Abstract
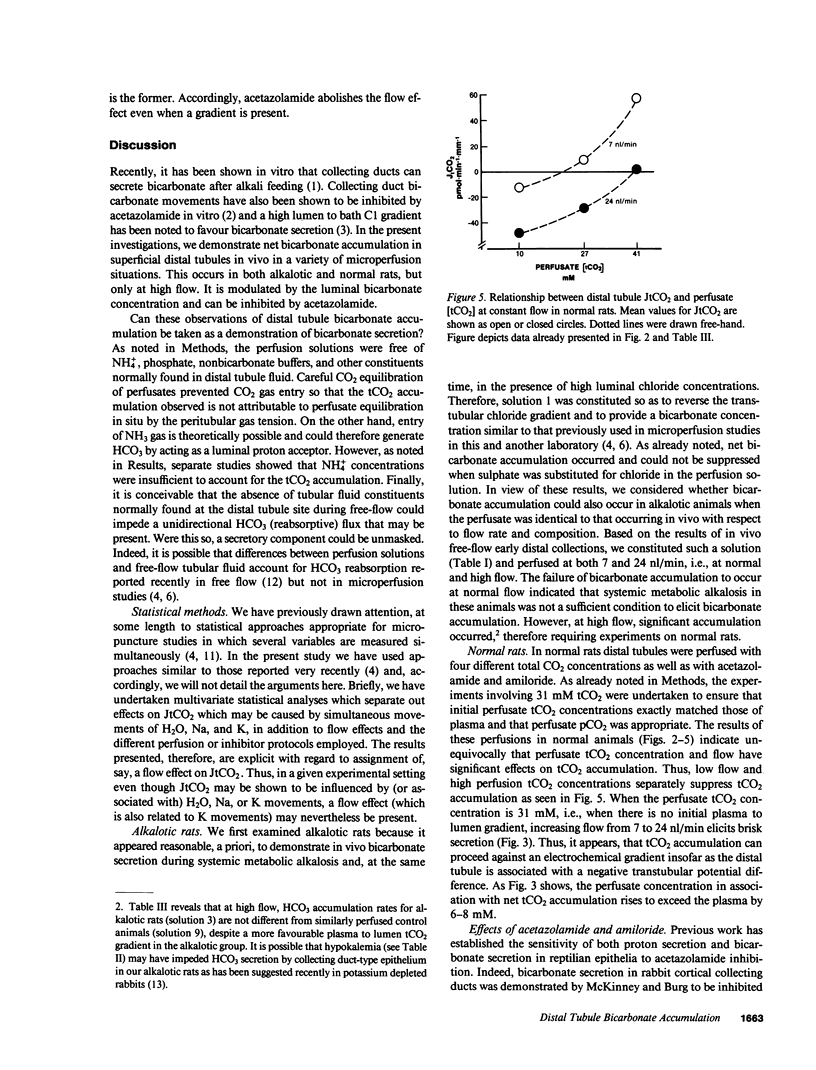
We have performed microperfusion studies on distal tubules of normal and alkalotic rats in an attempt to demonstrate in vivo bicarbonate secretion. All perfusion solutions were free of phosphate and other nonbicarbonate buffers. In both normal and alkalotic rats, distal perfusions elicited significant tCO2 entry only at high flow (24 nl/min). Even when perfusate tCO2 concentration closely matched plasma tCO2 concentration (30 mM tCO2), significant tCO2 entry again occurred at high flow. This was associated with a rise of the perfusate tCO2 concentration, which indicated net entry of tCO2 against a concentration gradient. In this "symmetrical" perfusion situation, acetazolamide blockade prevented tCO2 entry. Accordingly: distal tubule tCO2 entry is demonstrable in both alkalotic and normal rats at high flow rates; increasing perfusate tCO2 concentration can suppress tCO2 entry; and entry can occur in the absence of a gradient and this effect can be blocked by acetazolamide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burckhardt B. C., Cassola A. C., Frömter E. Electrophysiological analysis of bicarbonate permeation across the peritubular cell membrane of rat kidney proximal tubule. II. Exclusion of HCO3(-)-effects on other ion permeabilities and of coupled electroneutral HCO3(-)-transport. Pflugers Arch. 1984 May;401(1):43–51. doi: 10.1007/BF00581531. [DOI] [PubMed] [Google Scholar]
- Burckhardt B. C., Sato K., Frömter E. Electrophysiological analysis of bicarbonate permeation across the peritubular cell membrane of rat kidney proximal tubule. I. Basic observations. Pflugers Arch. 1984 May;401(1):34–42. doi: 10.1007/BF00581530. [DOI] [PubMed] [Google Scholar]
- Cheema-Dhadli S., Hamat R., Sonnenberg H., Halperin M. A micromethod to measure ammonia. Kidney Int. 1981 Jan;19(1):80–82. doi: 10.1038/ki.1981.10. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Alpern R. J. Regulation of proximal bicarbonate reabsorption. Am J Physiol. 1984 Sep;247(3 Pt 2):F387–F395. doi: 10.1152/ajprenal.1984.247.3.F387. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Maddox D. A., Lucci M. S., Rector F. C., Jr Control of proximal bicarbonate reabsorption in normal and acidotic rats. J Clin Invest. 1979 Nov;64(5):1168–1180. doi: 10.1172/JCI109570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla J. H., Bonduris D. N., Dumbauld S. L., Luke R. G. Segmental chloride and fluid handling during correction of chloride-depletion alkalosis without volume expansion in the rat. J Clin Invest. 1984 Jan;73(1):96–106. doi: 10.1172/JCI111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Austt J., Good D. W., Burg M. B., Knepper M. A. Deoxycorticosterone-stimulated bicarbonate secretion in rabbit cortical collecting ducts: effects of luminal chloride removal and in vivo acid loading. Am J Physiol. 1985 Aug;249(2 Pt 2):F205–F212. doi: 10.1152/ajprenal.1985.249.2.F205. [DOI] [PubMed] [Google Scholar]
- Good D. W., Wright F. S. Luminal influences on potassium secretion: sodium concentration and fluid flow rate. Am J Physiol. 1979 Feb;236(2):F192–F205. doi: 10.1152/ajprenal.1979.236.2.F192. [DOI] [PubMed] [Google Scholar]
- Laski M. E., Warnock D. G., Rector F. C., Jr Effects of chloride gradients on total CO2 flux in the rabbit cortical collecting tubule. Am J Physiol. 1983 Feb;244(2):F112–F121. doi: 10.1152/ajprenal.1983.244.2.F112. [DOI] [PubMed] [Google Scholar]
- Levine D. Z. An in vivo microperfusion study of distal tubule bicarbonate reabsorption in normal and ammonium chloride rats. J Clin Invest. 1985 Feb;75(2):588–595. doi: 10.1172/JCI111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D. Z., Byers M. K., McLeod R. A., Luisello J. A., Raman S. Loop of Henle bicarbonate accumulation in vivo in the rat. J Clin Invest. 1979 Jan;63(1):59–66. doi: 10.1172/JCI109278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S. A., de Moura J. L. Incorporation of cytoplasmic vesicles into apical membrane of mammalian urinary bladder epithelium. Nature. 1982 Jun 24;297(5868):685–688. doi: 10.1038/297685a0. [DOI] [PubMed] [Google Scholar]
- Lucci M. S., Pucacco L. R., Carter N. W., DuBose T. D., Jr Evaluation of bicarbonate transport in rat distal tubule: effects of acid-base status. Am J Physiol. 1982 Oct;243(4):F335–F341. doi: 10.1152/ajprenal.1982.243.4.F335. [DOI] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate secretion by rabbit cortical collecting tubules in vitro. J Clin Invest. 1978 Jun;61(6):1421–1427. doi: 10.1172/JCI109061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney T. D., Burg M. B. Bicarbonate transport by rabbit cortical collecting tubules. Effect of acid and alkali loads in vivo on transport in vitro. J Clin Invest. 1977 Sep;60(3):766–768. doi: 10.1172/JCI108830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman S., Mousseau G., Levine D. Z. Statistical models for renal micropuncture studies. Am J Physiol. 1977 Oct;233(4):F349–F357. doi: 10.1152/ajprenal.1977.233.4.F349. [DOI] [PubMed] [Google Scholar]
- Star R. A., Burg M. B., Knepper M. A. Bicarbonate secretion and chloride absorption by rabbit cortical collecting ducts. Role of chloride/bicarbonate exchange. J Clin Invest. 1985 Sep;76(3):1123–1130. doi: 10.1172/JCI112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C. S., Granges F., Kirk G., Gordon D., Giebisch G. Effects of saline infusion on titratable acid generation and ammonia secretion. Am J Physiol. 1984 Sep;247(3 Pt 2):F506–F519. doi: 10.1152/ajprenal.1984.247.3.F506. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Frömter E. Cell pH of rat renal proximal tubule in vivo and the conductive nature of peritubular HCO3- (OH-) exit. Pflugers Arch. 1984 Nov;402(3):300–305. doi: 10.1007/BF00585513. [DOI] [PubMed] [Google Scholar]



