Abstract
Background
Rats lever-press for access to running wheels suggesting that wheel running by itself is reinforcing. Furthermore, pairings of an episode of wheel running and subsequent confinement in a specific environment can establish a conditioned place preference (CPP). This finding implies that the reinforcing effects of wheel running outlast the actual occurrence of physical activity, a phenomenon referred to as aftereffect of wheel running. Aftereffect-induced CPP involves Pavlovian conditioning, i.e. repeated pairings of the aftereffect of wheel running with a specific environment creates a learned association between aftereffect and environment and, in turn, a preference for that environment. Given the involvement of dopamine systems in mediating effects of Pavlovian stimuli on appetitive behavior, a role of dopamine in mediating aftereffect-induced CPP seems plausible. Here we assessed whether the mixed D1/D2 receptor antagonist flupenthixol (0.25 mg/kg, i.p.) can block the expression of an aftereffect-induced CPP.
Results
In line with earlier studies, our results demonstrate that rats displayed a conditioned preference for environments paired with the aftereffect of wheel running and further show that the magnitude of CPP was not related to the wheel running rate. Furthermore, we found that flupenthixol (0.25 mg/kg, i.p.) reduced locomotor activity but did not attenuate the expression of an aftereffect-induced CPP.
Conclusion
The expression of a CPP produced by the aftereffect of wheel running seems not to depend on dopamine D1/D2 receptor activation.
Keywords: Wheel running, Aftereffect, Reinforcement, Dopamine, Rat
Background
Considerable evidence suggests that wheel running in rodents has reinforcing effects. For instance, rats lever-press for access to running wheels, i.e., the opportunity to run acts as a reinforcer that maintains operant responding [1-3]. Interestingly, those rats that developed the highest rates of running during wheel access also maintained the most stable and highest rates of lever pressing [3]. Furthermore, unrestricted wheel running can produce compulsive patterns of responding suggesting that wheel running has parallels with drug addiction [4]. Consistent with this notion, wheel running can act as a hedonic substitution for other reinforcers and attenuate self-administration of cocaine [5]. Considerable evidence suggests that opioid and dopamine systems are involved in mediating reinforcing effects of wheel running [6]. For instance, brief periods of wheel running increased striatal dopamine metabolism [7] and prior experience with wheel running produced cross-tolerance to the rewarding effects of morphine [8].
Remarkably, wheel running not only reinforces the behavior that generates it but also produces a preference for the environment that follows it [2]. For instance, pairings of an episode of wheel running and subsequent confinement in a specific environment can establish a conditioned place preference (CPP). These findings imply that the reinforcing effects of wheel running outlast the actual occurrence of physical activity, a phenomenon referred to as aftereffect of wheel running [2,8-10]. As with reinforcing effects of wheel running by itself, reinforcing effects of the aftereffect of wheel running are supported by opioid systems. For instance, naloxone, a μ-opioid receptor antagonist, attenuated the acquisition and expression of an aftereffect-induced CPP [9].
CPP involves Pavlovian conditioning, i.e., repeated pairings of the aftereffect induced by wheel running with a specific environment creates a learned association between aftereffect and environment and, in turn, a preference for that environment [11]. It is well known that dopamine systems play a critical role in mediating effects of Pavlovian stimuli on appetitive behavior [12], e.g., dopamine receptor blockade attenuated conditioned approach behavior [13]. Furthermore, through activation of dopamine systems, Pavlovian stimuli can both amplify and direct instrumental responding, phenomena termed as general and outcome-selective Pavlovian-instrumental transfer [14-18]. However, the role of dopamine in aftereffect-induced CPP is unknown at present. Here we investigated whether a blockade of dopamine receptors by systemic administration of the mixed D1/D2 receptor antagonist flupenthixol can block the expression of a CPP established by pairings of the wheel running aftereffect with a specific environment. Given the crucial role of dopaminergic systems in mediating effects of Pavlovian stimuli on appetitive behavior, flupenthixol should attenuate the expression of an aftereffect-induced CPP.
Of note, monoamine systems have been implicated in exercise addiction in humans [19]. Environmental stimuli play a critical role in maintaining addictive behavior and the CPP paradigm provides an important tool to analyze behavioral effects of contextual stimuli associated with drug cues [11]. Therefore, the present study could provide clues as to whether dopamine systems mediate behavioral effects of contextual stimuli associated with the aftereffect of physical activity.
Results
Conditioned place preference
In the place preference test, rats preferred the chamber paired with the aftereffect of wheel running over the control chamber. Furthermore, the context identity did not influence place preference (Figure 1). A statistical analysis confirmed this description. A two-way ANOVA with aftereffect and context identity as factors revealed a significant effect of aftereffect (F(1,30) =16.18; p < 0.0005), no effect of context identity (F(1,30) =0.2; n.s.) and no aftereffect × context identity interaction (F(1,30) =0.9; n.s.). Furthermore, CPP magnitude did not correlate with wheel running rate. Individual distances covered during wheel running on the final training day and time spent in the chamber paired with the aftereffect did not correlate (Pearson’s r = −0.25; n.s).
Figure 1.
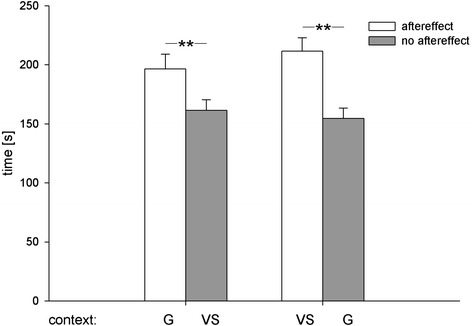
Aftereffect-induced CPP. Mean times (± SEM) rats spent in the chamber paired with the aftereffect of 2 h wheel running vs. the control chamber as a function of the chamber context (N = 32; G: grey; VS: vertical stripes). An ANOVA revealed a significant effect of aftereffect (**p < 0.0005), but no effect of chamber context and no aftereffect × chamber context interaction.
Flupenthixol effects on conditioned place preference
Results further demonstrate that, like saline controls, rats pretreated with flupenthixol displayed a preference for the chamber experienced under the aftereffect of wheel running over the chamber experienced without prior wheel running (Figure 2). A statistical analysis confirmed this description. An ANOVA with aftereffect and treatment as factors revealed a significant effect of aftereffect (F(1,30) =6.44; p < 0.05), but not of treatment (F(1,30) =0.67; n.s.) and no aftereffect × treatment interaction (F(1,30) =0.006; n.s.).
Figure 2.
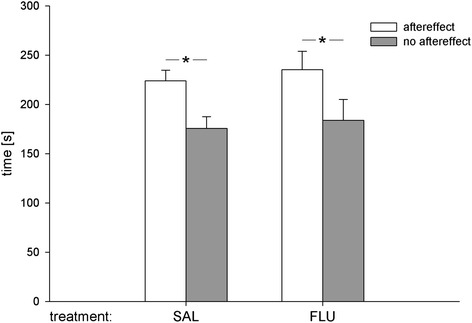
Effects of flupenthixol on aftereffect-induced CPP. Mean times (± SEM) spent in the chamber paired with the aftereffect of 2 h wheel running vs. the control chamber in animals that received saline (1 ml/kg, i.p., n = 16) or flupenthixol (0.25 mg/kg, i.p., n = 16) 60 min prior CPP testing. An ANOVA revealed a significant effect of aftereffect (*p < 0.05), but no effect of treatment and no aftereffect × treatment interaction.
Flupenthixol effects on locomotor activity
In a separate group of rats, we found that the dose of flupenthixol used in the CPP experiment reduced locomotor activity in an open field (Figure 3). Accordingly, an ANOVA on cumulated distance moved with treatment and time as factors revealed an almost significant effect of treatment (F(1,11) =4.69; p =0.053) and a significant treatment × time interaction (F(8,88) =2.06; p < 0.05).
Figure 3.
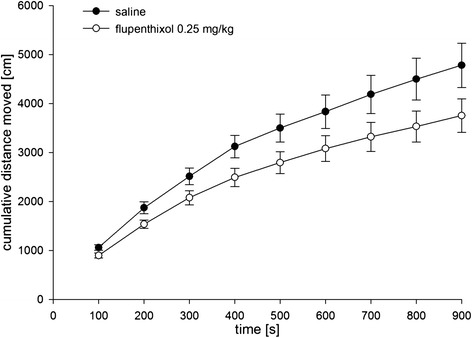
Effects of flupenthixol on locomotor activity. Mean cumulative distance (± SEM) moved in an open field in animals (n = 12) under saline (1 ml/kg, i.p.) vs. flupenthixol (0.25 mg/kg, i.p.) administered 60 min prior CPP testing. An ANOVA revealed a trend for an effect of treatment (F(1,11) =4.69; p =0.053) and a significant treatment × time interaction (F(8,88) =2.06; p < 0.05).
Discussion
Our results demonstrate that rats displayed a conditioned preference for environments paired with the aftereffect of wheel running and further show that the CPP magnitude was not related to the wheel running rate. Moreover, we found that the mixed dopamine D1/D2 receptor antagonist flupenthixol did not block the expression of CPP produced by the aftereffect of wheel running.
Consistent with previous studies using a similar protocol [2,8-10], the aftereffect generated by 2 h wheel running induced a CPP. The CPP effect size in our study was strong (r =0.59). Of note, earlier studies [2,8-10] used vertical and horizontal stripe chambers for CPP. Because rats tend to prefer vertical over horizontal stripe chambers [20], CPP may be influenced by context configuration even if the experimental design controlled for such a bias [2]. In our pilot studies, rats did not show spontaneous chamber preferences (monochrome grey vs. vertical black lines) suggesting that context configuration was balanced in the CPP apparatus used here. Importantly, data from the experiments reported here provided no evidence for an influence of context identity on aftereffect-induced CPP, i.e. statistical analysis demonstrated that the chamber context per se had no effect on CPP and did not interact with the aftereffect. Yet, due to considerable methodological differences in the experimental design (within vs. between subjects) and the place preference test system (2 vs. 3 chamber design), it is difficult to assess whether our balanced context configuration accounts for the, relative to previous studies [2,8-10], marked CPP effect size. Furthermore, as reported in other studies using 3-chamber CPP apparatus [21,22], animals spent some time in center compartment connecting the chambers. Nevertheless, reinforcing effects of a given stimulus such amphetamine can be reliably assessed in a CPP apparatus with either a 2- or 3-chamber design [23,24]. However, we cannot rule out that the use of a 3-chamber CPP apparatus influenced CPP effect size to some extent.
Our observation that the aftereffect of 2 h wheel running generated a robust CPP adds further support to the notion that the aftereffect is reinforcing as detected by a number of studies using various wheel running paradigms [2,10,25,26]. Remarkably, we found no evidence for a relationship between CPP magnitude and wheel running rate within 2 h. In line with this observation, higher rates of wheel running did not correlate with stronger CPP [2]. However, the relationship between CPP and reinforcing efficacy of stimuli bears a number of complexities. For instance, the magnitude of CPP induced by drug reinforcement in a given rat seems not to reflect the magnitude of its reinforcing efficacy measured by drug self-administration in the same rat [11]. Furthermore, while there is a large body of evidence suggesting that physical activity is a natural reinforcer in rodents [6], it is not clear whether there exist simple, e.g. linear, dose–response relationships between the intensity or duration of physical activity such as wheel running and its reinforcing efficacy and the resulting aftereffect. Studies in humans on associations between activity doses and affective responses also point to complex relationships between aerobic exercise and positive or negative affect that do not reflect simple inverted U-shaped curves [27,28].
We further demonstrated that the mixed dopamine D1/D2 receptor antagonist flupenthixol did not block the expression of an aftereffect-induced CPP. In addition, results show that the dose of flupenthixol used here (0.25 mg/kg) was behaviorally effective, i.e., reduced locomotor activity. In line with this notion, flupenthixol at 0.3 mg/kg but not at 0.15 mg/kg produced a within-session decline of locomotor activity [29]. Likewise, flupenthixol at 0.2 and 0.3 mg/kg was able to reduce the expression of CPP induced by testosterone [30] suggesting that under-dosing of flupenthixol seems unlikely to account for its failure to attenuate aftereffect-induced CPP. By contrast, our pilot experiments suggest that flupenthixol at doses of 0.4 mg/kg and higher may be not appropriate as they cause pronounced locomotor inhibition that interferes with CPP.
In the CPP paradigm used here, reinforcing aftereffects of a natural reinforcer such as wheel running was paired with exposure to specific environmental stimuli and this stimulus came to elicit a conditioned approach in the place preference test. The ability of a stimulus previously paired with reinforcement to acquire incentive motivational properties is mediated by Pavlovian learning and plays a key role not only in CPP but also in related phenomena such as Pavlovian-instrumental transfer [12]. The ventral striatum is modulated by dopamine and opioid systems [31] and represents a key element of the neural circuit that mediates expression of a CPP induced by natural reinforcers [32]. There is considerable evidence for a dopaminergic involvement in governing the effects of Pavlovian stimuli on appetitive behavior, e.g., dopamine receptor activation enhanced while dopamine receptor blockade attenuated conditioned approach behavior [13,33]. Furthermore, a number of studies point to a role of dopamine both in acquisition and expression of CPP generated by drug reinforcers. For instance, the acquisition of amphetamine-induced CPP requires both D1 and D2 receptor activation, while the expression of amphetamine-induced CPP selectively depends on D1 receptor activation [34]. However, a substantial number of studies suggests that dopamine depletion or dopamine receptor blockade failed to block CPP induced by cocaine, methylphenidate or nomifensine [35-39] pointing to an involvement of non-dopaminergic mechanisms in mediating reinforcing effects of dopaminergic drugs measured in CPP. As yet only a few studies examined the role of dopamine in the acquisition of CPP established by natural reinforcers. Results are mixed, e.g., dopamine D1/D2 receptor antagonists blocked the acquisition of food-reinforced CPP [40] but not CPP induced by paced mating behavior [41], while specific D2 receptor antagonists even facilitated the acquisition of food-reinforced CPP [42]. By contrast, the effects of dopamine receptor ligands on the acquisition of aftereffect-induced CPP are as yet unknown. Our results are, to our knowledge, the first to show that the expression of CPP induced by the aftereffect, i.e. a prominent natural reinforcer, does not depend on dopamine D1/D2 receptor activation. Further studies are required to assess whether or not the expression of CPP induced by other natural reinforcers than the aftereffect requires dopamine D1/D2 receptor activity. Moreover, it is possible that opioid rather than dopamine receptor activity supports the expression of aftereffect-induced CPP. It is well known that, by mediating the hedonic response to natural reinforcers, opioid receptor activity governs the acquisition of CPP [43]. For instance, opioid receptor antagonists attenuated the acquisition of a CPP induced by water [44] and the aftereffect of wheel running [9]. Furthermore, it has been speculated that the expression of CPP may also rely on opioid receptor activity, i.e., via an opioid receptor-mediated evocation of a hedonic response that elicits approach to the associated chamber [43]. Furthermore, recent data suggest that μ-opioid receptor-mediated signals not only govern hedonic responses but are also involved in mediating the incentive effects of Pavlovian stimuli on appetitive behavior [45]. Collectively, these findings point to the possibility that, by mediating both incentive and hedonic effects of Pavlovian stimuli, opioid receptor activation could support the expression of an aftereffect-induced CPP.
Conclusions
The aftereffect of wheel running for 2 h is reinforcing and can establish a CPP, however, expression of an aftereffect-induced CPP seems not to depend on dopamine D1/D2 receptor activation. Monoamine systems have been implicated in exercise addiction in humans [19]. As environmental stimuli play a critical role in maintaining addictive behavior the CPP paradigm provides an important tool to analyze behavioral effects of contextual stimuli associated with drug cues [11]. Our results suggest that dopamine D1/D2 receptor activity may not maintain exercise addiction in humans by mediating the behavioral effects of contextual stimuli associated with the aftereffect of physical activity.
Methods
All animal experiments were conducted according to the German Law on Animal Protection and approved by the local council of animal care (Regierungspraesidium Stuttgart).
Subjects
Male Sprague–Dawley rats (Janvier, Le Genest St. Isle, France, N = 32) weighing 271.6 ± 5.5 g at the beginning of the experiments were used for CPP experiments. They were housed in pairs in transparent cages (39 × 55 × 27 cm; Ebeco, Castrop-Rauxel, Germany) with standard metal lids. During a limited period of 4 weeks prior to the onset of behavioral experiments, cages were supplied with custom-made lids with an integrated running wheel (diameter: 31.5 cm, width: 10 cm). Rats had ad libitum access to water; food (standard maintenance chow; Altromin, Lage, Germany) was restricted to 15 g per animal and day. Temperature (21 ± 2°C) and humidity (45-50%) were kept constant in the animal house. A 14:10-h light–dark cycle (lights on at 8.00 a.m.) was used; behavioral experiments were performed during light period of the light–dark cycle.
A separate group of male Sprague–Dawley rats (Janvier, Le Genest St. Isle, France, n = 12) weighing 272.9 ± 2.8 g at the beginning of the experiment was used for a control experiment to assess the effects of flupenthixol on locomotor activity. They were housed in groups of four in transparent cages (39 × 55 × 27 cm; Ebeco, Castrop-Rauxel, Germany) with standard metal lids as described above.
Apparatus
Running wheels
Running wheels were used during conditioning enclosed by a transparent plastic box to prevent rats from leaving the running wheels. Running wheels (diameter 31.5 cm; 10 cm width) were identical to those used for habituation in the home cage.
Place preference conditioning apparatus
A 3-chamber place preference system was employed (TSE, Bad Homburg, Germany) which consisted of a “vertical” and “grey” chamber (each 30 cm long × 25 cm wide × 32 cm deep) connected by a center compartment (11 cm long × 25 cm wide × 32 cm deep). The vertical and grey chambers as well as the center compartment were distinct in wall patterns and floor textures. The vertical chamber included walls with vertical black and white stripes (2 cm width) and a metal floor with square holes (1 cm2, 2 cm apart), the grey chamber included grey walls and a metal floor with circular holes (diameter 0.5 cm, 5 cm apart), the center compartment had white walls and an unperforated metal floor. The lid of each chamber and of the center compartment consisted of a plastic panel. During place preference conditioning, access to the center compartment was blocked to confine a rat to a particular chamber.
Open field
Locomotor activity was recorded in an open field (68 × 68 cm). The testing area was illuminated by red light and surrounded by a cubicle providing optical and acoustical isolation. Locomotor activity was monitored by a video recording system (EthoVision XT 8.5, Noldus, Wageningen, Netherlands) and analyzed off-line.
Procedure
Place preference conditioning
Prior to the onset of place preference conditioning, rats were adapted in their home cages to the running wheels. During place preference conditioning, each rat received 6 pairings of one of both chambers (vertical or grey context) and the aftereffect of wheel running and 6 unpaired exposures to the other chamber with the different context. One pairing was given per day with paired and unpaired trials alternated. Chamber context/aftereffect pairings were counterbalanced across animals. During a paired trial, an animal was confined for 2 h in a running wheel integrated in a box and immediately thereafter placed into the respective chamber, during an unpaired trial, an animal was placed for 2 h in a small cage (42.5 cm × 26.5 cm × 25 cm) before being transferred into the respective chamber. Covered distances in running wheels were recorded from all animals on days 4–6 (N = 32), from a subgroup (n = 16) on days 1–3.
Place preference test
One day after completion of place preference conditioning, each animal was given a place preference test for 10 min with free access to both chambers. At the beginning of the place preference test, an animal was placed into the middle of the center compartment with the body position oriented to compartment wall. A chamber visit was counted if all 4 paws were within a particular chamber.
Place preference test under drug
Three days after the first place preference test, a second place preference test was performed as described above but with prior drug administration, i.e. animals received injections of either flupenthixol (0.25 mg/kg, n = 16) or saline (1 ml/kg, n = 16). Animals were assigned to treatment groups in a pseudo-random manner counterbalanced to chamber context.
Open field
Effects of flupethixol (0.25 mg/kg, i.p.) and vehicle (1 ml/kg, i.p.) on locomotor activity were tested 60 min after administration using a within-subjects design. The day before testing each animal was placed individually into the open field for habituation (10 min). On the subsequent test day 1, and 4 days later, on test day 2, locomotor activity was recorded in a 15 min session, respectively. On test days 1 and 2, each rat received counterbalanced administration of vehicle and flupenthixol. Cumulated distance moved in 100 s-bins is given.
Drugs
Flupenthixol (cis-(Z)-Flupenthixoldihydrochlorid, Sigma Aldrich) was dissolved in physiological saline (Braun, Melsungen Germany). Intraperitoneal injections were in a volume of 1 ml/kg 60 min. prior test onset.
Statistical analysis
Distance covered by each animal in the wheel running box within 2 h was recorded as well as the time spent in each chamber during the place preference test and the cumulative distance moved in the open field. Means ± standard error of the mean (SEM) are given. Data were subjected a repeated-measures analysis of variance (ANOVA). Based on a planned comparison using the t-statistic [46], the effect size r of the aftereffect on time spent in the paired vs. unpaired chamber was calculated according to the following equation: r = √t2/t2 + df [47]. Statistical significance was assessed against a type I error rate of 0.05. All statistical computations were carried out with STATISTICA 7.1 (StatSoft, Inc., Tulsa, USA).
Acknowledgements
We are grateful to I. Newar for assistance in animal husbandry, Dr. E. Scheibler for advice in research design, Dr. A. von Ameln-Meyerhofer for technical support and Prof. Dr. W. Schlicht for valuable comments prior to study design. We wish to dedicate this paper to the memory of our dear colleague and friend, Prof. Dr. Franziska Wollnik.
Abbreviation
- CPP
Conditioned place preference
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AT performed research, analyzed data, wrote the paper; WH designed research, analyzed data, wrote the paper. AT and WH read and approved the manuscript.
Contributor Information
Alexandra Trost, Email: alexandra.trost@gmx.de.
Wolfgang Hauber, Email: hauber@bio.uni-stuttgart.de.
References
- 1.Collier G, Hirsch E. Reinforcing properties of spontaneous activity in the rat. J Comp Physiol Psychol. 1971;77(1):155–160. doi: 10.1037/h0031588. [DOI] [PubMed] [Google Scholar]
- 2.Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Process. 2005;68(2):165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J Exp Anal Behav. 1993;60(1):219–238. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lattanzio SB, Eikelboom R. Wheel access duration in rats: I. Effects on feeding and running. Behav Neurosci. 2003;117(3):496–504. doi: 10.1037/0735-7044.117.3.496. [DOI] [PubMed] [Google Scholar]
- 5.Cosgrove KP, Hunter RG, Carroll ME. Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav. 2002;73(3):663–671. doi: 10.1016/S0091-3057(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 6.Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev. 2012;36(3):1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bliss EL, Ailion J. Relationship of stress and activity to brain dopamine and homovanillic acid. Physiol Pharmacol. 1971;10(20):1161–1169. doi: 10.1016/0024-3205(71)90276-1. [DOI] [PubMed] [Google Scholar]
- 8.Lett BT, Grant VL, Koh MT, Flynn G. Prior experience with wheel running produces cross-tolerance to the rewarding effect of morphine. Pharmacol Biochem Behav. 2002;72(1–2):101–105. doi: 10.1016/S0091-3057(01)00722-5. [DOI] [PubMed] [Google Scholar]
- 9.Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol Behav. 2001;72(3):355–358. doi: 10.1016/S0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- 10.Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34(1):87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- 11.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacol (Berl) 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 12.Cardinal RN. Neural systems of motivation. In: Koob GF, Le Moal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience, Three-Volume Set, 1-3 Volume 2, edn. Oxford: Elsevier Science; 2010. pp. 376–386. [Google Scholar]
- 13.Danna CL, Elmer GI. Disruption of conditioned reward association by typical and atypical antipsychotics. Pharmacol Biochem Behav. 2010;96(1):40–47. doi: 10.1016/j.pbb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickinson A, Smith J, Mirenowicz J. Dissociation of pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci. 2000;114(3):468–483. doi: 10.1037/0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- 15.Murschall A, Hauber W. Inactivation of the ventral tegmental area abolished the general excitatory influence of pavlovian cues on instrumental performance. Learn Mem. 2006;13(2):123–126. doi: 10.1101/lm.127106. [DOI] [PubMed] [Google Scholar]
- 16.Lex A, Hauber W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate pavlovian-instrumental transfer. Learn Mem. 2008;15(7):483–491. doi: 10.1101/lm.978708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbit LH, Janak PH, Balleine BW. General and outcome-specific forms of pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci. 2007;26(11):3141–3149. doi: 10.1111/j.1460-9568.2007.05934.x. [DOI] [PubMed] [Google Scholar]
- 18.Wassum KM, Ostlund SB, Balleine BW, Maidment NT. Differential dependence of pavlovian incentive motivation and instrumental incentive learning processes on dopamine signaling. Learn Mem. 2011;18(7):475–483. doi: 10.1101/lm.2229311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein A, Weinstein Y. Exercise, addiction-diagnosis, bio-psychological mechanisms and treatment issues. Curr Pharm Design. 2014;20:680–687. doi: 10.2174/13816128113199990614. [DOI] [PubMed] [Google Scholar]
- 20.Lett BT, Grant VL, Koh MT. Delayed backward conditioning of place preference induced by wheel running in rats. Learning Motiv. 2002;33:347–357. doi: 10.1016/S0023-9690(02)00002-4. [DOI] [Google Scholar]
- 21.Trezza V, Damsteegt R, Vanderschuren LJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol. 2009;19(9):659–669. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trezza V, Damsteegt R, Achterberg EM, Vanderschuren LJ. Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci. 2011;31(17):6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzig V, Capuani E, Kovar KA, Schmidt W. Effects of MPEP on expression of food‐, MDMA‐or amphetamine‐conditioned place preference in rats. Addict Biol. 2005;10(3):243–249. doi: 10.1080/13556210500223272. [DOI] [PubMed] [Google Scholar]
- 24.Tzschentke TM, Schmidt WJ. Blockade of morphine- and amphetamine-induced conditioned place preference in the rat by riluzole. Neurosci Lett. 1998;242(2):114–116. doi: 10.1016/S0304-3940(98)00023-8. [DOI] [PubMed] [Google Scholar]
- 25.Solomon RL. The opponent-process theory of acquired motivation: the costs of pleasure and the benefits of pain. Am Psychologist. 1980;35(8):691–712. doi: 10.1037/0003-066X.35.8.691. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed J, Buck S. The effect of regular aerobic exercise on positive-activated affect: a meta-analysis. Psychol Sport Exer. 2009;10(6):581–594. doi: 10.1016/j.psychsport.2009.05.009. [DOI] [Google Scholar]
- 28.Ekkekakis P, Petruzzello SJ. Acute aerobic exercise and affect: current status, problems and prospects regarding dose–response. Sports Med. 1999;28(5):337–374. doi: 10.2165/00007256-199928050-00005. [DOI] [PubMed] [Google Scholar]
- 29.Pitts SM, Horvitz JC. Similar effects of D(1)/D(2) receptor blockade on feeding and locomotor behavior. Pharmacol Biochem Behav. 2000;65(3):433–438. doi: 10.1016/S0091-3057(99)00249-X. [DOI] [PubMed] [Google Scholar]
- 30.Packard MG, Schroeder JP, Alexander GM. Expression of testosterone conditioned place preference is blocked by peripheral or intra-accumbens injection of alpha-flupenthixol. Horm Behav. 1998;34(1):39–47. doi: 10.1006/hbeh.1998.1461. [DOI] [PubMed] [Google Scholar]
- 31.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42(1):1–18. doi: 10.1016/0306-4522(91)90145-E. [DOI] [PubMed] [Google Scholar]
- 33.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Soc Neurosci. 2003;23(28):9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiroi N, White NM. The amphetamine conditioned place preference: differential involvement of dopamine receptor subtypes and two dopaminergic terminal areas. Brain Res. 1991;552(1):141–152. doi: 10.1016/0006-8993(91)90672-I. [DOI] [PubMed] [Google Scholar]
- 35.Spyraki C, Fibiger HC, Phillips AG. Cocaine-induced place preference conditioning: lack of effects of neuroleptics and 6-hydroxydopamine lesions. Brain Res. 1982;253(1–2):195–203. doi: 10.1016/0006-8993(82)90686-2. [DOI] [PubMed] [Google Scholar]
- 36.Martin-Iverson M, Ortmann R, Fibiger H. Place preference conditioning with methylphenidate and nomifensine. Brain Res. 1985;332(1):59–67. doi: 10.1016/0006-8993(85)90389-0. [DOI] [PubMed] [Google Scholar]
- 37.Mithani S, Martin-Iverson M, Phillips A, Fibiger H. The effects of haloperidol on amphetamine-and methylphenidate-induced conditioned place preferences and locomotor activity. Psychopharmacology. 1986;90(2):247–252. doi: 10.1007/BF00181251. [DOI] [PubMed] [Google Scholar]
- 38.Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27(46):12484–12488. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12(3–4):227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 40.Spyraki C, Fibiger HC, Phillips AG. Attenuation by haloperidol of place preference conditioning using food reinforcement. Psychopharmacology. 1982;77(4):379–382. doi: 10.1007/BF00432775. [DOI] [PubMed] [Google Scholar]
- 41.Garcia Horsman P, Paredes RG. Dopamine antagonists do not block conditioned place preference induced by paced mating behavior in female rats. Behav Neurosci. 2004;118(2):356–364. doi: 10.1037/0735-7044.118.2.356. [DOI] [PubMed] [Google Scholar]
- 42.Guyon A, Assouly-Besse F, Biala G, Puech AJ, Thiebot MH. Potentiation by low doses of selected neuroleptics of food-induced conditioned place preference in rats. Psychopharmacology. 1993;110(4):460–466. doi: 10.1007/BF02244653. [DOI] [PubMed] [Google Scholar]
- 43.Delamater AR, Sclafani A, Bodnar RJ. Pharmacology of sucrose-reinforced place-preference conditioning: effects of naltrexone. Pharmacol Biochem Behav. 2000;65(4):697–704. doi: 10.1016/S0091-3057(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 44.Agmo A, Galvan A, Talamantes B. Reward and reinforcement produced by drinking sucrose: two processes that may depend on different neurotransmitters. Pharmacol Biochem Behav. 1995;52(2):403–414. doi: 10.1016/0091-3057(95)00128-J. [DOI] [PubMed] [Google Scholar]
- 45.Pecina S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered ‘wanting’ for reward: entire core and medial shell mapped as substrates for PIT enhancement. European J Neurosci. 2013;37(9):1529–1540. doi: 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Field A. Discovering statistics using SPSS. London: SAGE Publications Ltd; 2009. [Google Scholar]
- 47.Rosenthal R. Meta-analytic procedures for social research, Vol. 6. London: SAGE Publications Ltd; 1991. [Google Scholar]


