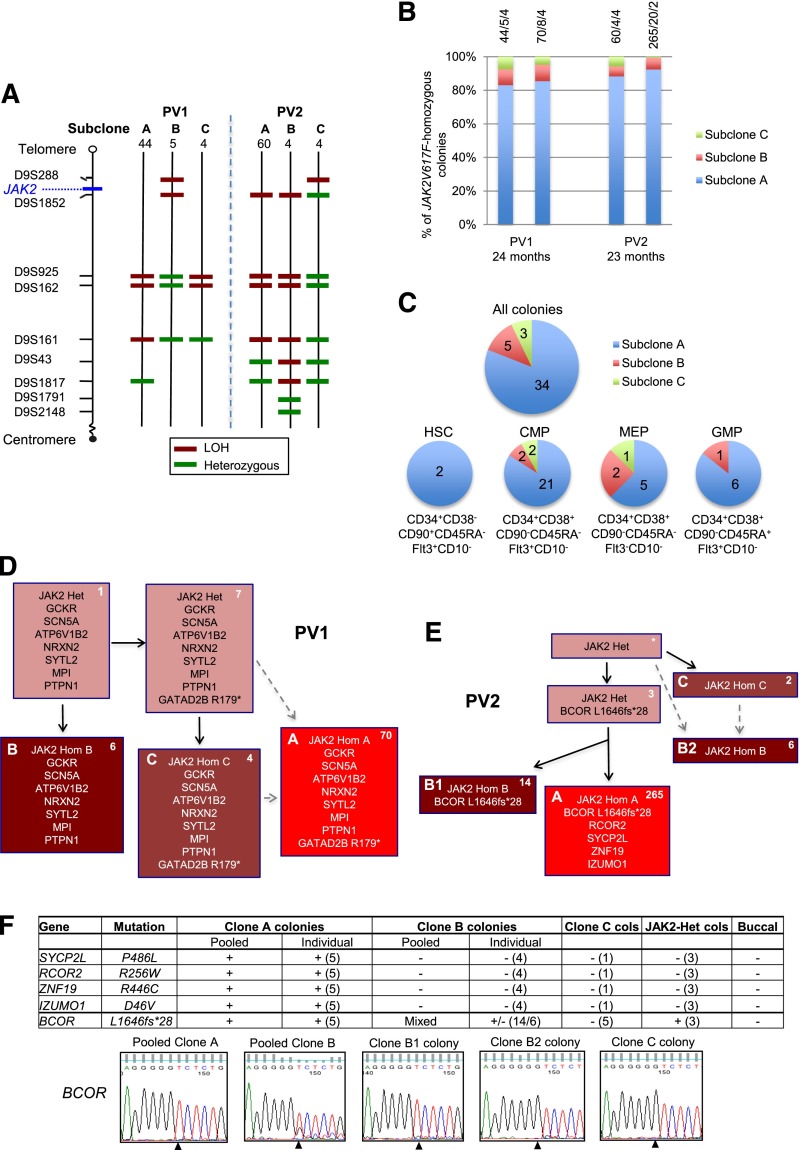
Figure 1.
Investigation of mechanisms for expansion of JAK2V617F-homozygous subclones in patients PV1 and PV2. (A) Microsatellite mapping of breakpoints for loss of heterozygosity (LOH) on 9p in JAK2V617F-homozygous BFU-E colonies from patients PV1 and PV2 (reported previously1). For microsatellite markers on chromosome 9 (left), 3 patterns were observed in colonies for each patient, indicating 3 distinct LOH breakpoints. Numbers of colonies per subclone are shown at the top. Green denotes a heterozygous marker; red denotes LOH. (B) Persistence of multiple JAK2V617F-homozygous subclones over time. JAK2V617F-homozygous BFU-E colonies grown at 2 time points (A and B) at 0.01 U/mL erythropoietin were genotyped for microsatellite markers on 9p. Bars are divided according to the number of colonies corresponding to subclones A, B, and C (panel A), with absolute numbers at the top (A/B/C). The time between assays is shown at the bottom. (C) Presence of multiple JAK2V617F-homozygous subclones in hematopoietic progenitor compartments. Patient PV1 was used because a high JAK2V617F allele burden (99% in granulocytes) permitted growth of many JAK2V617F-homozygous colonies. Colonies were cultured from fluorescence-activated cell sorted peripheral blood progenitors, and JAK2V617F-homozygous colonies were genotyped for microsatellite markers on 9p. Each pie chart is divided according to the number of colonies corresponding to subclones A, B, and C from panel A; for all colonies combined (top), and for HSC, common myeloid progenitor (CMP), megakaryocyte-erythroid progenitor (MEP), or granulocyte-monocyte progenitor (GMP) populations separately (bottom). Phenotypic definitions are shown. (D) Colony hierarchy for PV1. Mutant genes are shown in each box with the corresponding number of colonies at the top right in white. Pink boxes denote JAK2V617F-heterozygous colonies; red denotes JAK2V617F-homozygous, with different subclones shown by letters and red shades. Subclones C and A may have occurred sequentially (C then A, according to the LOH breakpoints) or may have arisen from the same JAK2V617F-heterozygous precursor (gray dashed arrows). Subclone B lacks the GATAD2B mutation and must have acquired 9p LOH independently. Full mutation details are shown in supplemental Table 2. (E) Colony hierarchy for PV2. Numbers of colonies from each subclone at a single time point are shown at the top right of each box in white, and the additional mutations detected in validation studies (on pooled or individual colonies, panel F) are shown. The BCOR wild-type clone B colonies are likely to represent an independent subclone (“B2”) with a similar 9p LOH breakpoint to the BCOR-mutant subclone (“B1”) (A,E: resolution of microsatellite mapping is 2.5 Mb). Subclones C and B2 may have arisen independently but could also have arisen sequentially (C then B2, according to the LOH breakpoints; gray dashed arrows). Subclone B1 has a more distal breakpoint than subclone A and cannot be an intermediate stage in its development. However, subclones A and B1 could have diverged either before 9p LOH or after an initial proximal LOH event. Additional variants were called by exome sequencing in both subclones A and B but were not pursued for validation (supplemental Table 2). *No colonies with this genotype were identified, but the presence of this intermediate can be inferred. Het, heterozygous; Hom, homozygous. (F) Genotyping data of 5 validated mutations in PV2 in pooled colonies from JAK2V617F-homozygous subclones, in individual colonies (number tested appears in parentheses) and in buccal DNA, from which the hierarchy in panel (E) is derived. +, presence of mutation; −, absence of mutation; MT, mutant allele; WT, wild-type allele. For clone C, 1 colony was genotyped for all mutations and an additional 4 from an earlier time point were genotyped for BCOR; given that all of these colonies are BCOR wild-type, the clone must be independent and wild-type for the other mutations. Capillary sequencing traces for the BCOR mutation are shown at the bottom; the mutation (del g) is present in pooled clone A and individual clone B1 colonies; pooled clone B colonies show a mixed trace; the wild-type sequence is present in clone B2 and C colonies. Note that the mutation is hemizygous (BCOR is X-linked; the patient is male).