Abstract
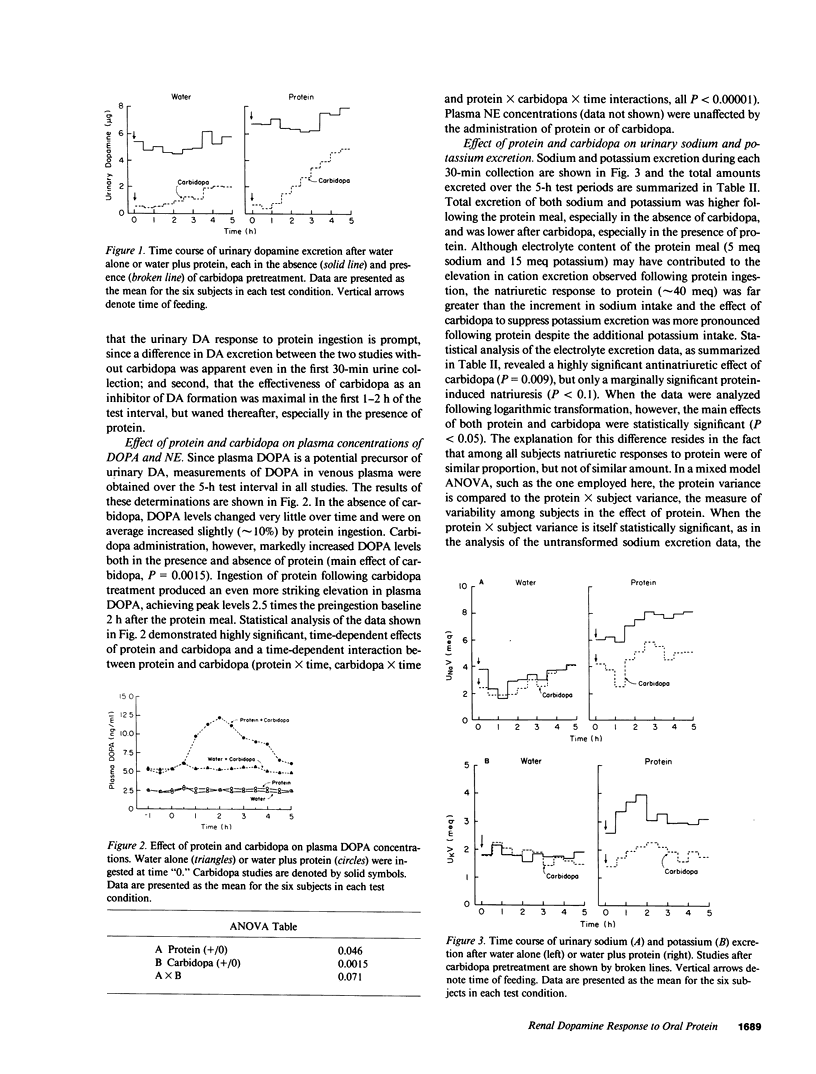
Since dietary protein increases urinary dopamine (DA) excretion in animals, this study was undertaken to assess the role of DA production in the acute changes in renal function following protein ingestion in man. Excretion of DA, sodium, potassium, water, solute, and creatinine were measured in six normal men in 30-min intervals over 5 h after oral ingestion of protein and/or carbidopa, an inhibitor of DA formation from 3,4-dihydroxyphenylalanine (DOPA). Overall, protein increased urinary DA 50% (P = 0.031) while carbidopa reduced it 70% (P less than 0.0001), although suppression of DA excretion by carbidopa was not uniform over the 5 h of observation. Carbidopa doubled the level of DOPA in venous plasma and greatly magnified the DOPA response to protein. Inhibition of decarboxylase activity reduced excretion of sodium, potassium, solute and water after protein ingestion. These results indicate that extraneuronal DOPA decarboxylation in kidney contributes to acute protein-induced changes in renal function in man and suggest a general role for the decarboxylation of circulating DOPA in the expression of dopaminergic effects on the kidney in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agharanya J. C., Alonso R., Wurtman R. J. Changes in catecholamine excretion after short-term tyrosine ingestion in normally fed human subjects. Am J Clin Nutr. 1981 Jan;34(1):82–87. doi: 10.1093/ajcn/34.1.82. [DOI] [PubMed] [Google Scholar]
- Agharanya J. C., Wurtman R. J. Effect of acute administration of large neutral and other amino acids on urinary excretion of catecholamines1,2. Life Sci. 1982 Mar 1;30(9):739–746. doi: 10.1016/0024-3205(82)90607-5. [DOI] [PubMed] [Google Scholar]
- Alexander R. W., Gill J. R., Jr, Yamabe H., Lovenberg W., Keiser H. R. Effects of dietary sodium and of acute saline infusion on the interrelationship between dopamine excretion and adrenergic activity in man. J Clin Invest. 1974 Jul;54(1):194–200. doi: 10.1172/JCI107743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines A. D., Craan A., Chan W., Morgunov N. Tubular secretion and metabolism of dopamine, norepinephrine, methoxytyramine and normetanephrine by the rat kidney. J Pharmacol Exp Ther. 1979 Jan;208(1):144–147. [PubMed] [Google Scholar]
- Ball S. G., Gunn I. G., Douglas I. H. Renal handling of dopa, dopamine, norepinephrine, and epinephrine in the dog. Am J Physiol. 1982 Jan;242(1):F56–F62. doi: 10.1152/ajprenal.1982.242.1.F56. [DOI] [PubMed] [Google Scholar]
- Ball S. G., Lee M. R. The effect of carbidopa administration on urinary sodium excretion in man. Is dopamine an intrarenal natriuretic hormone? Br J Clin Pharmacol. 1977 Apr;4(2):115–119. doi: 10.1111/j.1365-2125.1977.tb00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Reuss E., Higashi Y., Kaneda Y. Dopamine decreases fluid reabsorption in straight portions of rabbit proximal tubule. Am J Physiol. 1982 Jun;242(6):F634–F640. doi: 10.1152/ajprenal.1982.242.6.F634. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N., Arbogast L. A., Rhoades T. A., Schillo K. K., Pau K. Y., Jackson G. L. Plasma catecholamines in the chronically cannulated sheep fetus: predominance of L-dihydroxyphenylalanine. Endocrinology. 1983 Jul;113(1):216–221. doi: 10.1210/endo-113-1-216. [DOI] [PubMed] [Google Scholar]
- Bosch J. P., Saccaggi A., Lauer A., Ronco C., Belledonne M., Glabman S. Renal functional reserve in humans. Effect of protein intake on glomerular filtration rate. Am J Med. 1983 Dec;75(6):943–950. doi: 10.1016/0002-9343(83)90873-2. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Meyer T. W., Hostetter T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982 Sep 9;307(11):652–659. doi: 10.1056/NEJM198209093071104. [DOI] [PubMed] [Google Scholar]
- Bresnahan M. R., Hatzinikolaou P., Brunner H. R., Gavras H. Effects of tyrosine infusion in normotensive and hypertensive rats. Am J Physiol. 1980 Aug;239(2):H206–H211. doi: 10.1152/ajpheart.1980.239.2.H206. [DOI] [PubMed] [Google Scholar]
- Carey R. M., Drake C. R., Jr Dopamine selectively inhibits aldosterone responses to angiotensin II in humans. Hypertension. 1986 May;8(5):399–406. doi: 10.1161/01.hyp.8.5.399. [DOI] [PubMed] [Google Scholar]
- Cuche J. L., Marchand G. R., Greger R. F., Lang R. C., Knox F. G. Phosphaturic effect of dopamine in dogs. Possible role of intrarenally produced dopamine in phosphate regulation. J Clin Invest. 1976 Jul;58(1):71–76. doi: 10.1172/JCI108461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G. C., Kissinger P. T., Shoup R. E. Strategies for determination of serum or plasma norepinephrine by reverse-phase liquid chromatography. Anal Chem. 1981 Feb;53(2):156–159. doi: 10.1021/ac00225a006. [DOI] [PubMed] [Google Scholar]
- Finlay G. D., Whitsett T. L., Cucinell E. A., Goldberg L. I. Augmentation of sodium and potassium excretion, glomerular filtration rate and renal plasma flow by levodopa. N Engl J Med. 1971 Apr 15;284(15):865–870. doi: 10.1056/NEJM197104222841601. [DOI] [PubMed] [Google Scholar]
- GOLDBERG L. I., MCDONALD R. H., Jr, ZIMMERMAN A. M. SODIUM DIURESIS PRODUCED BY DOPAMINE IN PATIENTS WITH CONGESTIVE HEART FAILURE. N Engl J Med. 1963 Nov 14;269:1060–1064. doi: 10.1056/NEJM196311142692003. [DOI] [PubMed] [Google Scholar]
- Hansson C., Agrup G., Rorsman H., Rosengren E. Dopa and 5-S-cysteinyldopa in the serum of albino, black, and red guinea pigs. Acta Derm Venereol. 1980;60(2):155–156. [PubMed] [Google Scholar]
- Hostetter T. H. Human renal response to meat meal. Am J Physiol. 1986 Apr;250(4 Pt 2):F613–F618. doi: 10.1152/ajprenal.1986.250.4.F613. [DOI] [PubMed] [Google Scholar]
- Kaneda Y., Bello-Reuss E. Effect of dopamine on phosphate reabsorption in isolated perfused rabbit proximal tubules. Miner Electrolyte Metab. 1983;9(3):147–150. [PubMed] [Google Scholar]
- Krishna G. G., Danovitch G. M., Beck F. W., Sowers J. R. Dopaminergic mediation of the natriuretic response to volume expansion. J Lab Clin Med. 1985 Feb;105(2):214–218. [PubMed] [Google Scholar]
- LOVENBERG W., WEISSBACH H., UDENFRIEND S. Aromatic L-amino acid decarboxylase. J Biol Chem. 1962 Jan;237:89–93. [PubMed] [Google Scholar]
- MCDONALD R. H., Jr, GOLDBERG L. I., MCNAY J. L., TUTTLE E. P., Jr EFFECT OF DOPAMINE IN MAN: AUGMENTATION OF SODIUM EXCRETION, GLOMERULAR FILTRATION RATE, AND RENAL PLASMA FLOW. J Clin Invest. 1964 Jun;43:1116–1124. doi: 10.1172/JCI104996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald I. A., Lake D. M. An improved technique for extracting catecholamines from body fluids. J Neurosci Methods. 1985 May;13(3-4):239–248. doi: 10.1016/0165-0270(85)90072-x. [DOI] [PubMed] [Google Scholar]
- McClanahan M., Sowers J. R., Beck F. W., Mohanty P. K., McKenzie T. Dopaminergic regulation of natriuretic response to acute volume expansion in dogs. Clin Sci (Lond) 1985 Mar;68(3):263–269. doi: 10.1042/cs0680263. [DOI] [PubMed] [Google Scholar]
- Meyer M. B., McNay J. L., Goldberg L. I. Effects of dopamine on renal function and hemodynamics in the dog. J Pharmacol Exp Ther. 1967 Apr;156(1):186–192. [PubMed] [Google Scholar]
- Pelayo J. C., Fildes R. D., Eisner G. M., Jose P. A. Effects of dopamine blockade on renal sodium excretion. Am J Physiol. 1983 Aug;245(2):F247–F253. doi: 10.1152/ajprenal.1983.245.2.F247. [DOI] [PubMed] [Google Scholar]
- Rasmussen D. D., Ishizuka B., Quigley M. E., Yen S. S. Effects of tyrosine and tryptophan ingestion on plasma catecholamine and 3,4-dihydroxyphenylacetic acid concentrations. J Clin Endocrinol Metab. 1983 Oct;57(4):760–763. doi: 10.1210/jcem-57-4-760. [DOI] [PubMed] [Google Scholar]
- Smedes F., Kraak J. C., Poppe H. Simple and fast solvent extraction system for selective and quantitative isolation of adrenaline, noradrenaline and dopamine from plasma and urine. J Chromatogr. 1982 Aug 13;231(1):25–39. doi: 10.1016/s0378-4347(00)80506-x. [DOI] [PubMed] [Google Scholar]
- Sourkes T. L. Dopa decarboxylase: substrates, coenzyme, inhibitors. Pharmacol Rev. 1966 Mar;18(1):53–60. [PubMed] [Google Scholar]
- Suzuki H., Nakane H., Kawamura M., Yoshizawa M., Takeshita E., Saruta T. Excretion and metabolism of dopa and dopamine by isolated perfused rat kidney. Am J Physiol. 1984 Sep;247(3 Pt 1):E285–E290. doi: 10.1152/ajpendo.1984.247.3.E285. [DOI] [PubMed] [Google Scholar]
- Young J. B., Kaufman L. N., Saville M. E., Landsberg L. Increased sympathetic nervous system activity in rats fed a low-protein diet. Am J Physiol. 1985 May;248(5 Pt 2):R627–R637. doi: 10.1152/ajpregu.1985.248.5.R627. [DOI] [PubMed] [Google Scholar]



