Abstract
Thymic development requires bidirectional interaction or cross-talk between developing T cells and thymic stromal cells, a relationship that has been best characterized for the interaction between thymocytes and thymic epithelial cells (TECs). We have characterized here the requirement for similar cross-talk in the maintenance and function of thymic B cells, another population that plays a role in selection of developing thymic T cells. We found that maintenance of thymic B cells is strongly dependent upon the presence of mature single positive (SP) thymocytes and on the interactions of these T cells with specific antigen ligand. Maintenance of thymic B cell number is strongly dependent upon B cell-autonomous expression of CD40, but not MHCII, indicating that direct engagement of CD40 on thymic B cells is necessary to support their maintenance and proliferation. Thymic B cells can mediate negative selection of superantigen-specific self-reactive SP thymocytes, and we show that CD40 expression on B cells is critical for this negative selection. Cross-talk with thymic T cells is thus required to support the thymic B cell population through a pathway that requires cell-autonomous expression of CD40, and that reciprocally functions in negative selection of autoreactive T cells.
Introduction
Thymocytes undergo a series of developmental stages through interactions with major histocompatibility complex (MHC)-expressing antigen-presenting cells (APCs), resulting in the generation of mature T lymphocytes and selection of the T cell repertoire (1). APCs expressing a broad spectrum of self-antigens are responsible for the establishment of central tolerance through depletion of high affinity self-reactive T cells. This results in the selection of T cells expressing receptors recognizing a universe of foreign antigens in association with self MHC in the absence of autoreactivity. It has been well documented that medullary thymicepithelial cells (mTECs) and dendritic cells (DCs) are APCs that play important roles in the induction of central tolerance (2–6). Although B cells also reside in the thymus in normal mice and humans (7), less attention has been paid to the thymic B cell population. However, several reports have described a role for thymic B cells in thymocyte negative selection specific for endogenous mammary tumor virus (Mtv) superantigens and in model systems which have been genetically engineered so that antigen is specifically presented by B cells (8–10). In addition, it has recently been demonstrated that thymic B cells are capable of presenting naturally expressed self-antigens directly to T cells, performing as an efficient APC for antigens captured via B cell receptors (BCR) (11). These findings identify the importance of thymic B cells in shaping the T cell repertoire. Indeed, a deficiency of thymic B cells has been observed in animal models of autoimmune diseases such as diabetes and lupus, where it has been suggested that thymic B cells may participate in establishing central tolerance (12, 13).
The number of B cells in the normal mouse thymus is approximately 0.1–0.3% of thymocytes, similar to the number of DCs or TECs (14, 15), and it has been reported that the majority of these B cells develop intra-thymically (11). The mechanisms supporting homeostasis of thymic B cells are not well understood. Previous studies have shown that T cell blasts support in vitro proliferation of thymic B cells (15), suggesting that T cell presence is important for the regulation of the thymic B cell population. This led us to hypothesize that there is a bidirectional interaction or cross-talk between thymic T cells and thymic B cells similar to that reported between T cells and mTECs (16–20): that thymic B cells interact with T cells to mediate negative selection of autoreactive T cells, and thymic T cells in turn support maintenance of the thymic B cell population. We therefore addressed requirements that mediate the maintenance of the thymic B cell population by focusing on the interaction between thymic B and T cells, and we further studied the mechanism by which thymic B cells reciprocally influence thymocyte negative selection.
We found that the presence of SP T cells is important in supporting thymic B cells and that engaging SP T cells with specific antigen induces a robust increase in the thymic B cell population. In probing the specific interactions that support thymic B cells, we found that cell-autonomous expression of CD40 on B cells was critical for maintenance of the thymic B cell population, but surprisingly that cell autonomous MHCII expression was not required. Our studies further showed that thymic B cells reciprocally affect thymocytes through their CD40-dependent function in superantigen-mediated negative selection. CD40 thus plays a central role in the bidirectional cross-talk between thymic B and T cells, supporting the B cell population that in turn affects selection of the thymic T cell repertoire.
Materials and methods
Reagents
Anti-CD4, CD8, CD45.1 (Ly5.2), B220 (CD45R), IgMb, IgD, Bcl-2, Vβ3 (B20.6), Vβ8 (MR5-2), Vβ11 (MR11-1), Vβ12, GL7 and Fas mAbs and APC and Pecy7 Streptavidin were purchased from BD Biosciences (San Jose, CA). Anti-IgG1a-biotin, IgG1b-biotin mAbs and streptavidin-HRP were purchased from BD Biosciences. Anti-CD45.2 (Ly5.1) and I-A/I-E mAbs were purchased from BioLegend. Anti-CD19, CD11c, CD11b, CD86 and CD5 mAb were purchased from eBioscience (San Diego, CA). Anti-cleaved Caspase-3 (Asp175) mAb was purchased from Cell Signaling Technology Inc. (Danvers, MA). Alexa 594 Streptavidin was purchased from Life Technologies.
Mice
C57BL/6 (B6), BALB/c (BALB), B6.Ly5.2, and B6.Ly5.1/Ly5.2 mice were obtained from the Frederick Cancer Research Facility (Frederick, MD). CD40L KO, CD40 KO and CD80/86 KO mice on both a B6 background and a BALB background were obtained from The Jackson Laboratory (Bar Harbor, ME). BALB CD40L KO mice were generated by backcrossing the B6 CD40L KO mice on to a BALB background for 5 generations. BALB CDCD80/CD86 deficient mice were a generous gift from Arlene Sharpe. CD40/CD80/CD86 triple KO mice (T KO) on a B6 and BALB background were generated and housed at BioqualInc (Rockville, MD) and Frederick Cancer Research Facility. TCR transgenic mice, AND/PCC and OTII/RIP-OVA and TCRα KO mice on a B6 background were previously described (21–24). MHCII KO and Igha congenic mice were purchased from Jackson Laboratory (Bar Harbor, ME). μMT B cell KO mice on a BALB background were purchased from Charles River Laboratories Inc. (Frederick, MD). All mice were maintained at BioqualInc and Frederick Cancer Research Facility. Protocols for animal care and use were conducted consistent with the Guide for the Animal Care and Use of Laboratory Animals of National Institutes of Health. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the National Institutes of Health (IACUC protocol number: EIB-029, FCRF11-021). No surgery was performed, and humane endpoints were followed per IACUC guidelines.
Cell preparation and flow cytometry analysis
Single-cell suspensions were prepared from thymus, spleen, lymph nodes (LN), and blood, and resuspended in FACS buffer (0.2% BSA and 0.01% sodium azide in HBSS without phenol red). Cells were washed and then used for staining. Staining was performed by incubating cells with anti-FcRmAb 24G2 (to prevent FcR-mediated binding) and with various combinations of FITC-, PE-, APC-, Pacific blue- and/or biotin-conjugated Abs for 30–40 min. When biotin-conjugated antibodies were used, the cells were washed 3 times followed by a 10-min-incubation with Streptavidin conjugated to Alexa 594, Pe-Cy7 or APC. Dead cells were excluded using propidium iodide exclusion. flow cytometry analysis was performed using a FACS Calibur, FACS Aria, LSRII, or LSR/Fortessa flow cytometer (BD Bioscience, CA) and data were analyzed using FloJo flow cytometry analysis software (Tree Star, OR).
Enrichment of thymic cells and electronic cell sorting
Thymic CD8 or CD4 cells were enriched by depletion of CD4 or CD8 thymocytes using anti-CD4 or -CD8 magnetic microbeads (MiltenyiBiotec, Germany) following the manufacturer’s instructions. Thymic B cells were stained and sorted using a FACS Aria flow cytometer (BD Bioscience).
Generation and analysis of radiation bone marrow chimeras
Radiation bone marrow chimeras were prepared as previously described (25). Chimeras were generated by reconstituting lethally irradiated (950 rad) mice with 7.5×106 T-depleted bone marrow cells. Chimeric mice were analyzed 8–12 weeks following reconstitution.
Analysis of antibody responses
Mice were immunized with nitrophenyl (NP)-conjugated keyhole limpet hemocyanin (KLH) (NP-KLH) (100 μg) plus ImjectAlum (Pierce, Rockford, IL) intraperitoneally (i.p.). Germinal Center (GC) B cells (B220+ GL7+ Fas+) in spleen were analyzed by flow cytometry at day 7 after immunization. NP-specific GC B cells were detected with NP-PE. NP-specific IgG1 Ab in serum at 3 weeks after immunization was measured by ELISA. Briefly, NP-BSA was coated on ELISA plate (Immulon 4HBX, Thermo, Waltham, MA) overnight, washed (0.5% Tween in PBS), and serially diluted serum applied and incubated 2 hours at room temperature. Anti-mouse IgG1a or IgG1b-biotin Ab followed by streptavidin-HRP reaction was used to specifically detect allotype specific IgG1. After wash step, 2, 2′-Azino-di-(3-ethylbenzthiazoline-6-sulfonate) (ABTS) substrate (KPL) was added to the plate and enzyme reaction was stopped by ABTS HRP Stop Solution (KPL). Optical density at 405 nm was measured with FLUOstar OPTIMA plate reader and software (BMG LABTECH, Germany).
Cell cycle assay
Cell cycle was analyzed by 4′-6-diamidino-2-phenylindole (DAPI: Sigma-Aldrich, St Louis, MO) staining. Briefly, enriched thymic B cells were stained for B220 and CD19 and then, cells were fixed with Fixation/Permeabilization buffer (eBioscience) for 30 min in the dark following the manufacturer’s instruction. Fixed cells were stained with DAPI (0.5ug/5×106 cells) and incubated for 40 min at room temperature in the dark. Cells were washed with FACS buffer. DNA content was analyzed by LSRII flow cytometer (BD Bioscience), doublets were excluded by gating on DAPI-width vs DAPI-area, and cell fractions in G1, S and G2/M phase were analyzed using FloJo FACS analysis software.
Apoptosis Analysis
Apoptosis was analyzed by cleaved-Caspase-3 staining following the manufacturer’s instruction. Briefly, thymocytes and enriched thymic B cells were stained for CD4 SP T cells, CD4−CD8− double negative 3 (DN3) thymocytes and thymic B cells, respectively. Cells were fixed with Fixation/Permeabilization buffer (eBioscience) for 30 min in the dark following the manufacturer’s instruction. Cells were then stained with anti-cleaved Caspase-3 mAb (Alexa Fluor 488 conjugated) (0.5μg/1×106 cells) and incubated for 30 min at 4°Cin dark. Cells were washed with FACS buffer. DNA content was analyzed by FACS Calibur flow cytometer (BD Bioscience).
Real-time PCR
Thymic and splenic B cells and thymic DCs were sorted by FACS Aria. Total RNA was isolated by means of High Pure RNA isolation kit (Roche, Branchbrug, NJ) and TURBO DNAse (Life Technologies, Grand Island, NY). cDNA was generated using SuperScript III Fast-Strand (Life Technologies), and real-time PCR was performed using C1000 Touch Thermal Cycler (Bio-Rad Laboratories Ltd): 95°C for 5 min, 40 cycles of 95°C for 1 min, 62°C for 1 min and 72°C for 1 min, 72°C for 7 min to cool. Results were analyzed with SDS 2.3 software (Applied Biosystems, Grand Island, NY) based on deltaCT calculation method (26). The primer sequences of Mtv8 used for real-time PCR were (Forward: 5′-CCTAACATTCACCTCTCGTGTGTTTG-3′, Reverse: 5′-CCTATTCAAAGGATTCAAAGGAGCTG-3′). GAPDH was used for internal control: GAPDH (Forward: 5′-CCGGTGCTGAGTATGTCGTG -3′, Riverse: 5′-CAGTCTTCTGGGTGGCAGTG-3′).
In vitro superantigen-mediated negative selection
Superantigen-induced negative selection was induced in vitro as previously described with some modifications (27, 28). Briefly, thymic B cells from BALB WT and BALB CD40 KO mice and splenic B cells from B6 WT, BALB WT and BALB CD40 KO mice were sorted with FACS Aria. CD4+CD8lo thymocytes sorted from B6 WT were mixed with thymic or splenic B cells at a 3:1 ratio based on preliminary titration and co-cultured at 37°C, 5% CO2 for 24–36h. Cultured cells were then stained for surface markers followed by Live/Dead Fixable Aqua Dead Cell Stain (Life Technologies) for 30 min and analyzed by LSRFORTESSA flow cytometer (BD Bioscience) for frequency of CD4 SP T cells expressing each TCRVβ.
Statistical analysis
Statistical differences were assessed by Student’s t test for comparisons between 2 groups. Values given were obtained by two-tailed paired t tests for chimera results and non-paired t test for the other results. One-way ANOVA was performed to compare multiple groups. P values<0.05 were regarded as significant.
Results
Antigen recognition by SP T cells positively regulates the thymic B cell population
B220hi CD19hiIgM+CD11c− B cells represent 0.1–0.3% of total thymocytes, with no cells of this phenotype present in μMT B cell KO mice, confirming their B cell identity (Supplemental Fig. 1A). Analysis of WT mice of different ages showed that thymic B cell frequency increased significantly from 1 through 6 months of age, while absolute thymic B cell number was relatively stable, reflecting progressive decrease in total thymocyte number with age. (Supplemental Fig. 1B). The phenotype of thymic B cells is distinct from spleen, lymph nodes, and peripheral blood B cells, for example in expression of CD5, and differs from that of CD5+ peritoneal B cells in terms of IgD and CD11b expression (Supplemental Fig. 1C). Thymic B cells are thus not simply an unselected population of recirculating peripheral B cells.
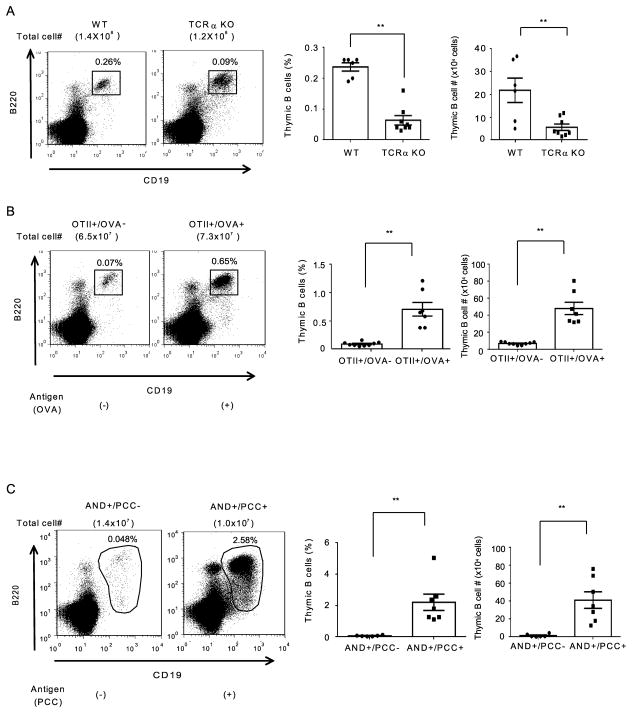
Previous reports that thymic B cells influence selection of thymic T cells (8–11) raised the question of whether interaction with T cells in turn affects the thymic B cell population, similar to the bidirectional ”cross-talk” described for interaction between SP T cells and mTECs (16, 18–20, 29, 30). We therefore examined TCRα KO mice lacking SP mature T cells to analyze the involvement of SP T cells in the regulation of thymic B cells. TCRα KO mice had a markedly reduced frequency and absolute number of thymic B cells compared with WT mice (Fig. 1A), indicating that SP T cells are required for the maintenance of thymic B cells.
Figure 1.
Antigen recognition by SP T cells positively regulates the thymic B cell population. Thymocytes from 10–12 week-old WT and TCRα KO mice, and 6–12 week-old OTII/RIP-OVA transgenic mice and AND/PCC TCR transgenic mice were stained with anti-B220 and anti-CD19 mAbs. Thymic B cell frequency and absolute number are shown. (A) Data are combined from 3 independent experiments. Total numbers of mice: WT: n=6 and TCRα KO: n=8. (B) Data are combined from 4 independent experiments, each using littermate mice. Total numbers of mice: OTII+/RIP-OVA− TCR transgenic mice: n=9 and OTII+/RIP-OVA+ TCR transgenic mice: n=7.(C) Data are combined from 5 independent experiments, each using littermate mice. Total numbers of mice: AND+/PCC− TCR transgenic mice: n=6 and AND+/PCC+ TCR transgenic mice: n=7. Dot plots: data are representative of the experiments. Bar graph: Mean ± SE. Statistical significance was determined using a Student’s non-paired two-tailed t-test. * P<0.05, ** P<0.01.
We further examined whether recognition of specific antigen by SP T cells has an effect on thymic B cell maintenance. We took advantage of two TCR transgenic mouse models to analyze thymic B cell frequency in the presence or absence of nominal specific antigen. In each of these systems, transgenic TCR recognition of high affinity MHCII-restricted nominal antigen results in negative selection of the TCR transgene-expressing T cells. OT-II mice express a TCR specific for chicken ovalbumin and RIP-OVA transgenics express OVA driven by rat insulin promoter (RIP), which is expressed by pancreatic is let cells but also by mTECs (23, 31). OT-II/RIP-OVA double transgenic mice expressing both TCR transgene and corresponding ligand OVA undergo negative selection of CD4 SP T cells (data not shown, (31)) and also had a substantially greater percent and absolute number of thymic B cells than mice expressing only the OT-II transgene, in absence of ligand (Fig. 1B). Thymic B cells in both OT-II and OT-II/RIP-OVA mice expressed IgM, indicating they are mature B cells. Thymic B cells in OT-II/RIP-OVA expressed higher levels of MHCII and CD86 than those in OT-II, suggesting that thymic B cells in OT-II/RIP-OVA are more highly activated than those in OT-II mice (Supplemental Fig. 2).
We also generated AND TCR/PCC double transgenic mice expressing TCR specific for pigeon cytochrome c (PCC) and PCC transgene driven by the ubiquitously expressed MHCI promoter. In these AND/PCC double transgenic mice, all cell types expressing MHCII could be capable of presenting PCC to AND TCR-expressing T cells and inducing negative selection of CD4 SP T cells (data not shown, (22)). AND/PCC mice similarly revealed a dramatic increase of thymic B cells relative to single transgenic AND mice (Fig. 1C). These results indicate that thymocyte activation by TCR recognition of antigen strongly influences the thymic B cell population.
Maintenance of thymic B cells does not require cell-autonomous MHCII expression
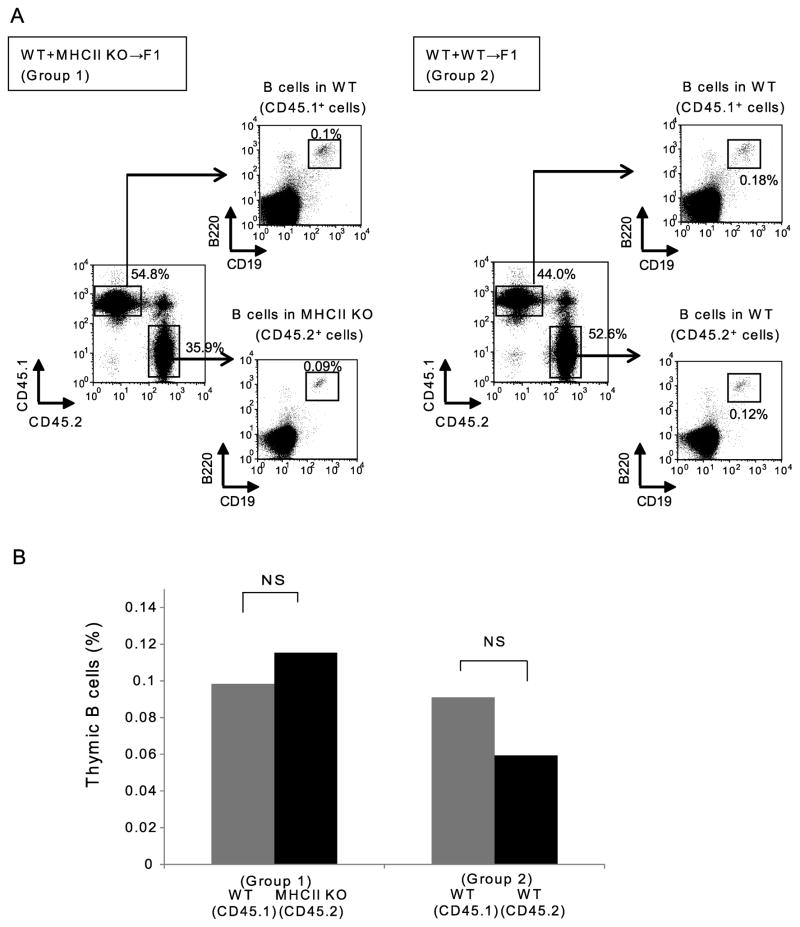
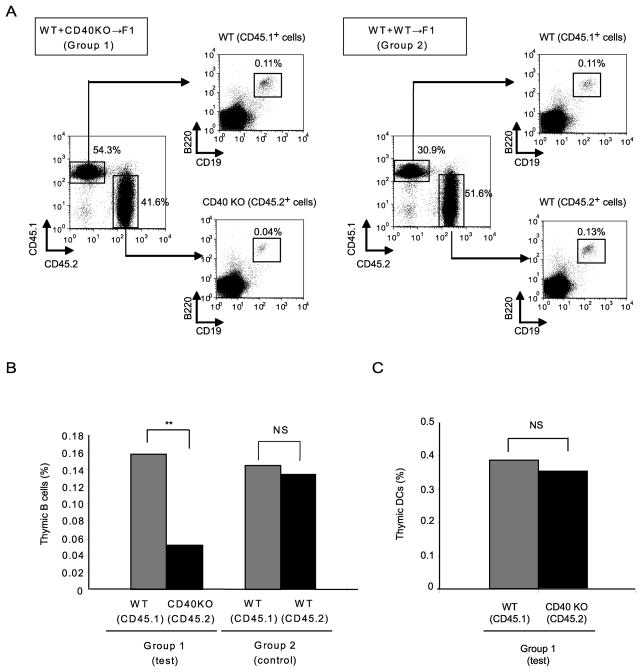
The T cell-dependent activation of peripheral B cells has been shown to require cognate interaction between T and B cells, involving both MHCII and CD40 expressed on responding B cells. Having observed in TCR knockout and transgenic model systems that T cell recognition of ligand is necessary for support of thymic B cells, we assessed in the context of normal T cell development and repertoire the requirements for expression of MHC and co-stimulatory molecules in supporting the thymic B cell population. We assessed the requirement for cell-autonomous expression of MHCII by B cells both in a T-dependent antibody response and in support of thymic B cells, using mixed BM chimeras. The mixed chimeras included two groups; an experimental group in which B6 WT (CD45.1) and B6 MHCII KO (CD45.2) donor-derived BM were used to reconstitute B6 WT (CD45.2 x CD45.1) recipients (Group 1) and a control mixed chimera in which B6 WT (CD45.1) and B6 WT (CD45.2) donors reconstituted B6 WT (CD45.2 x CD45.1) recipients (Group 2). To first test the requirement for expression of MHCII on B cells in T-dependent germinal center (GC) B cell responses, these chimeras were immunized with NP-OVA and assessed for MHCII requirements of T-dependent B cell immune responses. Analysis of NP-specific GC B cells by flow cytometry showed a strong predominance of WT over MHCII KO cells (Supplemental Fig. 3A and 3B), and analysis of anti-NP serum antibody in mixed allotype-congenic chimeras showed exclusive origin from WT B cells and not from MHCII-deficient B cells (Supplemental Fig. 3C). Thus, the MHCII-dependence of GC B cell response reflected a strong cell-autonomous requirement for MHCII. The requirements for MHCII expression for support of thymic B cells were assessed in non-immunized mice from the same chimeric groups. After 8–10 weeks, reconstituted chimeras were sacrificed and the frequency of the two donor-derived thymic B cell populations was analyzed by flow cytometry. In group 2 control chimeras, the frequency of thymic B cells did not differ significantly between B6 WT (CD45.1) donors and B6 WT (CD45.2) donors. Interestingly, in group 1 chimeras, the frequency of B6WT and B6 MHCII KO donor thymic B cells was also not significantly different (Fig. 2A and 2B). These results indicate that cell-autonomous expression of MHCII on thymic B cells is not required for their maintenance, in contrast to the requirements for cell-autonomous expression observed in GC B cell responses.
Figure 2.
Thymic B cells do not require cell-autonomous MHCII expression for their maintenance.(A) Thymocytes from mixed bone marrow chimeras were stained with anti-CD45.1 (WT) and anti-CD45.2 (WT or MHCII KO) Abs to distinguish the congenic donors. Both donor-derived thymocyte populations were further gated on B220+CD19+ thymic B cells. Group 1 (Left) Equal numbers of WT (CD45.1) + MHCII KO (CD45.2) bone marrow cells were used to reconstitute irradiated WT (CD45.1 x CD45.2) recipients; Group 2(Right) Equal numbers of WT (CD45.1) + WT (CD45.2) bone marrow cells were used to reconstitute irradiated WT (CD45.1 x CD45.2) recipients. Data are representative of 9 chimeras. (B) Thymic B cell frequency in each donor-derived population in Group 1 and Group 2. Data from two independent sets of chimeras consisting of 9 mice in each group were combined for this analysis. Statistical significance was determined using a Student’s paired two-tailed t-test. NS, not significant.
Thymic B cell maintenance is dependent upon CD40L-CD40 interaction
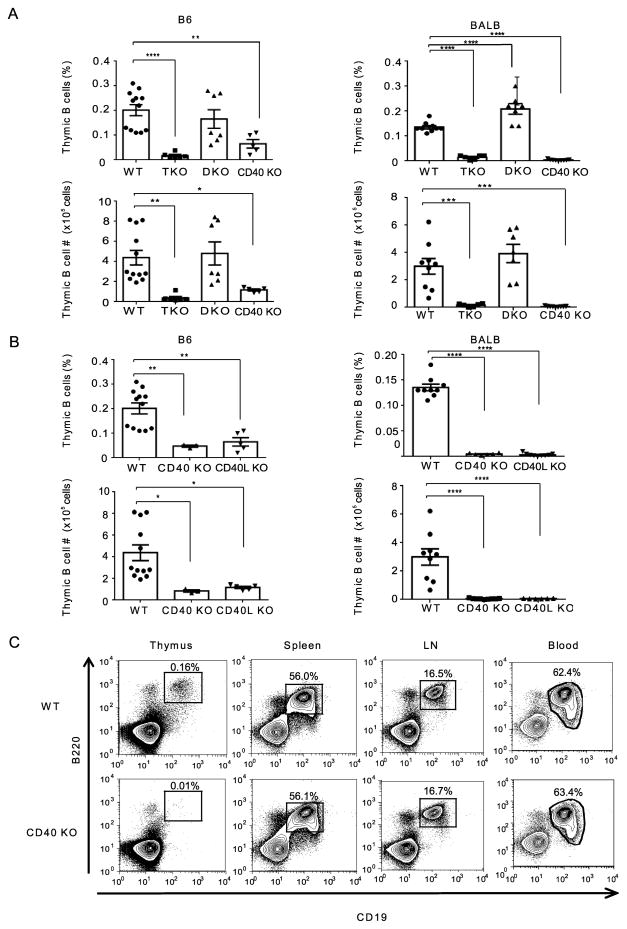
It has been demonstrated that activation of T cells by APCs generally involves at least two signaling events: one elicited by TCR recognition of peptide-MHC (p-MHC) complexes and the other by co-stimulatory molecules such as CD80/86/CD28 and CD40/CD40L (32, 33). We therefore next assessed the involvement of co-stimulatory signaling in the maintenance of thymic B cells. CD40/CD80/CD86 triple knockout (T KO) mice, deficient in both CD28-CD80/86 and CD40L-CD40 pathways, had profoundly lower thymic B cell frequency and fewer total thymic B cells than WT mice on both B6 and BALB backgrounds. When mice deficient in either CD28-CD80/86(D KO) or CD40L-CD40 (CD40 KO) interactions were compared, the frequency and number of thymic B cells in CD40 KO mice was dramatically reduced from WT levels, while CD80/86 KO (D KO) mice had thymic B cell populations which were not significantly different than WT mice (Fig. 3A). To confirm that the effect observed in CD40 KO mice reflected disruption of the CD40-CD40L pathway, we further examined thymic B cells in CD40L KO mice. Both B6 and BALB CD40L KO mice had greatly reduced frequencies of thymic B cells compared with B6 and BALB WT mice, respectively, resembling the CD40 KO effect in each strain (Fig. 3B). To analyze whether reduced B cell numbers in the absence of CD40 is unique to the thymus, we examined B cell frequency in thymus, spleen, lymph nodes (LN) and peripheral blood of CD40 KO and WT mice. As illustrated in Fig. 3C, B cell frequency in thymus was markedly lower in CD40 KO, compared with WT, whereas in spleen, LN and blood, there was no difference in B cell frequency between CD40 KO and WT, indicating that maintenance of B cells is highly dependent on CD40 signal in thymus but not in peripheral tissues. Taken together, these findings indicate that the CD40-CD40L signal pathway is highly and selectively important for the maintenance of thymic B cells.
Figure 3.
Thymic B cell maintenance is dependent upon CD40L-CD40 interactions.(A) Thymic B cell frequencies in WT, CD80/CD86/CD40 triple KO (T KO) CD80/CD86 double KO (D KO) and CD40 KO mice on a B6 background (Left) and BALB background (Right). (B) Thymic B cell frequencies in WT, CD40 KO mice and CD40L KO mice on B6 background (Left) and BALB background (Right). B6 WT: n=12, B6 T KO: n=6, B6 D KO: n=7, B6 CD40 KO: n=5 and B6 CD40L KO: n=3. Data are combined from 3 independent experiments. BALB WT: n=9, BALB T KO: n=6, BALB D KO: n=7, BALB CD40 KO: n=9 and BALB CD40L KO: n=6. Data are combined from 4 independent experiments. Mean ± SE. Statistical significance was determined using one-way ANOVA. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001. (C) Comparison of B cell frequencies between BALB WT and BALB CD40 KO mice in thymus, spleen, lymph nodes (LN) and blood. Data are representative of 3 independent experiments.
CD40 supports proliferation in thymic B cells
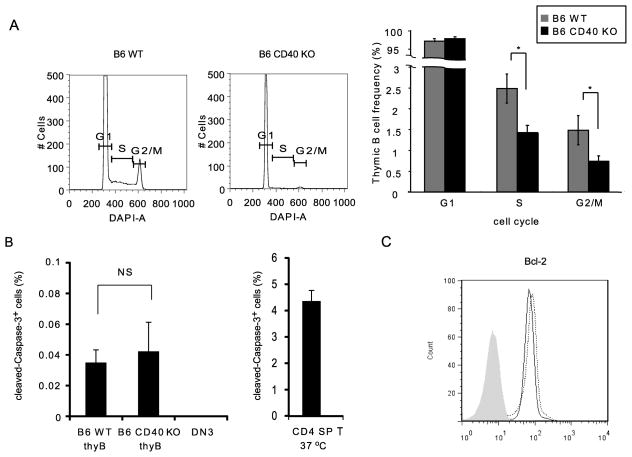
To investigate the mechanisms by which CD40 signals support the maintenance of thymic B cells, we determined whether the lower thymic B cell frequency in CD40 KO mice reflected deficient proliferation. We analyzed DNA content by DAPI staining of B220hiCD19hi thymic B cells in WT and CD40 KO mice. As shown in Fig. 4A, we found a decreased frequency of thymic B cells in the Sphase and G2/Mphase in CD40 KO mice, indicating that CD40 promotes cell cycle entry by thymic B cells. We next stained thymic B cells from WT and CD40 KO mice with cleaved-Caspase-3, a marker of apoptosis, and compared the frequency of cleaved-Caspase-3-expressing thymic B cells in WT and CD40 KO. We observed almost no cleaved-Caspase-3-expressing thymic B cells in CD40 KO mice or B6 WT mice, compared with cleaved-Caspase-3 expressing thymocytes cultured overnight as an apoptosis positive control (Fig 4B). We also examined Bcl-2 expression to assess possible impact on survival of thymic B cells, and found no difference in WT and CD40 KO mice (Fig 4C). Collectively, the results indicate that the lower thymic B cell frequency in CD40 KO mice reflects decreased thymic B cell cycle progression in the absence of CD40.
Figure 4.
CD40 supports proliferation in thymic B cells. CD4 cells were depleted from freshly explanted thymocytes pooled from 2–4 WT and CD40 KO mice using CD4 micro beads. CD4-depleted thymocytes were stained for B cell-surface molecules, B220 and CD19, fixed and permeabilized before intracellular staining. (A) Cell cycle analysis. CD4-depleted thymocytes were stained with DAPI for cell cycle analysis. Panels show DAPI staining profiles gated on B220+CD19+ thymic B cells from WT (Left) and CD40 KO (Right). Data are representative of 3 independent experiments. Graph shows thymic B cell frequency in each phase of cell cycles for WT (Gray) and CD40 KO (Black). Mean ± SE for n=3. Statistical significance was determined using a Student’s non-paired two-tailed t-test. * P<0.05. (B) Cleaved-Caspase-3 expression. Freshly explanted thymic B cells from WT and CD40 KO mice, freshly explanted WT double negative 3 thymocytes (DN3) and overnight incubated WT CD4SP T cells were stained with anti-cleaved-Caspase-3 Ab for apoptosis analysis. Graph showscleaved-Caspase-3 positive frequencies among B220+CD19+ thymic B cells, DN3 cells and CD4 SP T cells incubated overnight. CD4 SP T cells incubated overnight were used as an apoptosis positive control and freshly explanted DN3cells were used as an apoptosis negative control. Mean ± SE for n=3. Statistical significance was determined using a Student’s non-paired two-tailed t-test between WT and CD40 KO. NS, not significant.(C) CD4-depleted thymocytes from WT and CD40 KO mice were stained with anti-Bcl-2 mAb. Histogram shows Bcl-2 profile gated on B220+CD19+ thymic B cells. WT (Thick line) and CD40 KO (Dotted line), isotype control (Gray filled). Data are representative of 3 independent experiments.
Cell-autonomous CD40 expression is important for maintenance of thymic B cells
The defect in thymic B cells observed in CD40- or CD40L-deficient mice indicates that thymic B cell maintenance strongly depends on CD40. However, CD40 is expressed on multiple cell types, including B cells, DCs, macrophages, and mTECs in the thymus (34), so that CD40 on any of these cell types might influence the thymic B cell population. We previously demonstrated, using a WT/CD40 KO mixed chimera approach, that cell-autonomous expression of CD40 on peripheral B cells is essential for T cell-dependent antibody responses in vivo (35). Using this same approach we examined whether there is a cell-autonomous requirement for expression of CD40 on B cells in supporting thymic B cells. Mixed BM chimeras were generated in which B6 WT (CD45.1) and B6 CD40 KO (CD45.2) donor-derived BM cells (Group 1) or B6 WT (CD45.1) and B6 WT (CD45.2) BM donors (Group 2) co-exist in B6 WT (CD45.2 x CD45.1) recipients. After 8–12 weeks, reconstituted chimeras were sacrificed and the frequency of thymic B cells in both donor-derived populations was analyzed by flow cytometry. The frequency of thymic B cells derived from B6 CD40 KO donors was significantly lower than that derived from B6 WT donors, whereas in the control chimeras the frequency of thymic B cells did not differ significantly between B6 WT (CD45.1) donors and B6 WT (CD45.2) donors (Fig. 5A and 5B). There was no difference in the frequency of thymic DCs derived from B6 CD40 KO and B6 WT donors in mixed BM chimeras (Fig. 5C). These results indicate that cell-autonomous CD40 expression on thymic B cells is important for the maintenance of thymic B cells. The requirement for CD40 expression by thymic B cells is thus not solely the consequence of alterations in other cell populations that result from absence of CD40.
Figure 5.
Cell-autonomous CD40 expression is important for maintenance of thymic B cells. Group 1: BM cells from WT (CD45.1) and CD40 KO (CD45.2) were transplanted into irradiated WT (CD45.1 x CD45.2) mice. Group 2: BM cells from WT (CD45.1) and WT (CD45.2) were transplanted into WT (CD45.1 x CD45.2) mice as a control. Chimeras were analyzed for thymic B cell and DC frequencies 8–12 weeks after reconstitution. (A) Thymocytes from recipients were stained with anti-CD45.1 (WT) and anti-CD45.2 (WT or CD40 KO) mAbsto distinguish congenic donors. Each donor-derived thymocyte population was gated on B220+CD19+ thymic B cells or CD11c+ DCs. Group 1: left and Group 2: right. Graphs show (B) thymic B cell frequency in Group 1 and 2 and (C) thymic DC frequency in Group 1. Data from 3 independent sets of chimeras consisting of 13 mice in each group were combined for this analysis. Statistical significance was determined using a Student’s paired two-tailed t-test. ** P<0.01.
CD40 on B cells is essential for negative selection of superantigen-reactive T cells
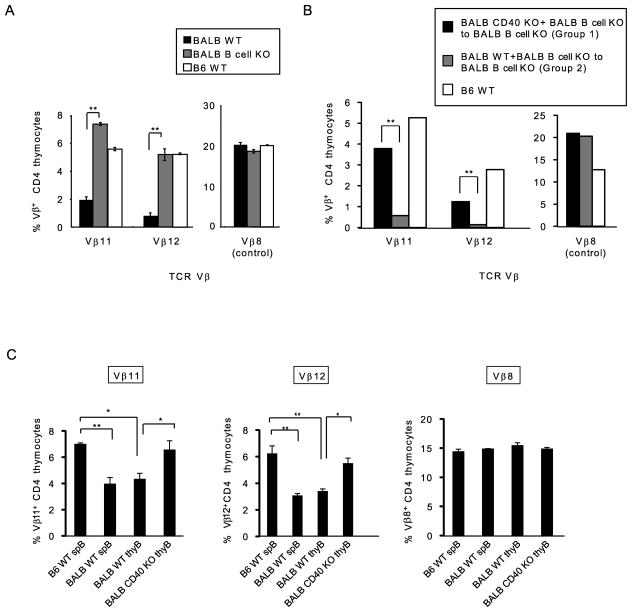
Our lab and others have previously demonstrated that CD40-CD40L interactions are required for negative selection of developing thymocytes which recognize mouse mammary tumor virus (Mtv)-encoded superantigen (25, 36). Having shown that CD40 expression on B cells is important for their thymic maintenance, we next examined the interrelationship of CD40-CD40L interactions, B cells and superantigen-mediated negative selection. We began by assessing superantigen-mediated negative selection in BALB WT and μMT B cell KO mice. In BALB WT mice, thymocytes expressing TCR that recognize endogenous retroviral Mtv antigens in association with MHCII (I-E) molecules are deleted during thymic development (37). BALB WT mice endogenously express Mtv-6, 8 and 9, and are MHCII I-E+. Therefore BALB mice delete thymocytes expressing TCRs specific for each Mtv product, including Vβ11 (Mtv-8 and 9) and Vβ12 (Mtv-8 and 9). Previous work has demonstrated that Mtv-8 and 9 are expressed only in B cells and not thymic DCs or TECs (38), and we therefore focused our studies on deletion of Vβ11 and Vβ12-expressing thymocytes. The frequency of Vβ8-expressing thymocytesis not influenced by Mtvs and can be measured as a control for the specificity of deletion. As illustrated in Fig. 6A, CD4+ SP thymocytes expressing Vβ11 and Vβ12 were deleted in BALB WT mice, relative to their expression levels in B6 WT mice (B6 WT mice express Mtv-8 and 9 but are MHCII I-E− and thus do not undergo superantigen-mediated negative selection). In contrast, in BALB μMT B cell KO mice, deletion of CD4+ SP thymocytes expressing Vβ11 and Vβ12 was impaired (Fig. 6A). These results indicate that B cells are necessary for efficient negative selection of T cells reactive with Mtv-8 and 9 superantigens.
Figure 6.
CD40 on B cells is essential for superantigen-mediated negative selection of CD4 T cells.
(A) Superantigen-mediated negative selection was analyzed in BALB WT, BALB B cell KO, and B6 WT mice. Thymocytes from BALB WT, BALB B cell KO and B6 WT mice were stained with anti-TCRVβ8, Vβ11 and Vβ12mAbs. Graph shows frequency of thymicCD4 SP T cells expressing TCRVβ8, Vβ11 and Vβ12 in BALB WT (Black), BALB B cell KO (Gray) and B6 WT (White). TCRVβ8-expressing CD4 SP T cells are shown as a control not affected by superantigen-specific deletion. Mean ± SE, n=3 per strain. Statistical significance was determined using a Student’s paired two-tailed t-test.** P<0.01.(B) BM cells from BALB B cell KO and BALB CD40 KO were transplanted into irradiated B cell KO mice, generating mice specifically deficient for expression of CD40 on B cells (Group 1). BM cells from BALB B cell KO and BALB WT were transplanted into B cell KO mice as a control (Group 2). Chimeras were studied 8–12 weeks after reconstitution. Frequency of thymic CD4 SP T cells expressing TCRVVβ8, Vβ11 and Vβ12 in Group 1 (B cell KO+CD40 KO BM donors) (Black), Group 2 (B cell KO+WT BM donors) (Gray), and control B6 WT (White). The graph shows mean ± SE for 4 mice per recipient in each group. Statistical significance was determined using a Student’s paired two-tailed t-test.** P<0.01.(C) In vitro negative selection was performed using BALB WT and BALBCD40 KO thymic B cells. B6 CD4+CD8lo DP thymocytes, BALB WT and CD40 KO thymic B cells and B6 and BALB splenic B cells were sorted, and thymocytes and B cells were co-cultured for 24–36h. The TCRVβ11- and TCRVβ12-expressing CD4 SP T cell frequencies were analyzed by flow cytometry to assess the T cell deletion efficiency induced by thymic B cells of BALB WT and BALB CD40 KO mice. BALB splenic B cells were used as a positive control and B6 splenic B cells were used as a negative control for superantigen-mediated negative selection. TCRVβ8-expressing CD4 SP T cell frequency was assessed as a control since these T cells are not deleted by superantigen-mediated negative selection. TCRVβ11 (Left), TCRVβ12 (Middle) and TCRVβ8 (Right). SpB: splenic B cells and thy B: thymic B cells. Data are representative of 3 independent experiments. Mean ± SE for n=3. Statistical significance was determined using one-way ANOVA. *P<0.05, ** P<0.01.
We next examined whether CD40 specifically expressed on B cells would be critical for superantigen-mediated negative selection. We generated mixed BM chimeras which were specifically deficient in CD40 expression on B cells by mixing BALB CD40 KO and BALB B cell KO donor-derived BM cells and injecting them into BALB B cell KO recipients (Group 1). Control (Group 2) chimeras differed only in that CD40 was expressed on all cell types, including B cells, using BALB WT and BALB B cell KO donor-derived cells to reconstitute BALB B cell KO recipients. After 8–12 weeks, reconstituted chimeras were sacrificed and analyzed for superantigen-mediated negative selection. Group 1 chimeras, in which B cells were CD40-deficient, had a marked defect in the deletion of CD4+ SP T cells expressing Vβ11 and Vβ12; whereas, in group 2 chimeras, in which B cells were CD40+, CD4+ SP T cells expressing Vβ11 and Vβ12 were efficiently deleted (Fig. 6B). These findings indicate that B cells, specifically B cells expressing CD40, are important for superantigen-mediated negative selection.
The profound defect of superantigen-mediated negative selection in the absence of CD40 on B cells could reflect a specific function of CD40 and/or could be the result of the reduced number of thymic B cells in CD40 KO mice (Supplemental Fig. 4A). To determine whether CD40 on thymic B cells is directly involved in mediating superantigen-specific negative selection, we analyzed negative selection in vitro. We compared the ability of WT and CD40 KO thymic B cells to mediate negative selection in CD4+CD8lo thymocytes from B6WT mice, which do not express I-E and are therefore not tolerant to Mtv superantigens (39, 40). Co-culture of B6 CD4+CD8lo DP thymocytes with BALB superantigen-expressing WT splenic B cells resulted in selective deletion of CD4 SP T cells expressing TCRVβ11 and 12, when compared to T cells cultured with non-superantigen-presenting B6 splenic B cells (Fig. 6C). Deletion induced by thymic B cells was similar to that induced by BALB splenic B cells (Fig. 6C), indicating that thymic B cells have the ability to induce negative selection. In contrast, the same number of CD40 KO BALB thymic B cells failed to induce deletion of TCRVβ11– and 12-expressing CD4 SP T cells (Fig. 6C). Superantigen-specific negative selection by thymic B cells is thus dependent upon CD40 expression.
CD40 might affect negative selection by B cells through regulation of superantigen-MHCII ligand expression on thymic B cells. WT and CD40 KO thymic B cells did not differ in cell surface MHCII expression, excluding differences in overall MHCII expression as a mechanism mediating the CD40 effect (Supplemental Fig. 4B). We analyzed mRNA expression of Mtv-8 (which mediates the deletion of TCRVβ11- and TCRVβ12-expressing thymocytes) on WT and CD40 KO thymic B cells. Real-time PCR showed that Mtv-8 mRNA expression on CD40 KO thymic B cells was not significantly different from that on WT thymic B cells (Supplemental Fig. 4C). These results indicate that the defect in superantigen-mediated negative selection in the absence of CD40 is not due to lower Mtv expression on thymic B cells, but rather that CD40 signals function to promote the ability of B cells to serve as effective antigen presenting cells for developing thymocytes.
Discussion
We have defined here the requirements for maintenance and function of the thymic B cell population and have demonstrated that thymic B cells and developing thymocytes engage in bidirectional cross-talk that substantially affects both populations. Thymic B cells are highly dependent upon mature SP thymocytes and upon recognition of specific antigen by these thymocytes. In defining the molecular interactions that mediate cross-talk between thymic B cells and developing T cells, we found that thymic B cells, but not other B cell populations, are uniquely dependent upon cell-autonomous expression of CD40, while there was no cell-autonomous role for MHCII. Reciprocally, negative selection of self-superantigen-specific T cells is highly dependent upon the presence of thymic B cells and specifically upon CD40 expression by thymic B cells. Thus, interaction between developing T cells and thymic B cells is critical for development of both populations, and cognate recognition of CD40 plays a central role in this cross-talk.
Previous work has identified situations in which thymic B cells are significantly affected as a result of perturbed thymocyte development. For example, disrupted Notch signaling is associated with a dramatic reduction in all subsets of thymocytes and a concomitant increase in thymic B cells that appear to derive, at least in part, from redirection of thymic settling progenitors from a T cell to B cell fate (41–44). On the other hand, mice in which T cell development is compromised because of a failure to express a functional TCR, rather than failure to adopt a T cell fate, display a significant decrease in thymic B cell numbers (45). These findings suggest that a normal thymic B cell population is linked to a normally developing thymocyte population. Our studies reported here address the cellular and molecular interactions that mediate the role of developing thymocytes in the maintenance of the thymic B cell population. We find that TCRα KO mice, which lack SP thymocytes, have a dramatic reduction in thymic B cell numbers. Further, in TCR transgenic mice, presence of the nominal Ag ligand for that TCR resulted in robust increase in the number of thymic B cells. Thus, maintenance of the thymic B cell population critically depends on TCR signaling in developing thymocytes.
The interaction of T and B cells has been extensively studied in thymic-dependent peripheral B cell responses. Here, immunoglobulin class switching and T-dependent antibody responses depend on cell-autonomous B cell expression of both CD40 and MHCII, as demonstrated by our own lab and others (35, 46, 47). This has been interpreted in a model of cognate interaction between T helper cells and B cells mediated by direct interaction with both p-MHC and CD40 on responding B cells. However, when we examined the cellular requirements for CD40 and MHCII on thymic B cells in the studies presented here, again using mixed BM chimeras, we found that B cells lacking CD40, but not those lacking MHCII, were at a disadvantage relative to their WT counterparts for maintenance in the thymus. Thus, support of thymic B cells requires direct interaction with CD40 on those B cells, but does not require direct or cognate recognition of MHCII. The finding of a cell-autonomous requirement for CD40 expression on thymic B cells is important in elucidating the mechanism by which CD40 functions in thymic B cell maintenance. CD40 is expressed by multiple cell types in the thymus, including DCs and mTECs, as well as B cells, and global deletion of CD40 could therefore have effects involving any of these cell types. It has in fact been demonstrated that CD40 plays an important role in the previously characterized cross-talk between thymic T cells and mTECs (19, 20). Moreover, a very recent study has reported the involvement of the CD40-CD40L pathway in cross-talk between thymic T cells and thymic DCs (48). The current finding of CD40 function on thymic B cells in mixed chimeras demonstrates a newly identified role of CD40 that is direct, and not the secondary consequence of CD40 effects on other populations, such as mTECs. This role of CD40 in maintaining B cells is specific to the thymic B cell population. Although T-dependent GC B cell responses are highly CD40-dependent, the overall homeostatic regulation of peripheral B cell number is not affected by CD40 deletion, identifying the uniqueness of CD40-dependent B cell regulation in the thymic environment. Schwartz et al (49) recently reported that CD4 T cells and CD40-CD40L interactions can play a role in selection and homeostasis of peripheral B cells. The effect of CD40 expression on peripheral B cells was not apparent in direct analysis of CD40-deficient mice, consistent with our observations reported here, but was observed during homeostatic B cell expansion under lymphopenic conditions, in competitive bone marrow chimeras relatively late after reconstitution, or in combination of CD40 deletion and impaired BCR signaling.
Our findings suggest that support for thymic B cells is independent of a requirement that they express specific Ag-MHC recognized by thymic T cells, and therefore independent of the B cell receptor (BCR) specificity required for efficient Ag presentation by B cells. The thymus would thus maintain a B cell population that expresses a diverse BCR repertoire capable of high affinity recognition and presentation of antigens encountered in the thymus, important for mediating negative selection and self-tolerance in developing T cells.
Previous studies have demonstrated that CD40 is important for superantigen-mediated negative selection of developing thymocytes (25, 36). Our observation that thymic B cells are profoundly reduced in the absence of CD40-CD40L interactions coupled with the finding that B cells are important in mediating deletion of superantigen-reactive thymocytes prompted us to further examine the role of CD40 in B cell-mediated negative selection. Results from our studies of in vivo and in vitro negative selection demonstrated that WT thymic B cells were significantly more effective than CD40 KO thymic B cells in eliciting deletion of superantigen-reactive thymocytes, despite equivalent Mtv mRNA levels and MHCII expression in these populations. Thus it appears that CD40 signaling is not only important for maintenance of thymic B cells but also for allowing these cells to effectively present antigens to developing thymocytes.
In addition to superantigen-mediated negative selection, B cells have been shown to mediate negative selection of developing T cells for other B cell-specific antigens. When MHCII I-Ea is expressed only on B cells, thymocytes expressing TCRVβ5 and Vβ11, which recognize I-Ea, are significantly decreased (8). Similarly, BMOG mice which were engineered to express the myelin oligodendrocyte glycoprotein peptide 35–55 of (MOGp33-55) selectively by B cells showed robust deletion of thymocytes expressing a TCR transgene specific for MOGp33-55(9). More recently, Perera et al. have provided evidence that thymic B cells can function to tolerize developing thymocytes via BCR-mediated uptake of self-antigens and presentation of peptides derived from these antigens. Taken together, these results as well as our own findings regarding deletion of superantigen-reactive thymocytes suggest that thymic B cells play a significant role in tolerization of the nascent T cell repertoire. In addition to involvement in negative selection, a recent study has reported that thymic B cells contribute to T cell tolerance by influencing regulatory T cells(50).
We have identified a bidirectional cross-talk between thymic B cells and SP T cells, and have demonstrated that CD40-CD40L interaction plays a central role in this cross-talk. Engagement of ligand by TCR expressed on developing T cells, and consequent activation of these T cells, is important in supporting thymic B cells. Notably, cell-autonomous expression of CD40, but not MHCII, on B cells is critical for this maintenance of the thymic B cell population. Thymic B cells reciprocally mediate CD40-dependent superantigen-specific negative selection, supporting central self-tolerance in developing thymocytes.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the NIH.
We thank Dr. Bishop and Dr. Takahama for critical reading of the manuscript and Tony Adams and Larry Granger for flow cytometry.
Abbreviations used in this article
- mTEC
medullary thymic epithelial cell
- SP
single positive
- DC
dendritic cell
- Mtv
mammary tumor virus
- LN
lymph node
- BM
bone marrow
- GC
germinal center
- NP-KLH
nitrophenyl-conjugated keyhole limpet hemocyanin
- RIP
rat-insulin promotor
- PCC
pigeon cytochrome c
- p-MHC
peptide-MHC
- PC
peritoneal cavity
- WT
wild type
References
- 1.Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see) Nature reviews Immunology. 2014;14:377–391. doi: 10.1038/nri3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohme J, Schuhbaur B, Kanagawa O, Benoist C, Mathis D. MHC-linked protection from diabetes dissociated from clonal deletion of T cells. Science. 1990;249:293–295. doi: 10.1126/science.2115690. [DOI] [PubMed] [Google Scholar]
- 3.Jenkinson EJ, Anderson G, Moore NC, Smith CA, Owen JJ. Positive selection by purified MHC class II+ thymic epithelial cells in vitro: costimulatory signals mediated by B7 are not involved. Developmental immunology. 1994;3:265–271. doi: 10.1155/1994/75434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doffinger R, Klein TC, Pepys MB, Casanova JL, Kyewski BA. The MHC class II-restricted T cell response of C57BL/6 mice to human C-reactive protein: homology to self and the selection of T cell epitopes and T cell receptors. Molecular immunology. 1997;34:115–124. doi: 10.1016/s0161-5890(97)00014-x. [DOI] [PubMed] [Google Scholar]
- 5.Klein L, Kyewski B. “Promiscuous” expression of tissue antigens in the thymus: a key to T-cell tolerance and autoimmunity? J Mol Med (Berl) 2000;78:483–494. doi: 10.1007/s001090000146. [DOI] [PubMed] [Google Scholar]
- 6.Hare KJ, Pongracz J, Jenkinson EJ, Anderson G. Modeling TCR signaling complex formation in positive selection. J Immunol. 2003;171:2825–2831. doi: 10.4049/jimmunol.171.6.2825. [DOI] [PubMed] [Google Scholar]
- 7.Kumamoto T, Inaba M, Imamura H, Nango K, Adachi Y, Than S, Inaba K, Kagawa T, Ikehara S. Characterization of B cells in human thymus. Immunobiology. 1991;183:88–93. doi: 10.1016/s0171-2985(11)80188-9. [DOI] [PubMed] [Google Scholar]
- 8.Kleindienst P, Chretien I, Winkler T, Brocker T. Functional comparison of thymic B cells and dendritic cells in vivo. Blood. 2000;95:2610–2616. [PubMed] [Google Scholar]
- 9.Frommer F, Heinen TJ, Wunderlich FT, Yogev N, Buch T, Roers A, Bettelli E, Muller W, Anderton SM, Waisman A. Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion. J Immunol. 2008;181:5748–5759. doi: 10.4049/jimmunol.181.8.5748. [DOI] [PubMed] [Google Scholar]
- 10.Frommer F, Waisman A. B cells participate in thymic negative selection of murine auto-reactive CD4+ T cells. PloS one. 2010;5:e15372. doi: 10.1371/journal.pone.0015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perera J, Meng L, Meng F, Huang H. Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17011–17016. doi: 10.1073/pnas.1313001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tullin S, Farris P, Petersen JS, Hornum L, Jackerott M, Markholst H. A pronounced thymic B cell deficiency in the spontaneously diabetic BB rat. J Immunol. 1997;158:5554–5559. [PubMed] [Google Scholar]
- 13.Andreu-Sanchez JL, Faro J, Alonso JM, Paige CJ, Martinez C, Marcos MA. Ontogenic characterization of thymic B lymphocytes. Analysis in different mouse strains. European journal of immunology. 1990;20:1767–1773. doi: 10.1002/eji.1830200822. [DOI] [PubMed] [Google Scholar]
- 14.Isaacson PG, Norton AJ, Addis BJ. The human thymus contains a novel population of B lymphocytes. Lancet. 1987;2:1488–1491. doi: 10.1016/s0140-6736(87)92622-5. [DOI] [PubMed] [Google Scholar]
- 15.Inaba M, Inaba K, Adachi Y, Nango K, Ogata H, Muramatsu S, Ikehara S. Functional analyses of thymic CD5+ B cells. Responsiveness to major histocompatibility complex class II-restricted T blasts but not to lipopolysaccharide or anti-IgM plus interleukin 4. The Journal of experimental medicine. 1990;171:321–326. doi: 10.1084/jem.171.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shores EW, Van Ewijk W, Singer A. Disorganization and restoration of thymic medullary epithelial cells in T cell receptor-negative scid mice: evidence that receptor-bearing lymphocytes influence maturation of the thymic microenvironment. European journal of immunology. 1991;21:1657–1661. doi: 10.1002/eji.1830210711. [DOI] [PubMed] [Google Scholar]
- 17.Surh CD, Ernst B, Sprent J. Growth of epithelial cells in the thymic medulla is under the control of mature T cells. The Journal of experimental medicine. 1992;176:611–616. doi: 10.1084/jem.176.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Ewijk W, Shores EW, Singer A. Crosstalk in the mouse thymus. Immunology today. 1994;15:214–217. doi: 10.1016/0167-5699(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 19.Jenkinson SR, Williams JA, Jeon H, Zhang J, Nitta T, Ohigashi I, Kruhlak M, Zuklys S, Sharrow S, Adams A, Granger L, Choi Y, Siebenlist U, Bishop GA, Hollander GA, Takahama Y, Hodes RJ. TRAF3 enforces the requirement for T cell cross-talk in thymic medullary epithelial development. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:21107–21112. doi: 10.1073/pnas.1314859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams JA, Zhang J, Jeon H, Nitta T, Ohigashi I, Klug D, Kruhlak MJ, Choudhury B, Sharrow SO, Granger L, Adams A, Eckhaus MA, Jenkinson SR, Richie ER, Gress RE, Takahama Y, Hodes RJ. Thymic medullary epithelium and thymocyte self-tolerance require cooperation between CD28-CD80/86 and CD40-CD40L costimulatory pathways. J Immunol. 2014;192:630–640. doi: 10.4049/jimmunol.1302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 22.Oehen S, Feng L, Xia Y, Surh CD, Hedrick SM. Antigen compartmentation and T helper cell tolerance induction. The Journal of experimental medicine. 1996;183:2617–2626. doi: 10.1084/jem.183.6.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. The Journal of experimental medicine. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunology and cell biology. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 25.Williams JA, Sharrow SO, Adams AJ, Hodes RJ. CD40 ligand functions non-cell autonomously to promote deletion of self-reactive thymocytes. J Immunol. 2002;168:2759–2765. doi: 10.4049/jimmunol.168.6.2759. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Ferrero I, Anjuere F, Azcoitia I, Renno T, MacDonald HR, Ardavin C. Viral superantigen-induced negative selection of TCR transgenic CD4+ CD8+ thymocytes depends on activation, but not proliferation. Blood. 1998;91:4248–4254. [PubMed] [Google Scholar]
- 28.Ferrero I, Anjuere F, Martin P, Martinez del Hoyo G, Fraga ML, Wright N, Varona R, Marquez G, Ardavin C. Functional and phenotypic analysis of thymic B cells: role in the induction of T cell negative selection. European journal of immunology. 1999;29:1598–1609. doi: 10.1002/(SICI)1521-4141(199905)29:05<1598::AID-IMMU1598>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J, Akiyama T, Takahama Y. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 34.van Kooten C, Banchereau J. CD40-CD40 ligand. Journal of leukocyte biology. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 35.Lumsden JM, Williams JA, Hodes RJ. Differential requirements for expression of CD80/86 and CD40 on B cells for T-dependent antibody responses in vivo. J Immunol. 2003;170:781–787. doi: 10.4049/jimmunol.170.2.781. [DOI] [PubMed] [Google Scholar]
- 36.Foy TM, Page DM, Waldschmidt TJ, Schoneveld A, Laman JD, Masters SR, Tygrett L, Ledbetter JA, Aruffo A, Claassen E, Xu JC, Flavell RA, Oehen S, Hedrick SM, Noelle RJ. An essential role for gp39, the ligand for CD40, in thymic selection. The Journal of experimental medicine. 1995;182:1377–1388. doi: 10.1084/jem.182.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herman A, Kappler JW, Marrack P, Pullen AM. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annual review of immunology. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 38.Moore NC, Anderson G, McLoughlin DE, Owen JJ, Jenkinson EJ. Differential expression of Mtv loci in MHC class II-positive thymic stromal cells. J Immunol. 1994;152:4826–4831. [PubMed] [Google Scholar]
- 39.Marodon G, Rocha B. Generation of mature T cell populations in the thymus: CD4 or CD8 down-regulation occurs at different stages of thymocyte differentiation. European journal of immunology. 1994;24:196–204. doi: 10.1002/eji.1830240131. [DOI] [PubMed] [Google Scholar]
- 40.Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH, Singer A. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nature immunology. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osborne BA. Transcriptional control of T cell development. Current opinion in immunology. 2000;12:301–306. doi: 10.1016/s0952-7915(00)00091-1. [DOI] [PubMed] [Google Scholar]
- 42.Robey EA, Bluestone JA. Notch signaling in lymphocyte development and function. Current opinion in immunology. 2004;16:360–366. doi: 10.1016/j.coi.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annual review of immunology. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 44.Jenkinson EJ, Jenkinson WE, Rossi SW, Anderson G. The thymus and T-cell commitment: the right niche for Notch? Nature reviews. Immunology. 2006;6:551–555. doi: 10.1038/nri1883. [DOI] [PubMed] [Google Scholar]
- 45.Tokoro Y, Sugawara T, Yaginuma H, Nakauchi H, Terhorst C, Wang B, Takahama Y. A mouse carrying genetic defect in the choice between T and B lymphocytes. J Immunol. 1998;161:4591–4598. [PubMed] [Google Scholar]
- 46.Sangster MY, Riberdy JM, Gonzalez M, Topham DJ, Baumgarth N, Doherty PC. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. The Journal of experimental medicine. 2003;198:1011–1021. doi: 10.1084/jem.20021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graham JP, Arcipowski KM, Bishop GA. Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunological reviews. 2010;237:226–248. doi: 10.1111/j.1600-065X.2010.00932.x. [DOI] [PubMed] [Google Scholar]
- 48.Spidale NA, Wang B, Tisch R. Cutting Edge: Antigen-Specific Thymocyte Feedback Regulates Homeostatic Thymic Conventional Dendritic Cell Maturation. J Immunol. 2014 doi: 10.4049/jimmunol.1400321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwartz MA, Kolhatkar NS, Thouvenel C, Khim S, Rawlings DJ. CD4+ T Cells and CD40 Participate in Selection and Homeostasis of Peripheral B Cells. J Immunol. 2014 doi: 10.4049/jimmunol.1400798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walters SN, Webster KE, Daley S, Grey ST. A Role for Intrathymic B Cells in the Generation of Natural Regulatory T Cells. J Immunol. 2014 doi: 10.4049/jimmunol.1302519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.