Abstract
Background
Oral Campylobacter species have been found to be associated with periodontitis progression. While the etiological significance of Campylobacter rectus is quite established, the association of C. gracilis, C. concisus, and C. curvus with health or disease remains contradictory.
Objectives
This study hypothesizes that the proportion of species within the Campylobacter genus rather than the absolute abundance of a single species is a suitable indicator for periodontitis progression.
Design
Subgingival plaque from 90 periodontitis patients and gingival sulcus fluid of 32 healthy individuals were subjected to a newly developed nested PCR approach, in which all Campylobacter spp. were amplified simultaneously. The resulting mixture of 16S-rRNA-gene-amplicons were separated by single-stranded conformation polymorphism (SSCP) gel electrophoresis, followed by sequencing and identification of excised bands and relative quantification of band intensities. In all samples, the abundance of selected periodontitis marker species was determined based on DNA hybridization on a microarray.
Results
The highly prevalent Campylobacter community was composed of varying proportions of C. rectus, C. gracilis, C. concisus, and C. curvus. Cluster analysis based on SSCP-banding pattern resulted in distinct groups which in turn coincided with significant differences in abundance of established periodontitis marker species (Tannerella forsythia, Porphyromonas gingivalis, and Fusobacterium nucleatum) and progression.
Conclusions
The shift in the Campylobacter community composition seems to display the general microbial community shift during clinical progression in a simplified manner. The focus on members of the Campylobacter in this study suggests that this genus can be an indicator of ecological changes in the subgingival oral microflora.
Keywords: oral Campylobacter, periodontitis progression, microbial ecology, SSCP, ParoCheck genechip
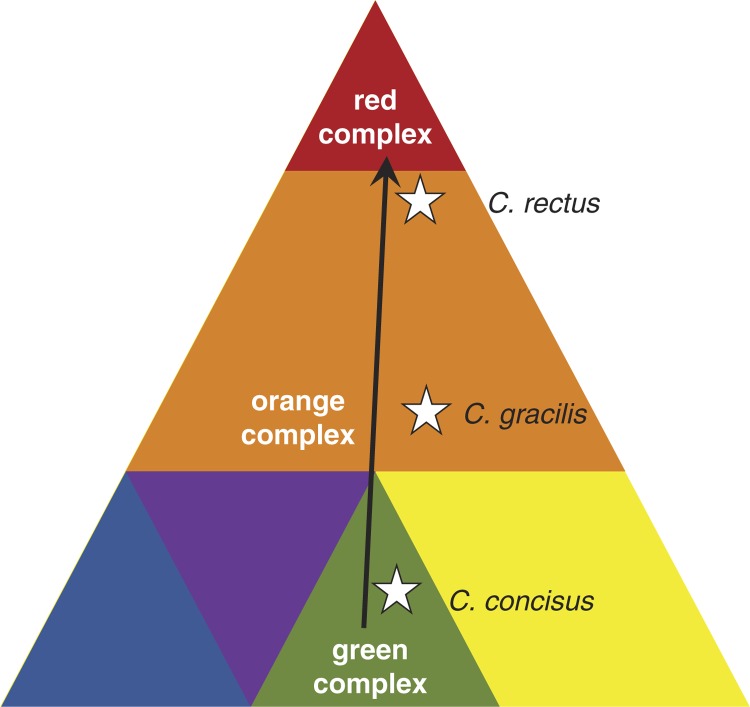
Periodontitis is an inflammatory disease of the periodontium, caused by different bacterial consortia, the so-called ‘microbial complexes’ (1). Socransky et al. visualized the progression of periodontitis as a pyramid with ‘colored’ components representing each a distinct microbial complex (2). While the complexes at the base of the pyramid (e.g. green and yellow complexes) comprise species that are thought to thrive under healthy conditions or at a very early state of periodontitis, the species of the orange complex become more dominant in an advanced stage and are thought to bridge base complexes and the red complex. The latter is forming the pyramidal roof at a late or final stage in progression.
Evidence exists that different oral Campylobacter species are associated with different stages of periodontitis progression. For instance, Campylobacter rectus and C. gracilis could be detected at elevated levels in diseased subgingival sites compared with healthy sites (3–6). Throughout the literature, C. rectus (former name Wolinella recta) is consistently described as being more prevalent and abundant in initial and established periodontitis. C. gracilis, although one of the most dominant Campylobacter species associated with periodontitis (4), was found according to other studies with higher prevalence and abundance in rather shallow subgingival sites (6, 7). The third most prevalent oral Campylobacter, C. concisus, seems to play an even more contradictory role in health and disease. While C. concisus is a recognized intestinal pathogen associated with Crohn's disease or gastroenteritis (8), at oral sites this species seems to be present predominantly in healthy subjects (6, 7).
According to Socransky's pyramid model, C. concisus is a member of the ‘green complex’, while C. gracilis and C. rectus are members of the ‘orange complex’. Based on correspondence analysis, C. gracilis showed more proximity to bacteria of the ‘green complex’ than to those of the ‘red complex’. In contrast, C. rectus showed strong association with members of the ‘red complex’ and further progression (1).
During periodontitis progression, the actual bacterial composition is thought to change gradually forming distinct microbial complexes. This may be accompanied by a continuous change of Campylobacter species within but also between each microbial complex (Fig. 1). Therefore, we hypothesize that the proportion of species within the genus Campylobacter, rather than the absolute abundance of a single species, is a suitable and dynamic parameter for periodontitis progression.
Fig. 1.
Pyramid of periodontitis complexes and the approximate positions (and the dynamic change) of typical Campylobacter species visualizing the hypothesis of this study. Modified from Ref. 2.
Material and methods
Subjects
Subgingival plaque samples of 90 consecutive periodontitis patients (aged 40–69, mean 52.7) were collected at the Clinic of Operative and Preventive Dentistry and Periodontology, Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen, Germany, for microbial analysis. In order to display the whole range of periodontitis-associated diseases, all patients that were diagnosed with ‘periodontitis’ were included. As a control, 32 samples of subgingival crevicular fluid were collected from dental students of the RWTH Aachen University Hospital (aged 21–33, mean 23.7) with a healthy periodontal status. Subjects who had taken antibiotics within the last 3 months were excluded. All participating subjects were informed about the study and signed an informed consent that had been approved in accordance with the guidelines of the Ethics Committee of the RWTH University Hospital, Aachen. From the periodontitis group, subgingival plaque was collected from 1 to 4 inflamed and deep periodontal pockets (>5 mm) using 1–4 sterile paper points ISO 45 (Alfred Becht GmbH, Offenburg, Germany). From the healthy control group, sulcus fluid was sampled from region 24 and 36 with the same method. Bacterial cells were recovered from paper points by vortexing with glass beads (diameter 1–2 mm) and subsequent centrifugation. All bacteria suspensions were stored frozen at −20°C until further analysis.
DNA extraction and chip hybridization
For each sample, DNA was extracted and purified with the Qiamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions with few changes as described previously (9). DNA was stored at −20°C until further use.
For abundance determination of Tannerella forsythia, Porphyromonas gingivalis, and Fusobacterium nucleatum (and seven other species not discussed here in depth) the ParoCheck® detection system (Greiner Bio One, Frickenhausen, Germany) based on universal 16S-rRNA-gene amplification and subsequent chip-hybridization was used. The amplification and hybridization were performed according to the instructions of the manufacturer which has also been described in detail previously by our group (9). Based on the resulting fluorescence signal, the signal-to-noise ratio (SNR) was calculated and used as an indication of species abundance.
Seminested PCR amplification
Purified DNA was subjected to a preamplification-PCR based on the universal forward primer 27F (5′-AGA GTT TGA TCM TGG CTC AG-3′) (10), and based on the reverse primer EPS914R specific to Epsilonproteobacteria (5′-GGT CCC CGT CTA TTC CTT-3′) (11), resulting in an amplicon length of 907 bp. PCR was carried out using 1 µl DNA template, 4 µl 10X PCR buffer, 1.6 µl DMSO, 0.8 µl of each primer (20 pmol/µl), 0.5 µl dNTPs (40 mM), and 0.5 µl Taq-polymerase in a final volume of 40 µl. After initial denaturation of 2 min at 95°C and subsequent 25 cycles (30 s at 95°C, 30 s at 60°C, 70 s at 72°C) a final elongation followed for 10 min at 72°C. One microliter of this PCR mixture was then subjected to a second PCR using the forward primer c-gsp8 (5′-GCG TGG AGG ATG ACA CTT TTC-3′) specific to the genus Campylobacter. The reverse primer EPS914R was kept identical as in the first-round PCR except that it was phosphorylated at the 5′ end. PCR was carried out using 4 µl 10X PCR buffer, 0.8 µl of each primer (20 pmol/µl), 1.6 µl dNTPs (40 mM), and 0.5 µl Taq-polymerase in a final volume of 40 µl. Starting with an initial denaturation of 2 min at 95°C, 32 cycles (30 s at 95°C, 30 s at 60°C, 40 s at 72°C) were followed by a final elongation for 10 min at 72°C. The length of the amplicon was 509 bp.
SSCP gel electrophoresis
Single-stranded conformation polymorphism (SSCP) is based on single-stranded DNA, which adopts a secondary/tertiary structure, determined by sequence-dependent intramolecular interactions. The migration through the gel matrix is affected by the secondary structure; it is hampered by spread out molecules and fast with compact molecules. The result of an SSCP electrophoresis is a pattern of bands in a lane, with each band ideally corresponding to one species/phylotype in the original community.
SSCP gel electrophoresis was performed with slight modifications according to the protocol from Schwieger and Tebbe (12). Briefly, in order to obtain single-stranded DNA, the phosphorylated reverse strand of the amplicons from the semi-nested PCR was digested by lambda exonuclease (New England Biolabs, Schwalbach, Germany) and further purified using the NucleoSpin Gel and PCR Clean-up Kit (Macherey-Nagel, Düren, Germany) following the manufacturer's instructions. Vacuum-dried single-stranded DNA was then resuspended in denaturating SSCP-loading buffer (47.5% formamide, 5 mM sodium hydroxide, 0.12% bromophenol blue, and 0.12% xylene cyanol), heated to 95°C for 5 min, and immediately chilled on ice. MDE-Gels for electrophoresis (6 ml 2X MDE®-Gel-Solution; Lonza, Cologne, Germany), 2 ml 10X TBE buffer, 12 ml bidistilled water, 8 µl TEMED (tetramethylethylenediamine), and 80 µl APS (ammonium persulfate, 10%) were cast between quadratic glass plates (20 cm) with 0.4 mm spacer. Running conditions were 400 V for 18 h at 20°C in 1X TBE buffer. After electrophoresis, the gels were silver stained with the protocol proposed by Bassam et al. (13).
Sequence analysis and identification of bands
To confirm the specificity of bands, three or more bands per running distance were excised and eluted in 30 µl gel-extraction buffer (10 mM Tris, 5 mM KCl, 1.5 mM MgCl2, 0.1% Triton X 100, pH 9.0) at 95°C for 20 min. One microliter of this eluate was used as template for the Campylobacter-specific PCR described above. The resulting amplicons were purified using the NucleoSpin Gel and PCR Clean-up Kit (Macherey-Nagel, Düren, Germany) and subsequently sequenced with the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Darmstadt, Germany) followed by purification using DyeEx 2.0 Spin Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The sequences were analyzed on the ABI PRISM™ 310 Genetic Analyser (Applied Biosystems, Darmstadt, Germany) and post edited with Bioedit (V. 7.1.3) (14). Sequences were then classified with the ‘sequence match’ function of the Ribosomal Database Project website (RDP) (15) and the ‘Nucleotide Blast’ tool of the NCBI (National Center for Biotechnology Information) website. Because C. rectus and C. showae cannot clearly be distinguished based on a 509 bp long amplicon, and because of the overall rareness of C. showae, corresponding sequences were consistently assigned to C. rectus.
Data analysis
Silver-stained SSCP gels were scanned with incident light conditions at 300 dpi. Resulting scans were edited with ImageJ software in four steps (16): 1) the image was inverted and rotated by 90° anticlockwise. 2) For noise reduction, the ‘median-filter’ option with one pixel radius was used. 3) For the background subtraction, we used the ‘sliding paraboloid’ option with 50 pixel radius. 4) Finally, we extracted the XY-data of the electropherogram with the ‘plot profile’ option. A self-programmed excel sheet was used to model Gauss’ distributions into the peaks of the electropherogram, and from the distributions’ parameters, the areas under the peaks could be calculated. The area of a species-specific peak divided by the total area of the electropherogram equals the relative proportion of this species inside the Campylobacter genus. The Pearson distance was calculated based on this relative proportion of species for each sample and subsequently used to create an UPGMA (Unweighted Pair Group Method with Arithmetic mean) tree with MEGA5 (17). Tests for significance were calculated with SPSS using the non-parametric Wilcoxon–Mann–Whitney Test.
Results
From 90 samples belonging to the periodontitis group (designated as P-001 to P-090), we were able to amplify Campylobacter spp. in 72 samples (80%) with our nested PCR approach. In contrast, only 19 out of 32 samples (59%) of the healthy control group (designated as KS-01 to KS-32) were Campylobacter positive. The overall abundance for Campylobacter, if present, was found to be between 3.2×101 and 1.5×105 cells/µl, measured by q-PCR (data not shown).
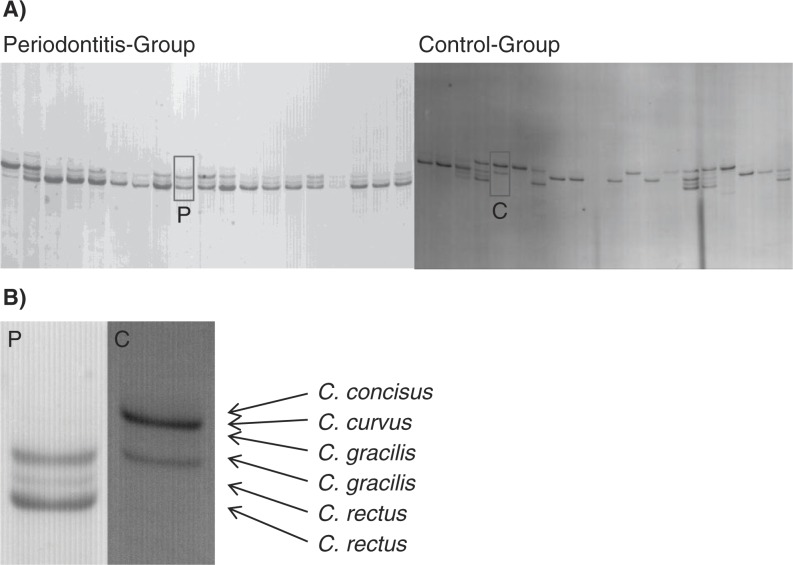
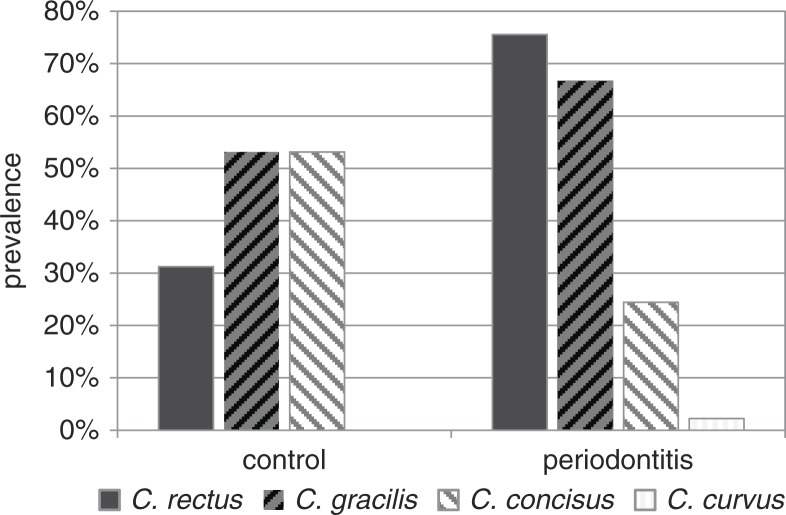
Subsequent SSCP analysis resulted in reproducible banding patterns with six major bands (Fig. 2). Excision of at least three bands per running distance and gel, followed by re-amplification, sequencing, and analysis with the seqmatch tool of the RDP-Database revealed that each band-height corresponded to one distinct oral Campylobacter species. However, the two species C. rectus and C. gracilis split up into double bands, with one major and one weaker band. All analyzed sequences belonged exclusively to the typical oral Campylobacter species, namely C. rectus, C. gracilis, C. concisus, and C. curvus, proving the PCR specificity. No atypical Campylobacter species or phylotypes were detected. In the periodontitis group, of 90 cases C. rectus was present in 68 cases, C. gracilis in 60 cases, C. concisus in 22 cases, and C. curvus in 2 cases. In the healthy control group, only 10 out of 32 cases showed C. rectus, 17 C. gracilis, and 17 C. concisus. C. curvus was not found in the control group (Fig. 3).
Fig. 2.
(A) SSCP gels illustrating examples of periodontitis group (left) and control group (right). (B) Enlargement of Fig. 2 A. Arrows indicate species-specific running distances.
Fig. 3.
Prevalence of different Campylobacter species in the periodontitis versus control group.
There were distinct differences between the band intensities, reflecting species abundance, of the diseased group and the healthy control group. In the diseased group, C. rectus showed clearly the most intense bands, while C. gracilis was less abundant and C. concisus only rarely present. In the control group, the bands of C. gracilis and C. concisus were the most intense ones, while C. rectus was only rarely present.
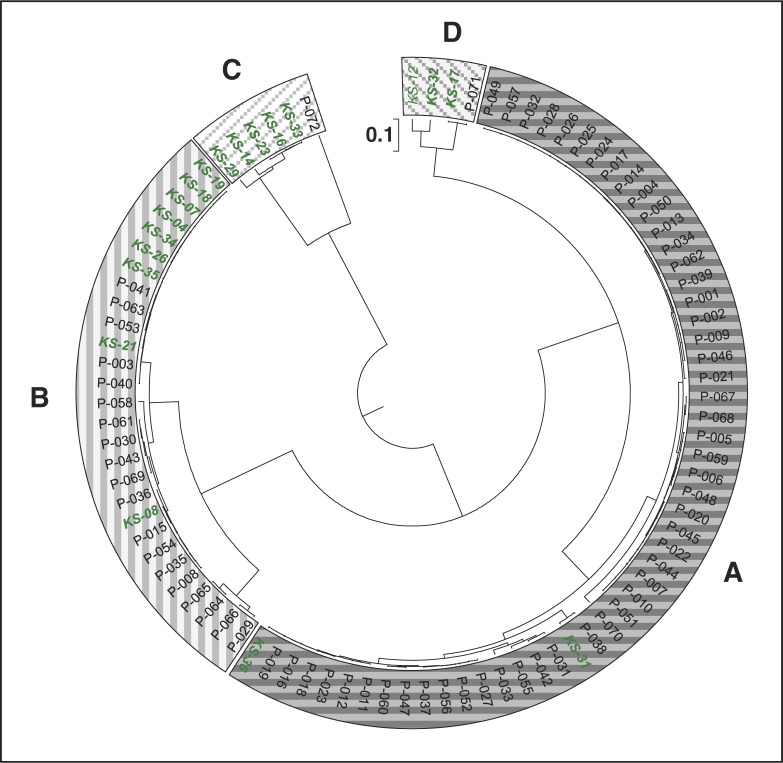
Cluster analysis based on the SSCP profiles resulted in four distinct main clusters (Fig. 4), which we refer to as ‘A to D’, which symbolize – according to our hypothesis – levels of an ecological shift among members of Campylobacter species: Cluster A is dominated by C. rectus with only low proportions of the other Campylobacter species. Most samples (71%) of the periodontitis group fall into this cluster but only two cases (KS-31 and KS-36) in the healthy controls. Cluster B is dominated by C. gracilis. Some members (26%) of the periodontitis group belong to this cluster as well as many members (47%) of the healthy control group. Cluster C is dominated by C. concisus and consists of members of the healthy control group only, except patient P-072. Cluster D exhibits elevated clusters of both C. rectus and C. concisus and is mainly composed of healthy members again with one exception (P-071).
Fig. 4.
Cluster analysis based on the SSCP banding pattern. Subjects of the periodontitis group (designated as P-001 to P-090) are depicted in black, control group (designated as KS-01 to KS-36) in green italic. Boxes indicate the four main clusters, referred to as ‘cluster A’ to ‘cluster D’, representing an ecological shift.
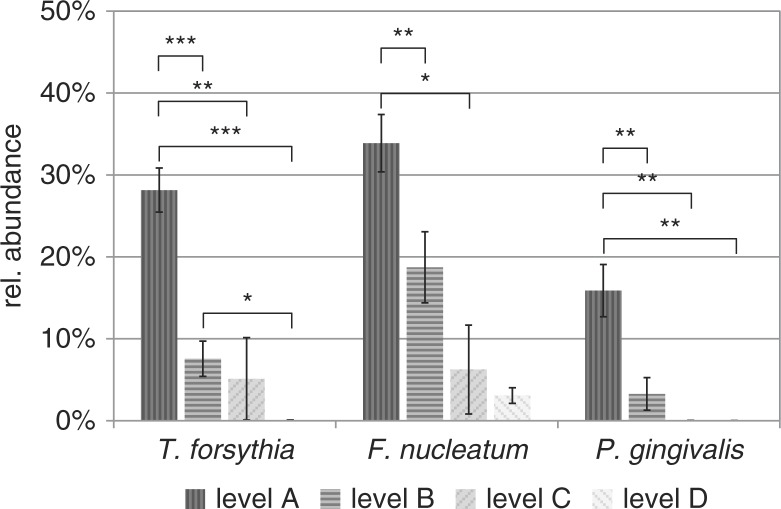
T. forsythia showed the highest mean abundance in cluster A (28.2%, highest SNR-value was set to 100%), a low abundance in cluster B (7.6%) and cluster C (5.1%), and very low abundance in cluster D (0.1%) (Fig. 5). The same clear decrease in abundance was confirmed by data from P. gingivalis (A: 15.9%, B: 3.3%, C: 0%, D: 0%) and for F. nucleatum (A: 33.9%, B: 18.7%, C: 6.2%, D: 3.1%).
Fig. 5.
Relative abundance of three prominent periodontitis marker species in proposed ecological shift clusters A–D. Highest occurring SNR value of each species was set to 100%. Asterisks indicate significant differences between ecological clusters (*p<0.05; **p<0.01; ***p<0.001).
Discussion
The importance of Campylobacter spp. in oral communities is emphasized by the high prevalence of species we found mainly in periodontitis but also in healthy subjects. This implies that the sheer presence of Campylobacter cannot function as an indicator for disease. However, the prevalence of different Campylobacter species (‘the Campylobacter-mix’) differs between the healthy and the periodontitis group, with C. rectus as the most prevalent species in periodontitis samples and C. gracilis and C. concisus primarily present in healthy controls. Using cultivation-based methods, Macuch and Tanner showed comparable occurrences of C. rectus and C. gracilis, with a low prevalence of Campylobacter in the healthy control group (3). Applying molecular methods C. rectus, C. gracilis, and C. concisus were found in both healthy and diseased subjects in different abundances but no study so far addressed the dynamic of species mix (18–20).
Although our PCR primers were designed broad enough to cover a wide range of members out of the genus Campylobacter, hitherto unknown (or at least for the oral cavity unusual) Campylobacter species were not found here. No taxa other than Campylobacter were found, underlining the specificity of our approach.
The existence of four major SSCP-based clusters reflecting distinct levels of an ecological shift was supported by concurrent shifts in abundance of T. forsythia, P. gingivalis, and F. nucleatum. These SSCP-based clusters differed markedly from each other in distinct features, 1) clusters A to C are dominated by different Campylobacter species, 2) the proportion of periodontitis subjects is increasing from cluster C to A, while the proportion of healthy subjects is decreasing from cluster C to A, 3) the abundance of prominent periodontitis marker species increases significantly from cluster C to A. Cluster D, represented by only three healthy controls and one patient (P-071), is characterized by low proportions of C. gracilis and an elevated proportion of C. concisus, but shows similarities to the ecological situation in cluster C, representing health.
In total, only four samples were grouped outside their expected cluster. In the ‘disease cluster A’, the two controls KS-31 and KS-36 grouped together with 51 patients. Interestingly, KS-31 showed the highest F. nucleatum abundance of all control samples, which might be indicative for an ecological disturbance preceding the onset of disease. However, we have no plausible explanation for the unusual clustering of KS-36. Patient P-071 clustering in the ‘healthy cluster D’ was devoid of P. gingivalis and T. forsythia which might indicate an ‘ecological recovery’ toward a healthy condition. Finally, patient P-072 clustering in the ‘healthy cluster C’ is unique by its ecological profile as it was the only case dominated by C. curvus. The ecological role of C. curvus in periodontitis was discussed in a few publications, and it can be concluded that it is more related with (initial) periodontitis than with health, which is in concordance with our data (3, 7). However, once more individuals have been tested, samples rich with C. curvus probably deserve their own cluster ‘E’.
In this pilot study, we used a periodontitis group and a control group that were not age-matched, hence we consider our results as preliminary but promising which warrants further investigation. We also want to mention that cross-sectional studies – even if including a high number of consecutive periodontitis patients and controls – have their limit to estimate the actual progression. Therefore, as a next step in order to verify the results, we will compare in a future study age-matched groups in a longitudinal study. In addition, the abundance of additional recognized periodontal marker bacteria, such as Treponema denticola, Aggregatibacter actinomycetemcomitans, and Prevotella intermedia, will be included for assessing the overall suitability of the Campylobacter ratios as a feasible and dynamic parameter for periodontitis progression.
Conflict of interest and funding
There is no conflict of interest in the present study for any of the authors.
References
- 1.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–4. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 2.Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 3.Macuch PJ, Tanner ACR. Campylobacter species in health, gingivitis, and periodontitis. J Dent Res. 2000;79:785–92. doi: 10.1177/00220345000790021301. [DOI] [PubMed] [Google Scholar]
- 4.Rawlinson A, Eley A, Bennett KW, Goodwin L. Bacteroides gracilis in periodontal health and disease. Microb Ecol Health Dis. 1994;7:201–5. doi: 10.3109/08910609409141355. [DOI] [Google Scholar]
- 5.Badger SJ, Tanner ACR. Serological studies of Bacteroides gracilis, Campylobacter concisus, Wolinella recta, and Eikenella corrodens, all from humans with periodontal disease. Int J Syst Bacteriol. 1981;31:446–51. doi: 10.1099/00207713-31-4-446. [DOI] [Google Scholar]
- 6.Haririan H, Andrukhov O, Bertl K, Lettner S, Kierstein S, Moritz A, et al. Microbial analysis of subgingival plaque samples compared with that of whole saliva in periodontitis patients. J Periodontol. 2013;85(6):819–28. doi: 10.1902/jop.2013.130306. [DOI] [PubMed] [Google Scholar]
- 7.Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43:3944–55. doi: 10.1128/JCM.43.8.3944-3955.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaakoush NO, Deshpande NP, Wilkins MR, Tan CG, Burgos-Portugal JA, Raftery MJ, et al. The pathogenic potential of Campylobacter concisus strains associated with chronic intestinal diseases. PLoS One. 2011;6:e29045. doi: 10.1371/journal.pone.0029045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vianna ME, Horz H-P, Gomes BPFA, Conrads G. Microarrays complement culture methods for identification of bacteria in endodontic infections. Oral Microbiol Immunol. 2005;20:253–8. doi: 10.1111/j.1399-302X.2005.00221.x. [DOI] [PubMed] [Google Scholar]
- 10.Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematics. Chichester: John Wiley & Sons; 1991. pp. 115–47. [Google Scholar]
- 11.Grote J, Jost G, Labrenz M, Herndl GJ, Jürgens K. Epsilonproteobacteria represent the major portion of chemoautotrophic bacteria in sulfidic waters of pelagic redoxclines of the Baltic and Black Seas. Appl Environ Microbiol. 2008;74:7546–51. doi: 10.1128/AEM.01186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwieger F, Tebbe CC. A new approach to utilize PCR–single-strand-conformation polymorphism for 16S rRNA gene-based microbial community analysis. Appl Environ Microbiol. 1998;64:4870–6. doi: 10.1128/aem.64.12.4870-4876.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassam BJ, Gresshoff PM. Silver staining DNA in polyacrylamide gels. Nat Protoc. 2007;2:2649–54. doi: 10.1038/nprot.2007.330. [DOI] [PubMed] [Google Scholar]
- 14.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic acids symposium series. 1999;41:95–8. Available from: http://jwbrown.mbio.ncsu.edu/JWB/papers/1999Hall1.pdf [cited 21 August 2014]. [Google Scholar]
- 15.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–43. [Google Scholar]
- 17.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bik EM, Long CD, Armitage GC, Loomer P, Emerson J, Mongodin EF, et al. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010;4:962–74. doi: 10.1038/ismej.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen RF, Harrington CS, Kortegaard HE, On SL. A PCR-DGGE method for detection and identification of Campylobacter, Helicobacter, Arcobacter and related Epsilobacteria and its application to saliva samples from humans and domestic pets. J Appl Microbiol. 2007;103:2601–15. doi: 10.1111/j.1365-2672.2007.03515.x. [DOI] [PubMed] [Google Scholar]