Abstract
Purpose
An intracranial aneurysm, with or without subarachnoid hemorrhage (SAH), is a relevant health problem. The rupture of an intracranial aneurysm is a critical concern for individual health; even an unruptured intracranial aneurysm is an anxious condition for the individual. The aim of this guideline is to present current and comprehensive recommendations for the management of intracranial aneurysms, with or without rupture.
Materials and Methods
We performed an extensive literature search, using Medline. We met in person to discuss recommendations. This document is reviewed by the Task Force Team of the Korean Society of Interventional Neuroradiology (KSIN).
Results
We divided the current guideline for ruptured intracranial aneurysms (RIAs) and unruptured intracranial aneurysms (UIAs). The guideline for RIAs focuses on diagnosis and treatment. And the guideline for UIAs focuses on the definition of a high-risk patient, screening, principle for treatment and selection of treatment method.
Conclusion
This guideline provides practical, evidence-based advice for the management of patients with an intracranial aneurysm, with or without rupture.
Keywords: Intracranial aneurysm, Management, Guideline
An intracranial aneurysm, with or without subarachnoid hemorrhage (SAH), is a relevant health problem. Preliminary reports about guidelines dealing with diagnosis and treatment of ruptured and unruptured intracranial aneurysms were published in Neurointervention (official journal of Korean Society of Interventional Neuroradiology, KSIN) in 2007 [1, 2]. And, there was a recent update of guidelines for neurointerventional procedures, which was also published in Neurointervention in 2013 [3].
Currently, there is a need to clarify the clinical practice guidelines covering this issue. We focus on diagnosis and treatment for ruptured intracranial aneurysms (RIAs) and defining high-risk patients, screening, principle for treatment and selection of treatment method of unruptured intracranial aneurysms (UIAs). We performed an extensive literature search, using Medline. We met in person to discuss recommendations. This document is reviewed by the Task Force Team of the Korean Society of Interventional Neuroradiology (KSIN).
Diagnosis and treatment of ruptured intracranial aneurysms (RIAs)
Diagnosis of aneurysmal subarachnoid hemorrhage (SAH)
Noncontrast head computed tomography (CT) remains the cornerstone for diagnosis of acute SAH [4, 5, 6]. The sensitivity of CT in the first 3 days after SAH remains very high (close to 100%), after which it decreases moderately during the following few days [7]. After 5 to 7 days, the rate of negative CT increases sharply, and lumbar puncture is often required to show xanthochromia.
However, advances in magnetic resonance imaging (MRI) of the brain, particularly the use of fluid-attenuated inversion recovery, proton density, diffusion-weighted imaging, and gradient echo sequences, can often allow the diagnosis of SAH to be made when a head CT scan is negative and there is clinical suspicion of SAH, possibly avoiding the need for lumbar puncture. The role of MRI in perimesencephalic SAH is controversial [8, 9, 10].
Recommendations
1. Acute diagnostic workup should include noncontrast head CT, which, if nondiagnostic, should be followed by lumbar puncture [5].
2. Magnetic resonance imaging (fluid-attenuated inversion recovery, proton density, diffusion-weighted imaging, and gradient echo sequences) may be reasonable for the diagnosis of SAH in patients with a nondiagnostic CT scan, although a negative result does not obviate the need for cerebrospinal fluid analysis [5].
3. Lumbar puncture must be performed in a case of clinically suspected SAH, if CT or MRI does not confirm the diagnosis [11].
Diagnosis of ruptured intracranial aneurysms (RIAs)
Compared with digital subtraction angiography (DSA), computed tomography angiography (CTA) has many advantages. It is non-invasive, fast, and has fewer limitations concerning equipment or manpower, and it is possible to examine unstable patients easily. On the other hand, CTA has disadvantages, which are the requirements of radiation and contrast. Furthermore, it has a low sensitivity for detecting small aneurysms less than 3 mm, and a low negative predictive value relative to DSA [12, 13, 14, 15]. Therefore, in the case of a negative finding of CTA, controversy about whether or not to perform a DSA exists [16, 17]. However, because of the development of CTA-MMBE (multisection CTA combined with matched mask bone elimination) or dual energy CTA, high sensitivity can be expected using a relatively low radiation dose [18, 19].
Magnetic resonance angiography (MRA) has the advantage that it does not give radiation to the patients. There are many disadvantages, however, including the need for a long examination time, motion artifacts, and low sensitivity and specificity [20].
There are controversies about performing a DSA to decide the treatment modality. In one series, 95.7% of patients with SAH were referred for treatment on the basis of CTA [21]. But, patients who are not diagnosed by CTA required DSA. The sensitivity of 3D angiography is far superior to 2D angiography [22, 23]. DSA using 2D and 3D is considered to be the diagnostic modality, and is an essential part of the diagnosis.
Because flat-panel volumetric CT can be checked in an angio suite instantly, it could be used to monitor changes in the patient, such as hydrocephalus or rebleeding [24].
Recommendations
1. CTA may be considered in the workup of SAH. If an aneurysm is detected by CTA, this study may help guide the decision for the type of aneurysm repair, but if CTA is inconclusive, DSA is still recommended (except possibly in the instance of classic perimesencephalic SAH) [5].
2. DSA with 3-dimensional rotational angiography is indicated for detection of an aneurysm in patients with SAH (except when the aneurysm was previously diagnosed by a noninvasive angiogram) and for planning treatment (to determine whether an aneurysm is amenable to coiling or to expedite microsurgery) [5].
3. DSA of all cerebral arteries should be performed, if a bleeding source was not found on CTA and the patient has a typical basal SAH pattern on CT [11].
4. If no aneurysm was found, CTA or DSA should be repeated as described below: SAH without aneurysm [11].
Treatment of ruptured intracranial aneurysms (RIAs)
Since the invention of detachable coils by Guglielmi et al. in 1991 [25], endovascular treatment of aneurysms has become increasingly accepted and has been applied to a growing fraction of patients. After the International Subarachnoid Aneurysmal Trial (ISAT), the first multicenter randomized study on endovascular coiling [26], the method has grown up to be the main treatment modality for aneurysm treatment. However, there is still a controversy over which modality of treatment should be chosen for an intracranial aneurysm, considering the aneurysm location, shape and patient's condition. Studies on the pros and cons about coiling are still under investigation now.
In ISAT, the rate of death and disability at 1 year after treatment was presented as 24% in coiling group and 31% in clipping group [26].
The main reason for higher mortality in the clipping group over the coiling group (22% vs. 16%) was thought to be that long treatment time and high complication rate during surgical clipping influenced the result [27,28]. After treatment, seizure was frequent in the clipping group. However, the rebleeding rate was higher in the coiling group (2.9% vs. 0.9%) [26]. In the coiling group, neck diameter and dome size were related to incomplete treatment and rebleeding [25]. Furthermore, a very small size aneurysm (below 3 mm) was related to failure of treatment [29].
Recommendations
1. Surgical clipping or endovascular coiling of the ruptured aneurysm should be performed as early as feasible in the majority of patients to reduce the rate of rebleeding after SAH [5].
2. Complete obliteration of the aneurysm is recommended whenever possible [5].
3. Determination of aneurysm treatment, as judged by both experienced neurovascular surgeons and neurointerventionalists, should be a multidisciplinary decision based on characteristics of the patient and the aneurysm [5].
4. For patients with ruptured aneurysms judged to be technically amenable to both endovascular coiling and neurosurgical clipping, endovascular coiling should be considered [5].
5. In the absence of a compelling contraindication, patients who underwent a coiling or clipping of a ruptured aneurysm should be examined by follow-up vascular imaging (timing and modality to be individualized), and strong consideration should be given to retreatment, either by repeat coiling or microsurgical clipping, if there is a clinically significant (e.g., growing) remnant [5].
6. In general, the decision on whether to clip or coil depends on several factors related to 2 major components:
(1) Patient: age, comorbidity, presence of ICH, SAH grade, aneurysm size, location and configuration, as well as status of collaterals
(2) Procedure: competence, technical skills and availability
7. Factors in favor of operative intervention (clipping) are: younger age, presence of space occupying ICH, and aneurysm-specific factors such as:
- location: middle cerebral artery and pericallosal aneurysm
- wide aneurysm neck
- arterial branches exiting directly out of the aneurysmal sac
- other unfavorable vascular and aneurysmal configuration for coiling [11]
8. Factors in favor of endovascular intervention (coiling) are: age above 70 years, space occupying ICH not present, and aneurysm-specific factors such as posterior circulation, small aneurysm neck and unilobar shape [11]
9. Stenting of a ruptured aneurysm is associated with increased morbidity and mortality, and should only be considered when less risky options have been excluded [5].
Screening and treatment of unruptured intracranial aneurysms (UIAs)
Screening of unruptured intracranial aneurysm
SAH due to the rupture of an intracranial aneurysm usually has a poor prognosis despite the recent advances in management [30].
Prevention of rupture would be of great importance to reduce the mortality and morbidity of SAH caused by intracranial aneurysm, and screening tests for high-risk populations are being considered [31].
Definition of high risk populations for screening of unruptured aneurysm
When 2 first- through third-degree relatives have an intracranial aneurysm, the risk of harboring an unruptured aneurysm was 8% with a relative risk of 4.2 [32, 33]. In the familial intracranial aneurysm, this is associated with SAH at a younger age and a high incidence of multiple aneurysms [32].
Variable conditions associated with intracranial aneurysms include moya-moya disease, pituitary adenoma [34], sickle cell disease [35], fibromuscular dysplasia, systemic lupus erythematosus [36], coarctation of aorta, and cerebral arteriovenous malformation. Genetic conditions associated with intracranial aneurysms include autosomal dominant polycystic kidney disease(ADPKD) [37, 38, 39], Marfan's syndrome [40], neurofibromatosis type I [41], multiple endocrine neoplasia type I [42], pseudoxanthoma elasticum [43,44], hereditary hemorrhagic telangiectasia [45] and type IV Ehlers-Danlos syndrome [46]. For example, aneurysms occur in 10-22.5% of ADPKD patients. ADPKD patients may need a screening test for unruptured intracranial aneurysms [37, 38, 39].
In other conditions, screening test may be considered on an individual basis. In patients who have been treated for a ruptured aneurysm, the annual rate of new aneurysm formation is 1-2% per year [47], and patients with multiple intracranial aneurysms may be particularly susceptible to new aneurysm formation [48].
Screening for unruptured intracranial aneurysms in the general population without a family history of SAH is currently not supported due to low cost-effectiveness [49].
Recommendations
1. Screening for unruptured intracranial aneurysms may be considered for individuals who have 2 or more first-degree relatives with an intracranial aneurysm.
2. Screening for unruptured intracranial aneurysms may be considered for patients with ADPKD.
3. In patients with previous SAH due to aneurysmal rupture, regular screening for detecting new aneurysms should be considered.
4. Screening for unruptured intracranial aneurysms may not be considered for patients with a negative family history of SHA and no known risk factors and/or genetic factors for an intracranial aneurysm.
Screening methods for unruptured intracranial aneurysms
As noninvasive imaging for screening, three-dimensional time-of-flight magnetic resonance angiography (MRA) or computed tomography angiography (CTA) may be useful [50, 51]. But, catheter angiography is the recommended the gold standard when it is clinically imperative to know if an aneurysm exists [4].
Treatment for unruptured intracranial aneurysm
Introduction
The management of an unruptured intracranial aneurysm should be determined based on the natural history of the lesion, but data about the natural history of unruptured intracranial aneurysms are limited. All aspects of patient-specific factors of age, co-morbidity, and health condition and aneurysm-specific factors of size, location, and morphology should be taken into account for the treatment decision. Aneurysmal clipping has been the standard method for treatment. However, with the technological advances in devices, endovascular treatment has been used with increasing frequency. The selection of a treatment method for an unruptured intracranial aneurysm should be individualized based on patient's factors, aneurysmal factors, and facility and performance of centers.
In 2013, Greving et al. presented the PHASES score for prediction of risk of rupture of intracranial aneurysms [52]. The scoring system was developed from a pooled analysis of individual patient data from 8382 participants in six prospective cohort studies. Predictors included age, hypertension, history of SAH, aneurysm size, aneurysm location, and geographical region, and were independently associated with the rupture risk of an intracranial aneurysm. According to the PHASES score, a high PHASES score corresponds to a great 5-year risk of aneurysm rupture (Table 1). It is not yet complete, but this study is the first proposal to reliably predict the long-term risk of aneurysm rupture and a risk prediction chart could serve as a valuable aid for treatment of an UIA.
Table 1.
PHASES Aneurysm Risk Score
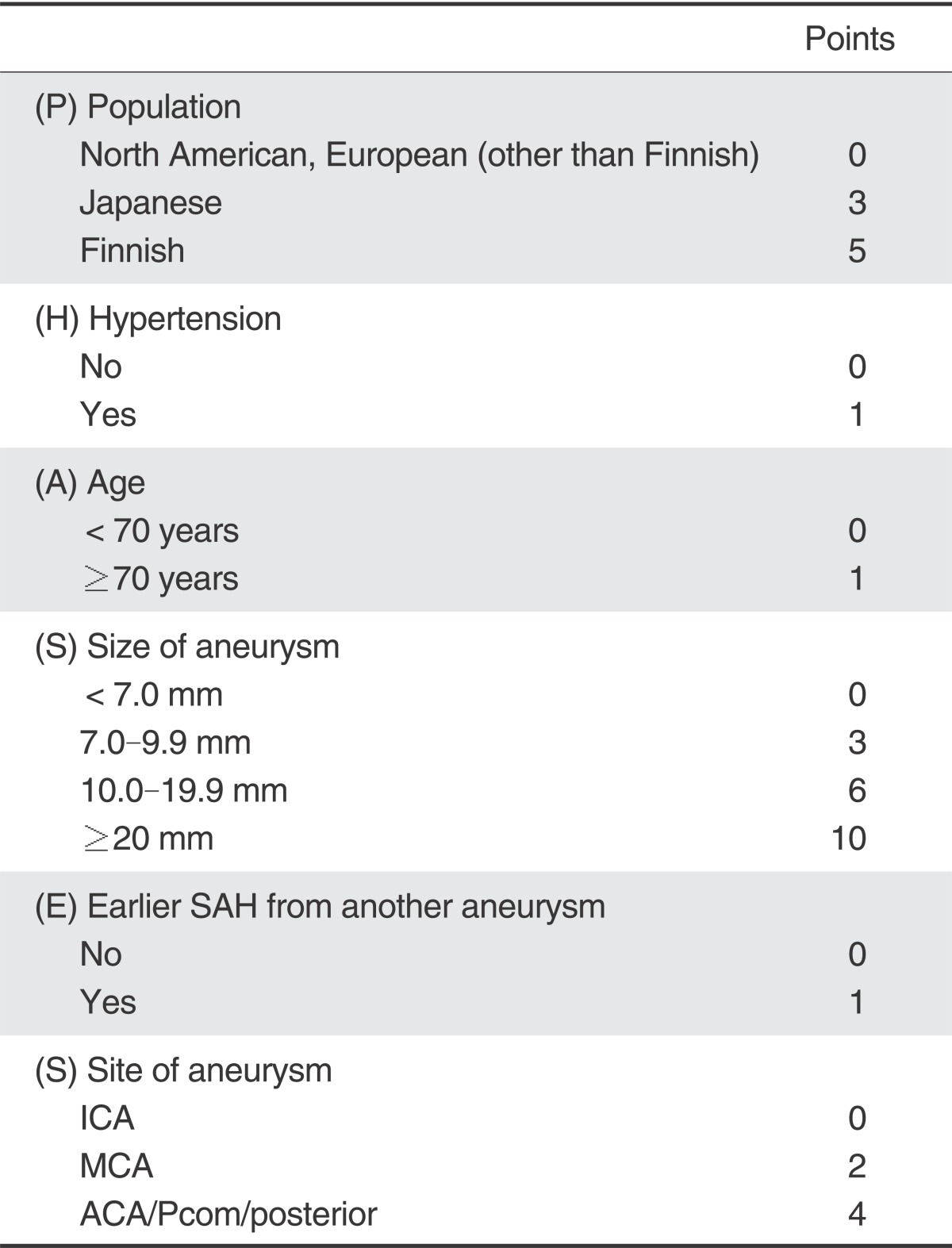
To calculate the PHASES risk score for an individual, the number of points associated with each indicator can be added up to obtain the total risk score. SAH=subarachnoid haemorrhage. ICA=internal carotid artery. MCA=middle cerebral artery. ACA=anterior cerebral arteries (including the anterior cerebral artery, anterior communicating artery, and pericallosal artery). Pcom=posterior communicating artery. posterior=posterior circulation (including the vertebral artery, basilar artery, cerebellar arteries, and posterior cerebral artery). Reference [52].
General principles
Aneurysm size was an important predictor of rupture risk and could be considered preferentially in determining whether to treat. In ISUIA (International Study of UIAs) published in 2003, calculating the total risk of rupture for patients demonstrates that for aneurysms 7 to 12 mm, 13 to 24 mm, and greater than 25 mm in diameter, the yearly rupture rates are 1.2, 3.1, and 8.6%, respectively [53]. Small, single incidental aneurysms less than 5 mm in diameter should be managed conservatively. However, treatment of a small aneurysm would be considered a relative rupture risk according to risk factors like location, history of SAH, symptomatic intracranial aneurysm, family history of aneurysm, and aneurysm with a multilobule or bleb.
SUAVe (Small Unruptured Aneurysm Verification), published in 2010, was a prospective study to assess the annual risk of rupture of UIAs less than 5 mm in diameter [54]. In SUAVe, the overall annual risk rate of rupture was demonstrated to be 0.54%/year (single unruptured aneurysms: 0.34%/year, multiple unruptured aneurysms: 0.95%/year). And patients <50 years of age (P=0.046; hazard ratio, 5.23; 95% CI,1.03 to 26.52), aneurysm diameter of ≥4.0 mm (P=0.023; hazard ratio, 5.86; 95% CI, 1.27 to 26.95), hypertension (P=0.023; hazard ratio, 7.93; 95% CI, 1.33 to 47.42), and aneurysm multiplicity (P=0.0048; hazard ratio, 4.87; 95% CI, 1.62 to 14.65) were found to be significant predictive factors for rupture of small aneurysms. Results of this study showed that the rupture risk of a small cerebral aneurysm in Japan was higher compared with that of ISUIA.
And, in UCAS (Unruptured Cerebral Aneurysm Study of Japan), reported in 2011, the overall rate of rupture of cerebral aneurysms was 0.95% annually, and anterior-circulation small aneurysms had a higher rupture rate than that of ISUIA [55].
In addition, treatment could be considered in patients with severe psychological disturbances secondary to harboring an unruptured aneurysm.
Symptomatic UIAs should be treated in principle. For patients at high risk of treatment because of co-morbid medical conditions, old age, or location and morphology of the aneurysm, the risks and benefits of treatment should be weighed in the treatment decision. However, the treatment decision should be determined after taking into account the patient-specific factors of age, co-morbidity, and health condition and aneurysm-specific factors of size, location, and morphology. Treatment is not generally recommended for asymptomatic extradural intracranial aneurysms. Long-term follow-up is recommended after treating an UIA. In particular, for patients managed with endovascular treatment, angiographic follow-up is recommended to detect incomplete occlusion or recurrence.
For patients with an UIA who are managed conservatively without treatment, treatment of high blood pressure, cessation of smoking, and regular noninvasive angiographic follow-up, even without symptoms, are recommended.
Frequent Aspirin use may confer a protective effect for risk of intracranial aneurysm rupture. In a case-control study on the protective effect of Aspirin for patients with an untreated intracranial aneurysm, patients who used Aspirin 3 times weekly to daily had a significantly lower odds of hemorrhage (adjusted OR, 0.27; 95% CI, 0.11 - 0.67; P=0.03) compared with those who never take aspirin [56]. However, further clinical investigation is needed to confirm this effect. UIAs generally are monitored annually with MRA or CTA for 2 to 3 years and then every 2 to 5 years thereafter if the UIAs are clinically and radiographically stable [57].
Recommendations
1. For patients with an unruptured intracranial aneurysm that are managed conservatively without treatment, treatment of high blood pressure, cessation of smoking, and regular vascular imaging follow-up, even without symptoms, are recommended.
2. Treatment is not generally recommended for an asymptomatic extradural intracranial aneurysm.
3. Symptomatic UIAs should be treated in principle.
4. Considering the natural course of asymptomatic UIA, treatment might be considered for patients who have a life expectancy of more than 10-15 years and have one or more of following conditions.
(1) Size of 5 mm or more
(2) Size under 5 mm at high risk of rupture
(4) Aneurysm located in the posterior circulation, anterior communicating artery, or posterior communicating artery [53, 54, 55].
(5) History of previous subarachnoid hemorrhage [53]
(6) Aneurysm undergoing increase in size or change in morphology during follow-up
(7) Patients with age less than 50 years, hypertension, and multiple aneurysms [54]
(8) Aneurysm with high aspect ratio (the ratio of aneurysm height to neck width) or high size ratio (the ratio of aneurysm size to the parent artery size), or aneurysm with multilobule or bleb [58, 59]
(9) Patients who have anxiety or depression because of the diagnosis of an aneurysm
5. It is recommended that the treatment decision for an UIA should be determined after taking into account the patient-specific factors of age, comorbidity, and health condition and aneurysm-specific factors of size, location, and morphology. The facility and performance of centers also should be considered for the selection of the treatment method. In the decisionmaking process, informed consent should be obtained after providing sufficient explanation to the patient or the patient's family.
6. In the decision-making process, the PHASES score may be considered for predicting a patient's risk of aneurysm rupture.
Selection of treatment modality for an unruptured intracranial aneurysm
The most appropriate treatment option for any UIA is that which provides an optimal balance of procedural safety and long-term efficacy based on patient and aneurysm characteristics. Currently, there are two available options for treating UIAs, surgical clipping and endovascular coiling.
The patient-specific factors, facility and performance of centers should be considered for the selection of treatment method.
Surgical treatment
Traditionally, surgical clipping has been deemed as being highly efficacious, but carrying greater risk due to the neurological complications associated with open neurosurgery. In safety concerns associated with surgical treatment, according to ISUIA reported in 2003, 1 year-morbidity and 1 year-mortality after clipping was 10.1% in cases without a history of SAH and 12.6% in cases with a history of SAH [53]. In domestic data of a retrospective study reported in 2010, there was a 30 day surgery-related mortality rate of 0.4% and a 30-day morbidity rate of 8.4% [60]. However, complication rates of surgical clipping differ according to aneurysm size, location and patient's age [59, 60, 61]. Moroi et al. reported that the surgical morbidity and mortality rates were 0% for ACA and MCA UIAs less than 10 mm in size [61]. Krisht et al. at 2006 suggested that surgical treatment may represent a superior approach to conservative management in patients with life expectancies greater than 10 years [62].
Endovascular treatment
During last two decades, endovascular surgery for treatment of intracranial aneurysms has been developing rapidly. Growing evidence seems to indicate that endovascular coiling carries lower risks than surgical clipping for UIAs. In a systematic review of 30 studies including 1397 unruptured aneurysms treated with detachable coils, morbidity and mortality were 7% and 0.6%, respectively [63]. In another systematic review of 176 unruptured aneurysms in 149 patients treated with detachable coils, morbidity and mortality were 2.6% and 1.3% [64]. In Japanese data of retrospective study, the 30-day mortality and overall morbidity including mortality was 0.2% and 6.3% for coiling [61]. In a 2007 retrospective study from 429 hospitals in 18 states in the US, neurosurgical cases had 70% greater odds of an adverse outcome, 30% increased hospital charges, and 80% longer length of stay compared with endovascular cases [65]. However, further large size, prospective studies are needed for endovascular treatment of unruptured aneurysms. And, the long-term efficacy and durability of endovascular treatment for unruptured aneurysms remains to be determined.
While endovascular treatment of UIAs is now widely used, certain aneurysmal morphologies and anatomical features, particularly a wide neck, render some aneurysms technically difficult to treat endovascularly. To facilitate endovascular coiling of aneurysms with broad necks, Moret et al. extended a previously utilized temporary balloon-inflation technique to the treatment of UIAs and named it balloon remodeling [66]. Another adjunctive therapy for wide-neck UIAs is stent-assisted coiling. Recently, flow diversion emerged as a new concept [67]. The role of a flow diverter is expected.
Recommendations of selection of treatment modality
1. Surgical aneurysm clipping and endovascular treatment yield comparable results. And the selection of treatment should be determined upon consideration of the risks of treatment and recurrence rate.
2. Long-term follow-up is recommended after treating an UIA. In particular, for patients managed with endovascular treatment, angiographic follow-up is recommended to detect incomplete occlusion or recurrence.
Conclusions
This guideline provides practical, evidence-based advice for the management of patients with an intracranial aneurysm with or without subarachnoid hemorrhage. But, these guidelines cannot provide the answer for every clinical situation and should not take precedence over the clinical judgment of responsible physicians for individual patients. The final judgment regarding the care of a particular patient must be made by the physician and patient in light of circumstances specific to that patient.
References
- 1.Lee MS. Guideline for management of ruptured aneurysm: preliminary report. Neurointervention. 2007;2:36–42. [Google Scholar]
- 2.Kim KH. Guideline for management of unruptured intracranial aneurysms: preliminary report. Neurointervention. 2007;2:43–49. [Google Scholar]
- 3.Shin SH, Kwon SC, Suh DC. Recent update of guidelines for neurointerventional procedures. Neurointervention. 2013;8:68–72. doi: 10.5469/neuroint.2013.8.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bederson JB, Connolly ES, Jr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 5.Connolly ES, Jr, Rabinstein AA, Carhuanpoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Assocation. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 6.Mayberg MR, Batjer HH, Dacey R, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1994;25:2315–2328. doi: 10.1161/01.str.25.11.2315. [DOI] [PubMed] [Google Scholar]
- 7.Cortnum S, Sorensen P, Jorgensen J. Determining the sensitivity of computed tomography scanning in early detection of subarachnoid hemorrhage. Neurosurgery. 2010;66:900–902. [PubMed] [Google Scholar]
- 8.Fiebach JB, Schellinger PD, Geletneky K, et al. MRI in acute subarachnoid haemorrhage; findings with a standardised stroke protocol. Neuroradiology. 2004;46:44–48. doi: 10.1007/s00234-003-1132-8. [DOI] [PubMed] [Google Scholar]
- 9.Kidwell CS, Wintermark M. Imaging of intracranial haemorrhage. Lancet Neurol. 2008;7:256–267. doi: 10.1016/S1474-4422(08)70041-3. [DOI] [PubMed] [Google Scholar]
- 10.Shimoda M, Hoshikawa K, Shiramizu H, Oda S, Matsumae M. Problems with diagnosis by fluid-attenuated inversion recovery magnetic resonance imaging in patients with acute aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2010;50:530–537. doi: 10.2176/nmc.50.530. [DOI] [PubMed] [Google Scholar]
- 11.Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrahge. Cerebrovasc Dis. 2013;35:93–112. doi: 10.1159/000346087. [DOI] [PubMed] [Google Scholar]
- 12.Donmez H, Serifov E, Kahriman G, Mavili E, Durak AC, Menku A. Comparison of 16-row multislice CT angiography with conventional angiography for detection and evaluation of intracranial aneurysms. Eur J Radiol. 2011;80:455–461. doi: 10.1016/j.ejrad.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Jayaraman MV, Mayo-Smith WW, Tung GA, et al. Detection of intracranial aneurysms: multi-detector row CT angiography compared with DSA. Radiology. 2004;230:510–518. doi: 10.1148/radiol.2302021465. [DOI] [PubMed] [Google Scholar]
- 14.Kadri S, Brunel H, Bourbotte G, Delort P, Lust S, Bonafe A. Can multislice helical computed tomography replace conventional angiography in the diagnosis of non traumatic subarachnoid hemorrhage? J Neuroradiol. 2006;33:45–50. doi: 10.1016/s0150-9861(06)77227-3. [DOI] [PubMed] [Google Scholar]
- 15.McKinney AM, Palmer CS, Truwit CL, Karagulle A, Teksam M. Detection of aneurysms by 64-section multidetector CT angiography in patients acutely suspected of having an intracranial aneurysm and comparison with digital subtraction and 3D rotational angiography. AJNR Am J Neuroradiol. 2008;29:594–602. doi: 10.3174/ajnr.A0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agid R, Andersson T, Almqvist H, et al. Negative CT angiography findings in patients with spontaneous subarachnoid hemorrhage: When is digital subtraction angiography still needed? AJNR Am J Neuroradiol. 2010;31:696–705. doi: 10.3174/ajnr.A1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinjikji W, Kallmes DF, White JB, Lanzino G, Morris JM, Cloft HJ. Inter- and intraobserver agreement in CT characterization of nonaneurysmal perimesencephalic subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2010;31:1103–1105. doi: 10.3174/ajnr.A1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romijn M, Gratama van, van Walderveen MA, et al. Diagnostic accuracy of CT angiography with matched mask bone elimination for detection of intracranial aneurysms: Comparison with digital subtraction angiography and 3D rotational angiography. AJNR Am J Neuroradiol. 2008;29:134–139. doi: 10.3174/ajnr.A0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang LJ, Wu SY, Niu JB, et al. Dual-energy CT angiography in the evaluation of intracranial aneurysms: Image quality, radiation dose, and comparison with 3D rotational digital subtraction angiography. AJR Am J Roentgenol. 2010;194:23–30. doi: 10.2214/AJR.08.2290. [DOI] [PubMed] [Google Scholar]
- 20.White PM, Teasdale EM, Wardlaw JM, Easton V. Intracranial aneurysms: CT angiography and MR angiography for detection prospective blinded comparison in a large patient cohort. Radiology. 2001;219:739–749. doi: 10.1148/radiology.219.3.r01ma16739. [DOI] [PubMed] [Google Scholar]
- 21.Agid R, Lee SK, Willinsky RA, Farb RI, terBrugge KG. Acute subarachnoid hemorrhage: Using 64-slice multidetector CT angiography to "triage" patients' treatment. Neuroradiology. 2006;48:787–794. doi: 10.1007/s00234-006-0129-5. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara H, Kato S, Akimura T, Suehiro E, Oku T, Suzuki M. Angiogram-negative subarachnoid hemorrhage in the era of three dimensional rotational angiography. J Clin Neurosci. 2007;14:252–255. doi: 10.1016/j.jocn.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 23.van Rooij WJ, Peluso JP, Sluzewski M, Beute GN. Additional value of 3D rotational angiography in angiographically negative aneurysmal subarachnoid hemorrhage: How negative is negative? AJNR Am J Neuroradiol. 2008;29:962–966. doi: 10.3174/ajnr.A0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doelken M, Struffert T, Richter G, et al. Flat-panel detector volumetric CT for visualization of subarachnoid hemorrhage and ventricles: Preliminary results compared to conventional CT. Neuroradiology. 2008;50:517–523. doi: 10.1007/s00234-008-0372-z. [DOI] [PubMed] [Google Scholar]
- 25.Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003;98:959–966. doi: 10.3171/jns.2003.98.5.0959. [DOI] [PubMed] [Google Scholar]
- 26.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 27.Bakker NA, Metzemaekers JD, Groen RJ, Mooij JJ, Van Dijk JM. International subarachnoid aneurysm trial 2009: endovascular coiling of ruptured intracranial aneurysms has no significant advantage over neurosurgical clipping. Neurosurgery. 2010;66:961–962. doi: 10.1227/01.NEU.0000368152.67151.73. [DOI] [PubMed] [Google Scholar]
- 28.Risselada R, Lingsma HF, Bauer-Mehren A, et al. Prediction of 60 day case-fatality after aneurysmal subarachnoid haemorrhage: Results from the International Subarachnoid Aneurysm Trial (ISAT) Eur J Epidemiol. 2010;25:261–266. doi: 10.1007/s10654-010-9432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannidis I, Lalloo S, Corkill R, Kuker W, Byrne JV. Endovascular treatment of very small intracranial aneurysms. J Neurosurg. 2010;112:551–556. doi: 10.3171/2008.8.17657. [DOI] [PubMed] [Google Scholar]
- 30.Graves EJ. National hospital discharge survey. Vital Health Stat 13. 1992:1–62. [PubMed] [Google Scholar]
- 31.Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–1347. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- 32.Raaymakers TW, Rinkel GJ, Ramos LM. Initial and follow-up screening for aneurysms in families with familial subarachnoid hemorrhage. Neurology. 1998;51:1125–1130. doi: 10.1212/wnl.51.4.1125. [DOI] [PubMed] [Google Scholar]
- 33.Ronkainen A, Miettinen H, Karkola K, et al. Risk of harboring an unruptured intracranial aneurysm. Stroke. 1998;29:359–362. doi: 10.1161/01.str.29.2.359. [DOI] [PubMed] [Google Scholar]
- 34.Jakubowski J, Kendall B. Coincidental aneurysms with tumours of pituitary origin. J Neurol Neurosurg Psychiatry. 1978;41:972–979. doi: 10.1136/jnnp.41.11.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preul MC, Cendes F, Just N, Mohr G. Intracranial aneurysms and sickle cell anemia: multiplicity and propensity for the vertebrobasilar territory. Neurosurgery. 1998;42:971–977. doi: 10.1097/00006123-199805000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Asai A, Matsutani M, Kohno T, Fujimaki T, Takakura K. Multiple saccular cerebral aneurysms associated with systemic lupus erythematosus--case report. Neurol Med Chir (Tokyo) 1989;29:245–247. doi: 10.2176/nmc.29.245. [DOI] [PubMed] [Google Scholar]
- 37.Xu HW, Yu SQ, Mei CL, Li MH. Screening for intracranial aneurysm in 355 patients with autosomal-dominant polycystic kidney disease. Stroke. 2011;42:204–206. doi: 10.1161/STROKEAHA.110.578740. [DOI] [PubMed] [Google Scholar]
- 38.Huston J, 3rd, Torres VE, Sulivan PP, Offord KP, Wiebers DO. Value of magnetic resonance angiography for the detection of intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3:1871–1877. doi: 10.1681/ASN.V3121871. [DOI] [PubMed] [Google Scholar]
- 39.Mariani L, Bianchetti MG, Schroth G, Seiler RW. Cerebral aneurysms in patients with autosomal dominant polycystic kidney disease--to screen, to clip, to coil? Nephrol Dial Transplant. 1999;14:2319–2322. doi: 10.1093/ndt/14.10.2319. [DOI] [PubMed] [Google Scholar]
- 40.Schievink WI. Marfan syndrome and intracranial aneurysms. Stroke. 1999;30:2767–2768. doi: 10.1161/01.str.30.12.2759-g. [DOI] [PubMed] [Google Scholar]
- 41.Schievink WI, Riedinger M, Maya MM. Frequency of incidental intracranial aneurysms in neurofibromatosis type 1. Am J Med Genet A. 2005;134A:45–48. doi: 10.1002/ajmg.a.30475. [DOI] [PubMed] [Google Scholar]
- 42.Adachi K, Kudo M, Chen MN, Nakazawa S, Wakabayashi I. Cerebral aneurysm associated with multiple endocrine neoplasia, type 1--case report. Neurol Med Chir (Tokyo) 1993;33:309–331. doi: 10.2176/nmc.33.309. [DOI] [PubMed] [Google Scholar]
- 43.Munyer TP, Margulis AR. Pseudoxanthoma elasticum with internal carotid artery aneurysm. AJR Am J Roentgenol. 1981;136:1023–1024. doi: 10.2214/ajr.136.5.1023. [DOI] [PubMed] [Google Scholar]
- 44.Defillo A, Nussbaum ES. Intracranial aneurysm formation in siblings with pseudoxanthoma elasticum: case report. J Neurosurg Sci. 2010;54:105–107. [PubMed] [Google Scholar]
- 45.Maher CO, Piepgras DG, Brown RD, Jr, Friedman JA, Pollock BE. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke. 2001;32:877–882. doi: 10.1161/01.str.32.4.877. [DOI] [PubMed] [Google Scholar]
- 46.de Paepe A, van Landegem W, de Keyser F, de Reuck J. Association of multiple intracranial aneurysms and collagen type III deficiency. Clin Neurol Neurosurg. 1988;90:53–56. doi: 10.1016/s0303-8467(88)80010-6. [DOI] [PubMed] [Google Scholar]
- 47.David CA, Vishteh AG, Spetzler RF, Lemole M, Lawton MT, Partovi S. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg. 1999;91:396–401. doi: 10.3171/jns.1999.91.3.0396. [DOI] [PubMed] [Google Scholar]
- 48.Wermer MJ, Koffijberg H, van der Schaaf IC. Effectiveness and costs of screening for aneurysms every 5 years after subarachnoid hemorrhage. Neurology. 2008;70:2053–2062. doi: 10.1212/01.wnl.0000304372.01248.02. [DOI] [PubMed] [Google Scholar]
- 49.Bederson JB, Awad IA, Wiebers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Circulation. 2000;102:2300–2308. doi: 10.1161/01.cir.102.18.2300. [DOI] [PubMed] [Google Scholar]
- 50.Raaymakers TW. Aneurysms in relatives of patients with subarachnoid hemorrhage: frequency and risk factors. MARS Study Group. Magnetic Resonance Angiography in Relatives of patients with Subarachnoid hemorrhage. Neurology. 1999;53:982–988. doi: 10.1212/wnl.53.5.982. [DOI] [PubMed] [Google Scholar]
- 51.Kuehn BM. FDA warning: CT scans exceeded proper doses. JAMA. 2010;303:124. doi: 10.1001/jama.2009.1906. [DOI] [PubMed] [Google Scholar]
- 52.Greving JP, Wermer MJ, Brown RD, Jr, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13:59–66. doi: 10.1016/S1474-4422(13)70263-1. [DOI] [PubMed] [Google Scholar]
- 53.Wiebers DO, Whisnant JP, Huston J, 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 54.Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 2010;41:1969–1977. doi: 10.1161/STROKEAHA.110.585059. [DOI] [PubMed] [Google Scholar]
- 55.UCAS Japan Investigators. Morita A, Kirino T, Hashi K, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366:2474–2482. doi: 10.1056/NEJMoa1113260. [DOI] [PubMed] [Google Scholar]
- 56.Hasan DM, Mahaney KB, Brown RD, Jr, et al. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162. doi: 10.1161/STROKEAHA.111.619411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiebers DO. Unruptured intracranial aneurysms: natural history and clinical management. Update on the international study of unruptured intracranial aneurysms. Neuroimaging Clin N Am. 2006;16:383–390. doi: 10.1016/j.nic.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Morita A, Fujiwara S, Hashi K, Ohtsu H, Kirino T. Risk of rupture associated with intact cerebral aneurysms in the Japanese population: a systematic review of the literature from Japan. J Neurosurg. 2005;102:601–606. doi: 10.3171/jns.2005.102.4.0601. [DOI] [PubMed] [Google Scholar]
- 59.Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251–256. doi: 10.1161/01.str.29.1.251. [DOI] [PubMed] [Google Scholar]
- 60.Kim JE, Lim DJ, Hong CK, Joo SP, Yoon SM, Kim BT. Treatment of unruptured intracranial aneurysms in South Korea in 2006 : a nationwide multicenter survey from the korean society of cerebrovascular surgery. J Korean Neurosurg Soc. 2010;47:112–118. doi: 10.3340/jkns.2010.47.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moroi J, Hadeishi H, Suzuki A, Yasui N. Morbidity and mortality from surgical treatment of unruptured cerebral aneurysms at Research Institute for Brain and Blood Vessels-Akita. Neurosurgery. 2005;56:224–231. doi: 10.1227/01.neu.0000148897.28828.85. [DOI] [PubMed] [Google Scholar]
- 62.Krisht AF, Gomez J, Partington S. Outcome of surgical clipping of unruptured aneurysms as it compares with a 10-year nonclipping survival period. Neurosurgery. 2006;58:207–216. doi: 10.1227/01.NEU.0000194638.61073.FC. [DOI] [PubMed] [Google Scholar]
- 63.Lanterna LA, Tredici G, Dimitrov BD, Biroli F. Treatment of unruptured cerebral aneurysms by embolization with guglielmi detachable coils: case-fatality, morbidity, and effectiveness in preventing bleeding--a systematic review of the literature. Neurosurgery. 2004;55:767–775. doi: 10.1227/01.neu.0000137653.93173.1c. [DOI] [PubMed] [Google Scholar]
- 64.van Rooij WJ, Sluzewski M. Procedural morbidity and mortality of elective coil treatment of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2006;27:1678–1680. [PMC free article] [PubMed] [Google Scholar]
- 65.Higashida RT, Lahue BJ, Torbey MT, Hopkins LN, Leip E, Hanley DF. Treatment of unruptured intracranial aneurysms: a nationwide assessment of effectiveness. AJNR Am J Neuroradiol. 2007;28:146–151. [PMC free article] [PubMed] [Google Scholar]
- 66.Moret J, Cognard C, Weill A, Castaings L, Rey A. Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol. 1997;24:30–44. [PubMed] [Google Scholar]
- 67.D'Urso PI, Lanzino G, Cloft HJ, Kallmes DF. Flow diversion for intracranial aneurysms: a review. Stroke. 2011;42:2363–2368. doi: 10.1161/STROKEAHA.111.620328. [DOI] [PubMed] [Google Scholar]


