Abstract:
Despite the availability of predictive tools and treatment guidelines, anticoagulant therapies are underprescribed and many patients are undertreated for conditions that predispose to thromboembolic complications, including stroke. This review explores reasons for which physicians fear that the risks of anticoagulation may be greater than the potential benefit. The results of numerous clinical trials confirm that patients benefit from judiciously managed anticoagulation and that physicians can take various approaches to minimize risk. Use of stratification scores for patient selection and accurate estimation of stroke risk may improve outcomes; bleeding risk is less important than stroke risk. Adoption of newer anticoagulants with simpler regimens may help physicians allay their fears of anticoagulant use in patients with atrial fibrillation. These fears, although not groundless, should not overtake caution and hinder the delivery of appropriate evidence-based care.
Key Indexing Terms: Atrial fibrillation, Stroke, Oral anticoagulants
THE LANDSCAPE: ANTICOAGULATION INDICATIONS AND USE
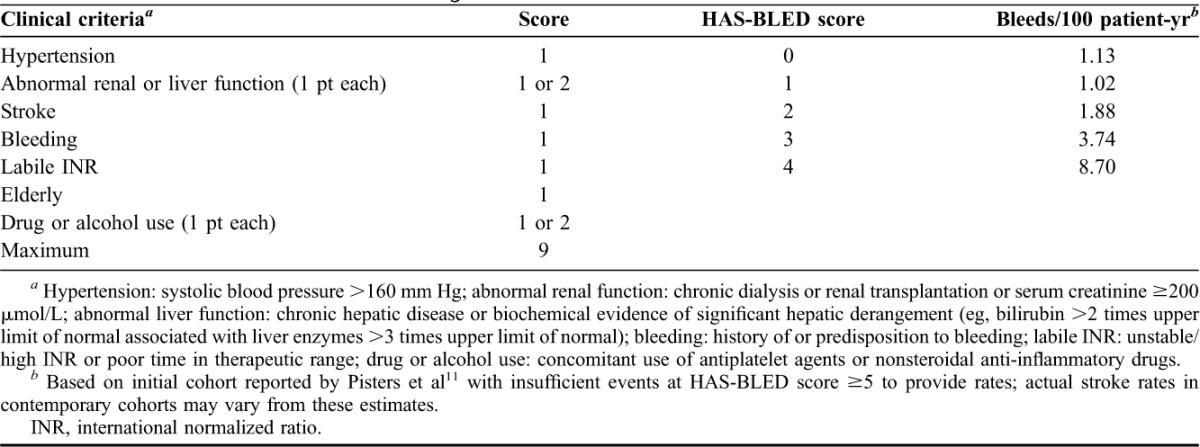
Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting approximately 2.4 million Americans and predisposing to a risk for ischemic stroke that is 2 to 5 times greater than that of age-matched controls.1–3 Stroke is the leading cause of adult disability, affecting 795,000 Americans annually.4 An estimated 69,165 of these strokes are attributable to AF.5,6 Every hour, approximately 8 Americans suffer from an ischemic stroke arising from AF.5,6 Currently, validated risk stratification schemes such as CHADS2 and CHA2DS2-VASc, based on other predisposing conditions, facilitate stroke prediction in patients with AF (Table 1).7,8 Oral anticoagulation can make a significant dent in this stroke risk in AF and is backed by evidence-based stroke prevention guidelines.8–10 Recently, schemes such as HAS-BLED (Table 2) have been developed to evaluate the risk of bleeding, the feared complication of oral anticoagulation therapy.11–13 Despite guidelines and tools, anticoagulation is underprescribed, which exposes patients with AF to the risk of debilitating strokes.3
TABLE 1.
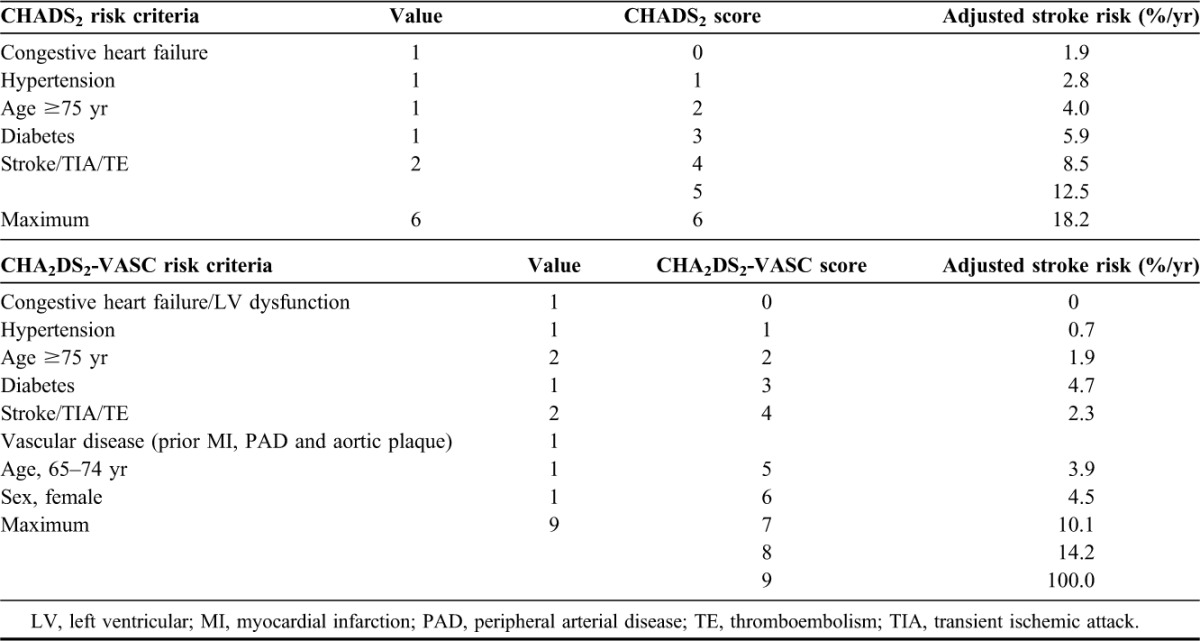
TABLE 2.
Clinical criteria for HAS-BLED bleeding risk score
Several studies have evaluated the prevalence of oral anticoagulation use in patients with AF.14–20 The rate of oral anticoagulation prescribing in patients with AF with a moderate-to-high risk of stroke ranged from 41% to 65%.14,21,22 Even after the elimination of patients with contraindications to anticoagulation, the rate of oral anticoagulation use did not increase.14,21,22 Among these studies, the National Anticoagulation Benchmark Outcomes Report (NABOR), a performance improvement program, investigated treatment gaps and predictors of warfarin use in a nationally representative AF population sample in the United States.14 Although risk factors indicated that 86% of patients had a high risk for stroke, only 55% of those at high risk received warfarin.14 High-risk stratification was not a positive predictor for warfarin use, and contraindications to warfarin did not account for the marked level of underuse.14 Another study examined Medicare Part D claims data for warfarin use among beneficiaries with nonvalvular AF (NVAF) in the context of current treatment guidelines.21 Among those at moderate-to-high stroke risk but not at high bleeding risk, 41.3% did not receive warfarin within 12 months of the index diagnosis.21 These real-world results showed that a significant proportion of Medicare beneficiaries in need of anticoagulation were not treated according to clinical guidelines, which led to an excessive rate of ischemic stroke in an at-risk population.21
The underuse of warfarin may stem from the drug's well-known limitations; however, compliance with guidelines may also be influenced by variables at system, physician, and patient levels.22 Newer oral anticoagulants may reduce the risk of stroke with a lower risk of adverse events than warfarin, but the need to understand why physicians deviate from anticoagulation guidelines “has implications that transcend therapeutic class.”22 This review explores possible explanations for withholding anticoagulant therapy. Such explanations frequently are based on fears that the risks are greater than any potential benefit of anticoagulants.23 Although it is undeniable that anticoagulant therapy may be associated with risk of bleeding, it is also evident from long experience, confirmed by objective analysis, that patients benefit from anticoagulation and that there are ways to minimize their bleeding risk. The choice of new oral anticoagulants with different mechanisms of action and simpler regimens may help persuade physicians and patients alike. It should be noted that the majority of studies to date with newer oral anticoagulants have focused on stroke risk factors in patients with NVAF. Although not as common, patients with valvular AF (VAF, ie, those with AF and rheumatic mitral stenosis or a prosthetic mitral valve) are also at risk for ischemic stroke.24 Although warfarin therapy (based on target International normalized ratio [INR]) has been reported as an effective means for stroke prevention,8 the role that newer anticoagulants might play in stroke prevention in patients with VAF has not been evaluated.
BARRIERS TO ADEQUATE ANTICOAGULATION: REAL AND PERCEIVED REASONS FOR UNDERTREATMENT
Physicians' Fears
Many physicians associate anticoagulant use with a heightened risk of bleeding.25 Death certificate data in 2003 and 2004 ranked anticoagulants first in the number of mentions of “deaths from drugs causing adverse effects in therapeutic use.”26 For a retrospective analysis of health care claims within a 4 million member managed care organization, patients diagnosed with AF were stratified into 2 cohorts: warfarin therapy (patients initiating warfarin) or warfarin candidates (eligible according to American College of Cardiology/American Heart Association/European Society of Cardiology guidelines but not receiving warfarin).27 During 2 years of follow-up, 4.7% experienced a hemorrhagic event.27 The incidence of intracranial hemorrhage was identical in both cohorts.27 There was no significant increase in risk for hemorrhage within the warfarin therapy group after adjustment for age, sex, and additional risk factors for hemorrhage.27 Although the study was not designed to determine why warfarin was underused despite indications for its use, the perceived risk of bleeding complications may have been a contributing factor.27 The investigators acknowledged that use of nonprescription antiplatelet agents may have contributed to the similarity in rates of hemorrhage and suggested that such similarity might also have resulted from conservative dosing and management of warfarin therapy, possibly with attainment of a lower INR than achieved in clinical trials.27 Earlier investigators noted that physicians treating patients with AF were more averse to cause harm in the form of warfarin-related hemorrhage than harm due to stroke resulting from failure to treat with warfarin.28 If physicians' treatment decisions are driven predominantly by historical concerns regarding an increased bleeding risk, conservative use and cautious dosing may deprive patients of the full benefit of anticoagulation.27
An Australian group randomly selected 1,000 family physicians, of whom 596 responded to a survey aimed at identifying barriers to the use of anticoagulation.29 Offered a choice of strategies for AF management, a minority (45.6%) of respondents selected warfarin in the presence of a minor risk for falls; even fewer (17.1%) said they would prescribe warfarin for a patient receiving treatment for a peptic ulcer.29 Prescribing patterns were influenced by physicians' experience and fear of bleeding events. For example, among the physicians surveyed, 15.8% reported having a patient with AF experience an intracranial hemorrhage while on anticoagulation, whereas 45.8% reported having patients who experienced ischemic stroke in the absence of anticoagulant therapy.29 The experience of intracranial hemorrhage in an anticoagulated patient with AF appeared to condition family physicians to feel responsible for this outcome, whereas they admitted to no such responsibility when presented with the more common experience of stroke in a patient with AF not receiving anticoagulation.29 The inculcation to “first do no harm” may condition physicians to feel more responsible for harm resulting from “commission” rather than “omission,” and this sense of culpability may lead them to shun therapies they associate with a risk of adverse events, even when the potential benefits are shown to be greater than the risks.29
Strikingly, similar results emerged from a population-based matched-pair analysis of 530 Canadian physicians' warfarin-prescribing patterns before and after adverse bleeding events in patients with AF. Warfarin-associated bleeding events negatively influenced warfarin prescribing throughout the 90-day study, whereas adverse events possibly related to underuse of warfarin seemed not to affect subsequent prescribing.30
Fear of bleeding is the factor common to clinicians providing warfarin therapy across countries and continents.23,31–33 Warfarin anticoagulation to conventional intensities increases the risk of intracranial hemorrhage 7- to 10-fold to an absolute rate of 1% annually for many stroke-prone patients.33 Most such hemorrhages (70%) are intracerebral hematomas, with patient-related risk factors (advanced age, prior stroke, hypertension and intensity of anticoagulation) overlapping those for stroke.33 Although intracranial bleeding is the most feared complication of anticoagulation, more common sites of bleeding are in fact the gastrointestinal (GI) tract, the genitourinary tract, and the soft tissues.34
After analyzing studies published between 1966 and 2002, Man-Son-Hing and Laupacis35 concluded that physicians' fears of the risk of bleeding associated with anticoagulant therapy are often exaggerated and unfound. Studies pursued when the intensity of anticoagulation was higher than current levels (target INR 3.0–4.5 versus 2.0–3.0) showed higher than expected rates of intracerebral hemorrhage in anticoagulated patients with a history of stroke, whereas recent studies based on the current less aggressive INR target have not confirmed those early findings.35 The salient issue is careful selection of patients and accurate estimation of stroke risk; anticoagulant-associated bleeding risk needs to be considered relevant to relatively few patients.35
Specific Fears: Falling, Upper GI Tract Bleeding and Stroke
Investigators and clinicians agree that there is an increased risk of potentially life-threatening bleeding complications associated with anticoagulant therapy, including bleeding into specific sites such as the GI tract or intracranial bleeding in patients with head trauma or uncontrolled hypertension.35 However, after a 1999 decision analysis, Man-Son-Hing et al36 asserted that a predisposition to falling, with the possibility of head trauma, is not a contraindication to the use of anticoagulant therapy in older patients with AF. They calculated that for the person with an average risk of AF-related stroke, the 5% annual risk of subdural hematoma from falling is so small that the person would have to fall 295 times in the course of a year to accrue greater risk than benefit from anticoagulant therapy.36 Similarly, systematic review by Man-Son-Hing and Laupacis35 demonstrated that in the era of routine testing and treatment for Helicobacter pylori infection, patients with a history of resolved upper GI tract bleeding seem to be at no higher risk of upper GI bleeding than those with a negative history.35
However, it should be noted that GI bleeding is one of the feared complications of anticoagulant therapy in patients who are frequently taking NSAIDs.37 Pooled results of a systematic analysis of 18 case-control and cohort studies performed between 1990 and 1999 demonstrated that persons taking nonsteroidal anti-inflammatory drugs (NSAIDs) without cytoprotection had a relative risk (RR) of 3.8 (95% confidence interval [CI], 3.6–4.1) for GI tract bleeding.38 The increased risk was maintained during treatment and returned to baseline on cessation of treatment.38 Results of randomized comparative trials showed that concomitant use of an NSAID with misoprostol or a proton pump inhibitor reduced the upper GI bleeding risk by approximately one half.39,40 Use of a cyclooxygenase-2 inhibitor-specific NSAID reduced the risk of upper GI tract bleeding to about half the rate associated with conventional NSAIDs.41 To determine how factors that increase the risk of major upper GI tract hemorrhage influence the choice of antithrombotic treatment for older patients with AF and an elevated risk for stroke, Man-Son-Hing and Laupacis42 conducted a systematic literature search of studies published between January 1966 and December 2000 in developing a decision-analytic model based on the risk of upper GI tract bleeding and stroke.42 The investigators determined the risk of anticoagulant-related bleeding in the presence of defined risk factors; that is, 3.8 times baseline for a patient taking a noncytoprotective NSAID and 2.4 times baseline for a patient taking warfarin, which resulted in a risk of 9.1 times baseline (3.8 × 2.4) for a patient taking both medications.42 Across several clinical scenarios, anticoagulation was the best therapy in terms of gain in quality-adjusted life-years for most of the older patients with AF.42 The main exception was patients with a low risk of AF-related stroke (because of an absence of clinical risk factors for stroke) combined with a high risk of upper GI tract bleeding (because of concomitant use of noncytoprotective NSAIDs).42 For such patients, acetylsalicylic acid (ASA) or no antithrombotic therapy appeared appropriate.42 For older patients with AF and a higher than average risk for upper GI tract bleeding, the choice of antithrombotic therapy for stroke prevention varied according to the magnitude of bleeding risk.42 The authors concluded that warfarin was no longer clearly the optimal antithrombotic therapy for older persons with a significantly higher risk of upper GI tract bleeding and/or lower risk for stroke who were concurrently taking a conventional NSAID.42
Lowering the Barriers to Effective Anticoagulation
In a 2011 editorial, Goldhaber43 addressed practical issues confronting physicians who provided day-to-day care of patients on anticoagulant therapy. Faced with a surfeit of information, clinicians lack a “unifying and reliable” source of information about developments in anticoagulant therapy. Goldhaber noted, for instance, important differences among 3 apparently authoritative sets of practice guidelines.44–46 There are also clinically important differences among various schemes for stratifying stroke risk in patients with AF.43 Experts fail to agree which tool is the most reliable for scoring bleeding risk during anticoagulant therapy.43 Not surprisingly, clinicians are uncertain how to balance the risk of thromboembolic events against the risk of bleeding and consequently, for fear of causing harm, err on the side of caution. Other stratification schemes are available that can help clinicians estimate patients' who fall risk and make better selection of candidates for anticoagulation even when a risk for falls is present.47,48
A positive direction for future therapy emerged from a study by Banerjee et al49 who modeled differing scenarios using a real-world cohort derived from the Danish National Patient Registry. In patients with a CHADS2 score ≥1 or CHA2DS2-VASc score ≥2, warfarin offered a net clinical benefit in preventing stroke, but the newer Food and Drug Administration (FDA)–approved agents, dabigatran, rivaroxaban and apixaban, offered greater net clinical benefit than warfarin.49 The authors suggest that their findings may encourage physicians to use anticoagulation in patients stratified by a relatively simple risk-scoring system (Figure 1) and raise awareness of the advantages of a new generation of anticoagulants compared with warfarin.49
FIGURE 1.
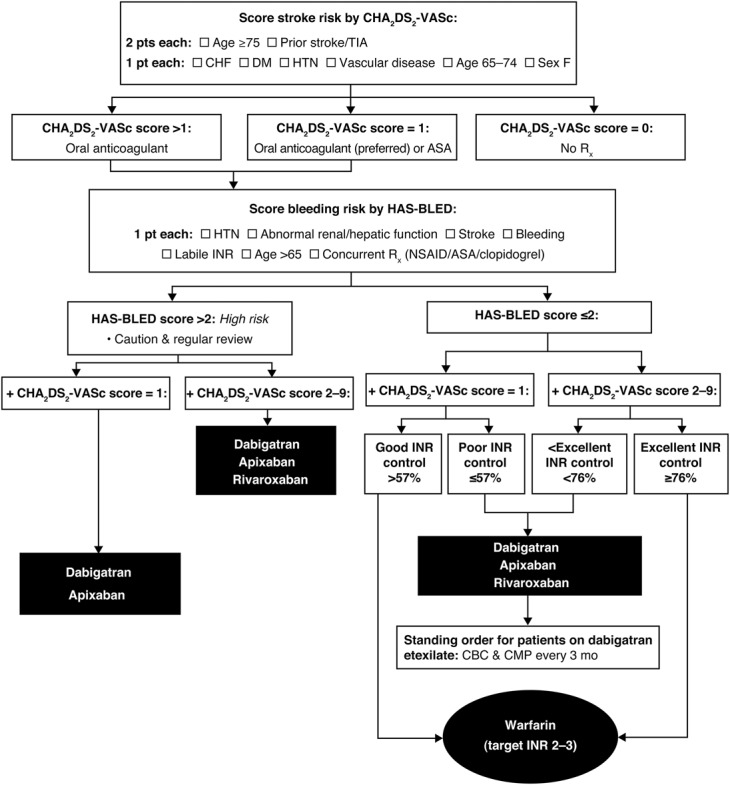
Algorithm for choice of anticoagulant in patients with nonvalvular AF. Relative effectiveness based on post hoc modeling using the Danish National Registry.47 AF, atrial fibrillation; ASA, acetylsalicylic acid; CHF, congestive heart failure; DM, diabetes mellitus; HTN, hypertension; INR, international normalized ratio; NSAID, nonsteroidal anti-inflammatory drug; TIA, transient ischemic attack.
Primary care physicians may overlook hypertension as a powerful determinant of intracerebral hemorrhage risk, whereas hypertensive patients are often unaware of symptoms until they suffer a catastrophic complication, such as ischemic stroke or intracerebral hemorrhage.34,50 Providing effective antihypertensive therapy by the physician, and close adherence to therapy by the patient, are essential to reduce the risk of intracerebral hemorrhage associated with anticoagulation in patients with hypertension patients.34,50 Advances in neuroimaging with magnetic resonance are allowing unprecedented insights into brain pathology and predictive factors for intracerebral hemorrhage risk, but such techniques are not yet fully developed for routine clinical use.34,50 The impact of hypertension control in reducing the incidence of intracerebral hemorrhage has been demonstrated in a number of trials, notably the Perindopril Protection against Recurrent Stroke Study (PROGRESS).51 In patients at high risk for recurrent stroke (N = 6105, mean age 64 years), absolute rates of intracerebral hemorrhage dropped from 2% to 1% (RR reduction, 50%; 95% CI, 26–67), when mean blood pressure was reduced by 12/5 mm Hg from baseline.51
A history of stroke should not be considered a contraindication to anticoagulant treatment: a prior stroke increases the risk of another stroke for a patient with AF.35 For most patients, nonfatal and extracranial bleeds are far less clinically significant than strokes of any severity, and patients at risk who are not adequately anticoagulated may be more likely to have embolic strokes.34,49 The American Hospital Association/American Stroke Association's 2012 science advisory on the use of oral antithrombotic agents for stroke prevention in NVAF did not directly address physicians' fears regarding anticoagulation.52 However, in reiterating the FDA's rationale for approving a higher but not a lower dose of a new oral anticoagulant, dabigatran etexilate, the advisory alluded to a prior observation that “the irreversible effects of strokes and systemic emboli have greater clinical significance than nonfatal bleeding.”52–54 Superior stroke prevention with the higher dose, as the FDA's approval indicates, is a more desirable outcome than a lower rate of nonfatal bleeding with the lower dose.53,54
A recent analysis of outcomes in a retrospective cohort helps to illuminate the RRs, benefits and optimal timing for reinitiating warfarin therapy after a major gastrointestinal bleeding (GIB) event.55 Anticoagulation therapy with warfarin was reinitiated in 653/1,329 (49.1%) of the patients, which was associated with a decreased risk of thromboembolism (hazard ratio [HR], 0.71; and 95% CI, 0.54–0.93; P = 0.01), and decreased mortality (HR, 0.67; 95% CI, 0.56–0.81; P < 0.0001).55 Of note, the recurrence rate of major GIB events was not affected by the reinitiation of warfarin anticoagulation therapy, with an HR of 1.18 (95% CI, 0.94–1.10).55 Although limited by the retrospective design of this study, this analysis suggests that restarting warfarin anticoagulant therapy 7 days or later after a major GIB event is associated with lower rates of thromboembolism and increased survival, with no increase in GIB event rates.55 Physicians may be hesitant to treat with anticoagulants if they lack confidence in the patient's ability to adhere to a medication regimen.35 The many challenges of warfarin use, a lengthy dose-adjustment period, the need for frequent sampling and regular anticoagulation monitoring, the difficulty of maintaining the INR within the therapeutic range, dietary restrictions, raise barriers to optimal anticoagulation in at-risk populations.25,32,34 The educational approach developed by Garcia et al25 for the Anticoagulation Forum (Table 3) offers a comprehensive and practical education model that may be adapted to encourage patients' adherence in any therapeutic area.
TABLE 3.
Fifteen key points for patient education about oral anticoagulant therapy

Although treatment with the newer oral anticoagulants is more expensive than with older therapy, such as warfarin, these agents may offer adherence advantages because they have simpler dosing regimens and fewer requirements for dose adjustment, anticoagulation monitoring, or dietary restrictions. Because there are no available reversal agents for these newer therapies, patients and prescribers should be aware that optimal protocols to address bleeding event complications are still under development, whereas new and specific reversal therapies are currently in clinical trials.56,57
Conventional Anticoagulation
Warfarin
Warfarin received FDA approval in 1954 and is the most widely prescribed oral anticoagulant drug in North America.58,59 It induces anticoagulant activity by antagonizing vitamin K-dependent synthesis of clotting factors in the liver.58 Although the antiplatelet agent ASA has been found to reduce the risk of stroke by 21%, long-term therapy with warfarin is associated with a stroke risk reduction of 68%.60,61 Warfarin, slow to take effect and difficult to dose, has been the only available oral anticoagulant for the last half century.25,58,61 The response to warfarin is subject to genetic variations and drug–drug interactions,61,62 and the therapeutic effect is susceptible to dietary variations,61 some of which, such as consumption of green and leafy vegetables, might otherwise be considered positive behaviors for stroke risk reduction. Warfarin is also a leading cause of emergency department visits and preventable costs.63,64 The low acquisition cost of warfarin may be counterbalanced by the costs of laboratory monitoring to ensure that the anticoagulant effect remains within the narrow safe range and by the costs of managing potentially catastrophic complications of therapy.64,65 Despite its shortcomings, warfarin remains an important drug for long-term anticoagulant therapy worldwide.66 An estimated 7 million people in the United States and Europe have either paroxysmal or persistent AF, for which anticoagulant therapy is indicated to reduce the risk of stroke.66 However, the recent approval of new oral anticoagulants offers therapeutic alternatives that are at least as effective as warfarin and offer a potential for safer and more easily managed anticoagulation.67–71
New Oral Anticoagulants
Three new oral anticoagulants have recently been approved in the United States for the reduction of stroke risk in patients with AF: dabigatran, rivaroxaban and apixaban.10,32,69–71 These agents have been compared with warfarin; to date, head-to-head comparisons are lacking.32
Dabigatran Etexilate
Dabigatran etexilate, an oral direct thrombin inhibitor, was approved by the FDA in 2010 to reduce the risk of stroke and systemic embolism (SSE) in patients with NVAF.72,73 Dabigatran etexilate is administered in a fixed dose of 150 mg twice daily for most patients, with a dose of 75 mg twice daily recommended for patients with creatinine clearance (CrCl) of 15 to 30 mL/min.69 The phase 3 Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial compared open-label dose-adjusted warfarin with 2 blinded doses (150 mg twice a day or 110 mg twice a day) of dabigatran etexilate for the primary outcome of SSE in 18,113 patients with NVAF.72,73 For warfarin-treated patients (n = 6022), the INR was within therapeutic range (2.0–3.0) a mean 64% of the study period.72 Updated results adjudicated in a blinded fashion according to the study protocol demonstrated that the dose of 150 mg twice daily of dabigatran etexilate was superior to warfarin for the prevention of SSE (HR, 0.66; 95% CI, 0.53–0.82; P < 0.001 for superiority), with a similar risk of the primary safety end point of major bleeding (3.1% and 3.4% per year, respectively; P = 0.31).72,73 Lower rates of intracranial hemorrhage were reported for dabigatran 110 mg twice daily and 150 mg twice daily than for warfarin (0.2%, 0.3%, and 0.7%, respectively; P < 0.001 for either dabigatran dose versus warfarin).74 Similar rates of SSE were recorded in the dabigatran etexilate 110 mg twice daily and warfarin groups (1.54% and 1.71% a year, respectively; P = 0.30); dabigatran etexilate 110 mg twice daily was associated with a lower rate of major bleeding than warfarin (2.9% versus 3.6% per year, respectively; P = 0.003).72,73 The FDA did not approve the 110 mg twice daily dose; hence, it is not available for clinical use in the United States.53,69 The concomitant use of dabigatran etexilate with P-glycoprotein (P-gp) inducers (eg, rifampin) reduces exposure to dabigatran and should generally be avoided.75 In patients with moderate renal impairment, concomitant administration of dabigatran etexilate with P-gp inhibitors (eg, dronedarone and systemic ketoconazole) is not recommended, as coadministration is expected to increase exposure to dabigatran.75 No drug–food interactions are reported. Routine blood coagulation monitoring is not required during dabigatran etexilate therapy, but when necessary, the extent of anticoagulation may be estimated by measuring the activated partial prothrombin time or the ecarin clotting time.75
Rivaroxaban
An oral factor Xa inhibitor, rivaroxaban, was approved by the FDA in 2011 to reduce the risk of SSE in patients with NVAF and subsequently for additional indications.10,70 To reduce the risk of SSE in patients with NVAF, rivaroxaban is administered at a dose of 20 mg/d to patients with CrCl ≥50 mL/min; for patients with CrCl 15 to 50 mL/min, the recommended dose is 15 mg/d.10,75,76 Rivaroxaban and warfarin were compared for efficacy in 14,264 patients with NVAF in the phase 3 Rivaroxaban Once-daily oral direct Factor Xa inhibition compared with vitamin K antagonism for the prevention of stroke and Embolism Trial in AF (ROCKET-AF).76 The results suggest that rivaroxaban may be an alternative to warfarin for patients with AF with a moderate-to-high risk of stroke.32 Rivaroxaban was noninferior to warfarin for preventing SSE both in the intent-to-treat population (2.1% and 2.4% a year, respectively; P < 0.0001 for noninferiority) and the per-protocol population (HR, 0.88; 95% CI, 0.74–1.03; P < 0.001 for noninferiority and P = 0.12 for superiority).76 Among patients treated with warfarin, INR values were within therapeutic range (2.0–3.0) for 55% of the time.76 The incidence of the primary safety end point, major and clinically relevant nonmajor bleeding, was similar in the rivaroxaban and warfarin groups (3.6% and 3.4%, respectively; P = 0.44).76 The reported rate of intracranial hemorrhage for rivaroxaban (0.5%) was lower than that for warfarin (0.7%). Major GIB was more common in the rivaroxaban group: 224 bleeding events (3.2%) compared with 154 in the warfarin group (2.2%, P < 0.001).76 As with dabigatran, routine blood coagulation monitoring is not required.75 Rivaroxaban should not be used concurrently with other anticoagulants or combined P-gp and strong cytochrome P450 3A4 (CYP3A4) inhibitors and inducers.75
Apixaban
In 2012, this orally administered factor Xa inhibitor received FDA approval to reduce the risk of SSE in patients with NVAF.71 In clinical trials, apixaban was given as a fixed dose of 2.5 mg or 5 mg twice daily without routine coagulation monitoring.32,77,78 The recommended dose is 5 mg twice daily; for patients with ≥2 of the following characteristics: age, 80 years or older, body weight ≤60 kg and serum creatinine ≥1.5 mg/dL, the recommended dose is 2.5 mg twice daily.75 The utility of apixaban for stroke prevention in NVAF was evaluated in 2 large, randomized, double-blind trials: Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE)77 and Apixaban Versus Acetylsalicylic Acid to Prevent Strokes (AVERROES).78 The ARISTOTLE trial compared apixaban with warfarin for the prevention of SSE in patients with NVAF and ≥1 additional risk factor for stroke.77 Apixaban reduced SSE by 21% compared with warfarin (HR, 0.79; 95% CI, 0.66–0.95; P = 0.01).77 Apixaban treatment compared with warfarin resulted in less major bleeding (2.1% and 3.1%, respectively; P < 0.001) and intracranial hemorrhage (0.33% and 0.8%, respectively). Apixaban was better tolerated than warfarin, and fewer patients were withdrawn from therapy.77 In AVERROES, the efficacy of apixaban 5 mg twice daily was compared with ASA (81–325 mg once daily) for the prevention of SSE in 5,599 patients with NVAF who were not candidates for warfarin treatment.78 The trial was stopped early on the recommendation of the Data and Safety Monitoring Board when clear benefits favoring apixaban became evident regarding stroke reduction (HR, 0.45; 95% CI, 0.32–0.62; P < 0.001).78 Rates of major bleeding (1.4% versus 1.2%) and intracranial hemorrhage (0.4% versus 0.4%) noted with apixaban were similar to those observed with ASA. Apixaban was better tolerated than ASA, with significantly fewer discontinuations of study drug.78 Simultaneous use of strong inducers of CYP3A4 and P-gp reduces blood levels of apixaban, and concurrent use should be avoided.75 Concurrent use of an anticoagulant with an antiplatelet agent or an NSAID generally increases bleeding risk, and clopidogrel, in particular, should be used with caution if coadministered with apixaban.75
CONCLUSIONS
Fear of bleeding is a widely acknowledged reason that many physicians do not prescribe, and many patients at risk do not receive, the requisite preventative anticoagulant therapy. Although all anticoagulant therapies necessarily involve some degree of bleeding risk, anticoagulation-related bleeding risk may be lowered by consistent use of evidence-based stratification schemes designed to help physicians identify patients who require anticoagulation to reduce the risk of stroke, predict the risk of bleeding during anticoagulant therapy and take preventive measures accordingly. Medication adherence may be fostered with the use of one of the new fixed-dose anticoagulants rather than warfarin, which requires dose adjustment and monitoring to maintain anticoagulant effect within a narrow therapeutic window. Education plays a key role in minimizing patient susceptibility, helping patients understand the importance of lifelong anticoagulation and the need for consistent lifestyle measures and raising awareness of signs and symptoms of adverse events including bleeding. As more evidence emerges from clinical trials, clinical guidelines may be expected to converge and clarify optimal approaches to long-term anticoagulant care.
ACKNOWLEDGMENTS
The authors acknowledge the writing and editorial assistance of Rosemary Perkins of Envision Scientific Solutions, whose services were funded by Boehringer Ingelheim Pharmaceuticals, Inc.
Footnotes
The authors have no financial or other conflicts of interest to disclose.
REFERENCES
- 1.Rowan SB, Bailey DN, Bublitz CE, et al. Trends in anticoagulation for atrial fibrillation in the US. J Am Coll Cardiol 2007;49:1561–5. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 3.Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123:638–45. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013;127:143–52. [DOI] [PubMed] [Google Scholar]
- 5.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006;37:577–617. [DOI] [PubMed] [Google Scholar]
- 6.Freeman WD, Aguilar MI. Prevention of cardioembolic stroke. Neurotherapeutics 2011;8:488–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 8.Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation 2006;114:e257–354. [DOI] [PubMed] [Google Scholar]
- 9.Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran). Circulation 2011;123:1144–50. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt GH, Akl EA, Crowther M, et al. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 12.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011;58:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation 2004;110:2287–92. [DOI] [PubMed] [Google Scholar]
- 14.Waldo AL, Becker RC, Tapson VF, et al. ; NABOR Steering Committee. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol 2005;46:1729–36. [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Ann Intern Med 1999;131:927–34. [DOI] [PubMed] [Google Scholar]
- 16.Hylek EM, D'Antonio J, Evans-Molina C, et al. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke 2006;37:1075–80. [DOI] [PubMed] [Google Scholar]
- 17.Birman-Deych E, Radford MJ, Nilasena DS, et al. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke 2006;37:1070–4. [DOI] [PubMed] [Google Scholar]
- 18.Walker AM, Bennett D. Epidemiology and outcomes in patients with atrial fibrillation in the United States. Heart Rhythm 2008;5:1365–72. [DOI] [PubMed] [Google Scholar]
- 19.Williams CJ, Reynolds MW, Sander SD, et al. The extent of warfarin use and its effectiveness within atrial fibrillation patients from a US nationally representative sample. Presented at: American College of Cardiology 58th Annual Scientific Session; March 29-31, 2009; Orlando, FL.
- 20.Nieuwlaat R, Capucci A, Lip GYH, et al. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J 2006;27:3018–26. [DOI] [PubMed] [Google Scholar]
- 21.Fitch K, Broulette J, Penson BS, et al. Utilization of anticoagulant therapy in Medicare patients with nonvalvular atrial fibrillation. Am Health Drug Benefits 2012;5:157–68. [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy MF. Warfarin utilization in Medicare patients with nonvalvular atrial fibrillation: sentinel data from an administrative claims base. Am Health Drug Benefits 2012;5:167–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Schulman S, Beyth RJ. Risk of bleeding with long-term antithrombotic therapy in atrial fibrillation. Eur Heart J Suppl 2005;7(suppl C):C34–40. [Google Scholar]
- 24.Mohr JP, Albers GW, Amarenco P, et al. American Heart Association Prevention Conference. IV. Prevention and rehabilitation of stroke. Etiology of stroke. Stroke 1997;28:1501–6. [DOI] [PubMed] [Google Scholar]
- 25.Garcia DA, Witt DM, Hylek E, et al. Delivery of optimized anticoagulant therapy: consensus statement from the Anticoagulation Forum. Ann Pharmacother 2008;42:979–88. [DOI] [PubMed] [Google Scholar]
- 26.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med 2007;167:1414–9. [DOI] [PubMed] [Google Scholar]
- 27.Darkow T, Vanderplas AM, Lew KH, et al. Treatment patterns and real-world effectiveness of warfarin in nonvalvular atrial fibrillation within a managed care system. Curr Med Res Opin 2005;21:1583–94. [DOI] [PubMed] [Google Scholar]
- 28.Devereaux PJ, Anderson DR, Gardner MJ, et al. Differences between perspectives of physicians and patients on anticoagulation in patients with atrial fibrillation: observational study. BMJ 2001;323:1218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattellari M, Worthington J, Zwar N, et al. Barriers to the use of anticoagulation for nonvalvular atrial fibrillation: a representative survey of Australian family physicians. Stroke 2008;39:227–30. [DOI] [PubMed] [Google Scholar]
- 30.Choudhry NK, Anderson GM, Laupacis A, et al. Impact of adverse events on prescribing warfarin in patients with atrial fibrillation: matched pair analysis. BMJ 2006;332:141–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein JN, Greenberg SM. Should anticoagulation be resumed after intracerebral hemorrhage? Cleve Clin J Med 2010;77:791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katsnelson M, Sacco RL, Moscucci M. Progress for stroke prevention with atrial fibrillation: emergence of alternative oral anticoagulants. Circulation 2012;125:1577–83. [DOI] [PubMed] [Google Scholar]
- 33.Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke 1995;26:1471–7. [DOI] [PubMed] [Google Scholar]
- 34.Freeman WD, Aguilar M. Anticoagulation therapy for cardioembolic stroke prevention in the elderly: defining benefits and risks. Aging Health 2010;6:439–50. [Google Scholar]
- 35.Man-Son-Hing M, Laupacis A. Anticoagulant-related bleeding in older persons with atrial fibrillation: physicians' fears often unfounded. Arch Intern Med 2003;163:1580–6. [DOI] [PubMed] [Google Scholar]
- 36.Man-Son-Hing M, Nichol G, Lau A, et al. Choosing antithrombotic therapy for elderly patients who are at risk for falls. Arch Intern Med 1999;159:677–85. [DOI] [PubMed] [Google Scholar]
- 37.Delaney JA, Opatrny L, Brophy JM, et al. Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding. CMAJ 2007;177:347–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernández-Díaz S, García Rodríguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation. Arch Intern Med 2000;160:2093–9. [DOI] [PubMed] [Google Scholar]
- 39.Silverstein FE, Graham DY, Senior JR, et al. Misoprostol reduced serious gastrointestinal complications in patients with rheumatoid arthritis receiving nonsteroidal anti-inflammatory drugs: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 1995;123:241–9. [DOI] [PubMed] [Google Scholar]
- 40.Yeomans ND, Tulassay Z, Juhász L, et al. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiiflammatory drugs. N Engl J Med 1998;338:719–26. [DOI] [PubMed] [Google Scholar]
- 41.Langman MJ, Jensen DM, Watson DJ, et al. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA 1999;282:1929–33. [DOI] [PubMed] [Google Scholar]
- 42.Man-Son-Hing M, Laupacis A. Balancing the risks of stroke and upper gastrointestinal bleeding in older persons with atrial fibrillation. Arch Intern Med 2002;162:541–50. [DOI] [PubMed] [Google Scholar]
- 43.Goldhaber SZ. What's the “go to” anticoagulant for stroke prevention in atrial fibrillation? Thromb Haemost 2012;107:397–8. [DOI] [PubMed] [Google Scholar]
- 44.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. J Am Coll Cardiol 2011;57:e101–98. [DOI] [PubMed] [Google Scholar]
- 45.Camm AJ, Kirchhof P, Lip GYH, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360–420. [DOI] [PubMed] [Google Scholar]
- 46.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133(suppl 6):381S–453S. [DOI] [PubMed] [Google Scholar]
- 47.Gage BF, Birman-Deych E, Kerzner R, et al. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005;118:612–7. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs LG, Billett HH, Freeman K, et al. Anticoagulation for stroke prevention in elderly patients with atrial fibrillation, including those with falls and/or early-stage dementia: a single-center, retrospective observational study. Am J Geriatr Pharmacother 2009;7:159–66. [DOI] [PubMed] [Google Scholar]
- 49.Banerjee A, Lane DA, Torp-Pedersen C, et al. Net clinical benefit of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus no treatment in a “real world” atrial fibrillation population. Thromb Haemost 2012;107:584–9. [DOI] [PubMed] [Google Scholar]
- 50.Roob G, Lechner A, Schmidt R, et al. Frequency and location of microbleeds in patients with primary intracerebral hemorrhage. Stroke 2000;31:2665–9. [DOI] [PubMed] [Google Scholar]
- 51.Chapman N, Huxley R, Anderson C, et al. Effects of a perindopril-based blood pressure–lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke 2004;35:116–21. [DOI] [PubMed] [Google Scholar]
- 52.Furie KL, Goldstein LB, Albers GW, et al. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012;43:3442–53. [DOI] [PubMed] [Google Scholar]
- 53.Beasley BN, Unger EF, Temple R. Anticoagulant options—why the FDA approved a higher but not a lower dose of dabigatran. N Engl J Med 2011;364:1788–90. [DOI] [PubMed] [Google Scholar]
- 54.U.S. Food and Drug Administration. Summary Minutes of the Cardiovascular and Renal Drugs Committee. 2010. Available at: www.fda.gov/downloads/Advisory%20Committees/CommitteesMeetingMaterials/%20Drugs/CardiovascularandRenalDrugs%20AdvisoryCommittee/UCM236322.pdf. Accessed December 5, 2012.
- 55.Qureshi W, Mittal C, Patsias I, et al. Restarting anticoagulation and outcomes after major gastrointestinal bleeding in atrial fibrillation. Am J Cardiol 2014;113:662–8. [DOI] [PubMed] [Google Scholar]
- 56.Glund S, Stangier J, Schmohl M, et al. A specific antidote for Dabigatran: Immediate, complete and sustained reversal of dabigatran induced anticoagulation in healthy male volunteers. Dallas, TX: American Heart Association (AHA) 2013 Scientific Sessions; 2013. [Google Scholar]
- 57.Crowther M, Vandana M, Michael K, Genmin L, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of rivaroxaban-induced anticoagulation in Healthy subjects by Andexanet Alfa (PRT064445), an Antidote for Fxa inhibitors. 55th ASJ Annual Meeting and Exposition; November 15, 2013; New Orleans, LA.
- 58.Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med 2005;165:1095–106. [DOI] [PubMed] [Google Scholar]
- 59.Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449–57. [PubMed] [Google Scholar]
- 60.Atrial Fibrillation Investigators. The efficacy of aspirin in patients with atrial fibrillation: analysis of pooled data from 3 randomized trials. Arch Intern Med 1997;157:1237–40. [PubMed] [Google Scholar]
- 61.Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(suppl 3):204S–33S. [DOI] [PubMed] [Google Scholar]
- 62.Flockhart DA, O'Kane D, Williams MS, et al. Pharmacogenetic testing of CYP2C9 and VKORC1 alleles for warfarin. Genet Med 2008;10:139–50. [DOI] [PubMed] [Google Scholar]
- 63.Ryan F, Byrne S, O'Shea S. Managing oral anticoagulation therapy: improving clinical outcomes. J Clin Pharm Ther 2008;33:581–90. [DOI] [PubMed] [Google Scholar]
- 64.Ghate SR, Biskupiak J, Ye X, et al. All-cause and bleeding-related health care costs in warfarin-treated patients with atrial fibrillation. J Manag Care Pharm 2011;17:672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobs JM, Stessman J. New anticoagulant drugs among elderly patients: is caution necessary?: Comment on “The use of dabigatran in elderly patients”. Arch Intern Med 2011;171:1287–8. [DOI] [PubMed] [Google Scholar]
- 66.International Warfarin Pharmacogenetics Consortium. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med 2009;360:753–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ansell J. Factor Xa or thrombin: is factor Xa a better target? J Thromb Haemost 2007;5(suppl 1):60–4. [DOI] [PubMed] [Google Scholar]
- 68.Weitz JI. Factor Xa or thrombin: is thrombin a better target? J Thromb Haemost 2007;5(suppl 1):65–7. [DOI] [PubMed] [Google Scholar]
- 69.U.S. Food and Drug Administration. FDA News Release. October 19, 2010. FDA approves Pradaxa to prevent stroke in people with atrial fibrillation. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm230241.htm. Accessed May 2, 2013.
- 70.U.S. Food and Drug Administration. FDA News Release. November 4, 2011. FDA approves Xarelto to prevent stroke in people with common type of abnormal heart rhythm. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm278646.htm. Accessed May 2, 2013.
- 71.U.S. Food and Drug Administration. FDA News Release. December 28, 2012. FDA approves Eliquis to reduce the risk of stroke, blood clots in patients with non-valvular atrial fibrillation. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm333634.htm. Accessed May 2, 2013.
- 72.Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 73.Connolly SJ, Ezekowitz MD, Yusuf S, et al. ; Randomized Evaluation of Long-Term Anticoagulation Therapy Investigators. Newly identified events in the RE-LY trial. N Engl J Med 2010;363:1875–6. [DOI] [PubMed] [Google Scholar]
- 74.Hart RG, Diener H-C, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran. The RE-LY Trial. Stroke 2012;43:1511–7. [DOI] [PubMed] [Google Scholar]
- 75.Walenga JM, Adiguzel C. Drug and dietary interactions of the new and emerging oral anticoagulants. Int J Clin Pract 2010;64:956–67. [DOI] [PubMed] [Google Scholar]
- 76.Patel MR, Mahaffey KW, Garg J, et al. ; for the ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 77.Granger CB, Alexander JH, McMurray JJV, et al. ; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 78.Connolly SJ, Eikelboom J, Joyner C, et al. ; AVERROES Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–17. [DOI] [PubMed] [Google Scholar]


