–16. doi: 10.1007/BF00338186. [DOI] [PubMed] [Google Scholar]
- 8.Coughlin PB. Antiplasmin: The forgotten serpin? FEBS J. 2005;272:4852–4857. doi: 10.1111/j.1742-4658.2005.04881.x. [DOI] [PubMed] [Google Scholar]
- 9.Robinson BR, Houng AK, Reed GL. Catalytic life of activated factor xiii in thrombi. Implications for fibrinolytic resistance and thrombus aging. Circulation. 2000;102:1151–1157. doi: 10.1161/01.cir.102.10.1151. [DOI] [PubMed] [Google Scholar]
- 10.Weitz JI, Leslie B, Hirsh J, Klement P. Alpha 2-antiplasmin supplementation inhibits tissue plasminogen activator-induced fibrinogenolysis and bleeding with little effect on thrombolysis. J Clin Invest. 1993;91:1343–1350. doi: 10.1172/JCI116335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolev K, Lerant I, Tenekejiev K, Machovich R. Regulation of fibrinolytic activity of neutrophil leukocyte elastase, plasmin, and miniplasmin by plasma protease inhibitors. J Biol Chem. 1994;269:17030–17034. [PubMed] [Google Scholar]
- 12.Suri MF, Yamagishi K, Aleksic N, Hannan PJ, Folsom AR. Novel hemostatic factor levels and risk of ischemic stroke: The atherosclerosis risk in communities (aric) study. Cerebrovasc Dis. 2010;29:497–502. doi: 10.1159/000297966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marti-Fabregas J, Borrell M, Cocho D, Belvis R, Castellanos M, Montaner J, Pagonabarraga J, Aleu A, Molina-Porcel L, Diaz-Manera J, Bravo Y, Alvarez-Sabin J, Davalos A, Fontcuberta J, Marti-Vilalta JL. Hemostatic markers of recanalization in patients with ischemic stroke treated with rt-pa. Neurology. 2005;65:366–370. doi: 10.1212/01.wnl.0000171704.50395.ba. [DOI] [PubMed] [Google Scholar]
- 14.Houng AK, Wang D, Reed GL. Reversing the deleterious effects of alpha2-antiplasmin on tissue plasminogen activator therapy improves outcomes in experimental ischemic stroke. Experimental neurology. 2014;255C:56–62. doi: 10.1016/j.expneurol.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: A gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 16.Nagai N, De Mol M, Van Hoef B, Verstreken M, Collen D. Depletion of circulating alpha(2)-antiplasmin by intravenous plasmin or immunoneutralization reduces focal cerebral ischemic injury in the absence of arterial recanalization. Blood. 2001;97:3086–3092. doi: 10.1182/blood.v97.10.3086. [DOI] [PubMed] [Google Scholar]
- 17.Vivien D, Buisson A. Serine protease inhibitors: Novel therapeutic targets for stroke? J Cereb Blood Flow Metab. 2000;20:755–764. doi: 10.1097/00004647-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Weitz JI. Limited fibrin specificity of tissue-type plasminogen activator and its potential link to bleeding. J Vasc Interv Radiol. 1995;6:19S–23S. doi: 10.1016/s1051-0443(95)71243-x. [DOI] [PubMed] [Google Scholar]
- 19.Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur J, Zhao Z, Klein GM, Lo EH, Buchan AM. The neurotoxicity of tissue plasminogen activator? J Cereb Blood Flow Metab. 2004;24:945–963. doi: 10.1097/01.WCB.0000137868.50767.E8. [DOI] [PubMed] [Google Scholar]
- 21.Lijnen HR, Okada K, Matsuo O, Collen D, Dewerchin M. Alpha2-antiplasmin gene deficiency in mice is associated with enhanced fibrinolytic potential without overt bleeding. Blood. 1999;93:2274–2281. [PubMed] [Google Scholar]
- 22.Reed GL, 3rd, Matsueda GR, Haber E. Synergistic fibrinolysis: Combined effects of plasminogen activators and an antibody that inhibits alpha 2-antiplasmin. Proc Natl Acad Sci U S A. 1990;87:1114–1118. doi: 10.1073/pnas.87.3.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein c. Nat Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZG, Chopp M, Goussev A, Lu D, Morris D, Tsang W, Powers C, Ho KL. Cerebral microvascular obstruction by fibrin is associated with upregulation of pai-1 acutely after onset of focal embolic ischemia in rats. J Neurosci. 1999;19:10898–10907. doi: 10.1523/JNEUROSCI.19-24-10898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraft P, Gob E, Schuhmann MK, Gobel K, Deppermann C, Thielmann I, Herrmann AM, Lorenz K, Brede M, Stoll G, Meuth SG, Nieswandt B, Pfeilschifter W, Kleinschnitz C. Fty720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke. 2013;44:3202–3210. doi: 10.1161/STROKEAHA.113.002880. [DOI] [PubMed] [Google Scholar]
- 26.Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: Mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki Y, Nagai N, Umemura K, Collen D, Lijnen HR. Stromelysin-1 (mmp-3) is critical for intracranial bleeding after t-pa treatment of stroke in mice. J Thromb Haemost. 2007;5:1732–1739. doi: 10.1111/j.1538-7836.2007.02628.x. [DOI] [PubMed] [Google Scholar]
- 28.Rosell A, Lo EH. Multiphasic roles for matrix metalloproteinases after stroke. Curr Opin Pharmacol. 2008;8:82–89. doi: 10.1016/j.coph.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Lam CK, Yoo T, Hiner B, Liu Z, Grutzendler J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature. 2010;465:478–482. doi: 10.1038/nature09001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crumrine RC, Marder VJ, Taylor GM, Lamanna JC, Tsipis CP, Novokhatny V, Scuderi P, Petteway SR, Jr., Arora V. Safety evaluation of a recombinant plasmin derivative lacking kringles 2-5 and rt-pa in a rat model of transient ischemic stroke. Exp Transl Stroke Med. 2012;4:10. doi: 10.1186/2040-7378-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinsius T, Bogousslavsky J, Van Melle G. Large infarcts in the middle cerebral artery territory. Etiology and outcome patterns. Neurology. 1998;50:341–350. doi: 10.1212/wnl.50.2.341. [DOI] [PubMed] [Google Scholar]
- 32.Hara T, Mies G, Hossmann KA. Effect of thrombolysis on the dynamics of infarct evolution after clot embolism of middle cerebral artery in mice. J Cereb Blood Flow Metab. 2000;20:1483–1491. doi: 10.1097/00004647-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Butte AN, Houng AK, Jang IK, Reed GL. Alpha 2-antiplasmin causes thrombi to resist fibrinolysis induced by tissue plasminogen activator in experimental pulmonary embolism. Circulation. 1997;95:1886–1891. doi: 10.1161/01.cir.95.7.1886. [DOI] [PubMed] [Google Scholar]
- 34.Lee KN, Jackson KW, Christiansen VJ, Dolence EK, McKee PA. Enhancement of fibrinolysis by inhibiting enzymatic cleavage of precursor alpha2-antiplasmin. J Thromb Haemost. 2011;9:987–996. doi: 10.1111/j.1538-7836.2011.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser SR, Booth NA, Mutch NJ. The antifibrinolytic function of factor xiii is exclusively expressed through alpha-antiplasmin cross-linking. Blood. 2011;117:6371–6374. doi: 10.1182/blood-2011-02-333203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed GL, Matsueda GR, Haber E. Fibrin-fibrin and alpha 2-antiplasmin-fibrin cross-linking by platelet factor xiii increases the resistance of platelet clots to fibrinolysis. Trans Assoc Am Physicians. 1991;104:21–28. [PubMed] [Google Scholar]
- 37.Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: More than a thrombolytic. Trends in neurosciences. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Choudhri TF, Hoh BL, Zerwes HG, Prestigiacomo CJ, Kim SC, Connolly ES, Jr., Kottirsch G, Pinsky DJ. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting gp iib/iiia receptor-mediated platelet aggregation. J Clin Invest. 1998;102:1301–1310. doi: 10.1172/JCI3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ninomia T, Wang L, Kumar SR, Kim A, Zlokovic BV. Brain injury and cerebrovascular fibrin deposition correlate with reduced antithrombotic brain capillary functions in a hypertensive stroke model. J Cereb Blood Flow Metab. 2000;20:998–1009. doi: 10.1097/00004647-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994;25:1847–1853. doi: 10.1161/01.str.25.9.1847. discussion 1853-1844. [DOI] [PubMed] [Google Scholar]
- 41.Thomas GR, Thibodeaux H, Bennett WF, Refino CJ, Badillo JM, Errett CJ, Zivin JA. Optimized thrombolysis of cerebral clots with tissue-type plasminogen activator in a rabbit model of embolic stroke. The Journal of pharmacology and experimental therapeutics. 1993;264:67–73. [PubMed] [Google Scholar]
- 42.Morris DC, Zhang L, Zhang ZG, Lu M, Berens KL, Brown PM, Chopp M. Extension of the therapeutic window for recombinant tissue plasminogen activator with argatroban in a rat model of embolic stroke. Stroke. 2001;32:2635–2640. doi: 10.1161/hs1101.097390. [DOI] [PubMed] [Google Scholar]
- 43.de la Rosa X, Cervera A, Kristoffersen AK, Valdes CP, Varma HM, Justicia C, Durduran T, Chamorro A, Planas AM. Mannose-binding lectin promotes local microvascular thrombosis after transient brain ischemia in mice. Stroke. 2014;45:1453–1459. doi: 10.1161/STROKEAHA.113.004111. [DOI] [PubMed] [Google Scholar]
- 44.Pfeiler S, Massberg S, Engelmann B. Biological basis and pathological relevance of microvascular thrombosis. Thromb Res. 2014;133(Suppl 1):S35–37. doi: 10.1016/j.thromres.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto K, Loskutoff DJ. Fibrin deposition in tissues from endotoxin-treated mice correlates with decreases in the expression of urokinase-type but not tissue-type plasminogen activator. J Clin Invest. 1996;97:2440–2451. doi: 10.1172/JCI118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmeister HM, Szabo S, Kastner C, Beyer ME, Helber U, Kazmaier S, Wendel HP, Heller W, Seipel L. Thrombolytic therapy in acute myocardial infarction: Comparison of procoagulant effects of streptokinase and alteplase regimens with focus on the kallikrein system and plasmin. Circulation. 1998;98:2527–2533. doi: 10.1161/01.cir.98.23.2527. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmeister HM, Kastner C, Szabo S, Beyer ME, Helber U, Kazmaier S, Baumbach A, Wendel HP, Heller W. Fibrin specificity and procoagulant effect related to the kallikrein-contact phase system and to plasmin generation with double-bolus reteplase and front-loaded alteplase thrombolysis in acute myocardial infarction. Am J Cardiol. 2000;86:263–268. doi: 10.1016/s0002-9149(00)00911-5. [DOI] [PubMed] [Google Scholar]
- 48.Fujimura M, Gasche Y, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. 1999;842:92–100. doi: 10.1016/s0006-8993(99)01843-0. [DOI] [PubMed] [Google Scholar]
- 49.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 50.Makowski GS, Ramsby ML. Binding of latent matrix metalloproteinase 9 to fibrin: Activation via a plasmin-dependent pathway. Inflammation. 1998;22:287–305. doi: 10.1023/a:1022300216202. [DOI] [PubMed] [Google Scholar]
- 51.Hu K, Yang J, Tanaka S, Gonias SL, Mars WM, Liu Y. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281:2120–2127. doi: 10.1074/jbc.M504988200. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan KL, Mather T, DeMarco L, Solomon S. Effect of fibrin on endothelial cell production of prostacyclin and tissue plasminogen activator. Arteriosclerosis. 1989;9:43–49. doi: 10.1161/01.atv.9.1.43. [DOI] [PubMed] [Google Scholar]
- 53.Cuadrado E, Ortega L, Hernandez-Guillamon M, Penalba A, Fernandez-Cadenas I, Rosell A, Montaner J. Tissue plasminogen activator (t-pa) promotes neutrophil degranulation and mmp-9 release. J Leukoc Biol. 2008;84:207–214. doi: 10.1189/jlb.0907606. [DOI] [PubMed] [Google Scholar]
- 54.Reed GL, 3rd, Matsueda GR, Haber E. Inhibition of clot-bound alpha 2-antiplasmin enhances in vivo thrombolysis. Circulation. 1990;82:164–168. doi: 10.1161/01.cir.82.1.164. [DOI] [PubMed] [Google Scholar]
- 55.Matsuno H, Kozawa O, Okada K, Ueshima S, Matsuo O, Uematsu T. Inhibitors of fibrinolytic components play different roles in the formation and removal of arterial thrombus in mice. J Cardiovasc Pharmacol. 2002;39:278–286. doi: 10.1097/00005344-200202000-00015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.