Abstract
Purpose
To compare the refractive results of cataract surgery measured by applanation ultrasound and the new partial coherence interferometer, AL-scan.
Methods
Medical records of 76 patients and 104 eyes who underwent cataract surgery from January 2013 to June 2013 were retrospectively reviewed. Biometries were measured using ultrasound and AL-scan and intraocular lens power was calculated using the SRK-T formula. Automatic refraction examination was done 1 month after the operation, and differences between the ultrasound group and AL-scan group were compared and analyzed by mean absolute error.
Results
Mean axial length measured preoperatively by the ultrasound method was 23.53 ± 1.17 mm while the lengths measured using the AL-scan were 0.03 mm longer than that of the ultrasound group (23.56 ± 1.15 mm). However, there was not a significant difference in this finding (p = 0.638). Mean absolute error was 0.34 ± 0.27 diopters in the ultrasound group and 0.36 ± 0.31 diopters in AL-scan group, which showed no significant difference (p = 0.946) in precision of predicting postoperative refraction.
Conclusions
Although the difference was not statistically significant, intraocular lens calculations done by the AL-scan were nearly similar in predicting postoperative refraction compared to those of applanation ultrasound, however more precise measurements may be obtained if the axial length is longer than 24.4 mm. Except in the case of opacity in the media, which makes obtaining measurements with the AL-scan difficult, AL-scan could be a useful biometry in cataract surgery.
Keywords: Biometry, Interferometery, Phacoemulsification, Ultrasonography
Satisfaction of patients undergoing cataract surgery is dependent on precise predictions of refractory outcomes. Over the years, development of biometry, phacoemulsification, and intraocular lens (IOL) calculation enabled precise prediction of postoperative refractory status.
To obtain accurate IOL power, a number of factors are needed. These biometries include axial length, corneal refractive power, and anterior chamber depth. Among these factors, Olsen [1] reported that axial length plays a main role in determining postoperative refraction and is responsible for 54% of the actual refractive error. Axial length error of 100 µm translates to a postoperative refraction error of 0.28 diopters (D).
Until recently, axial length was measured by using applanation ultrasound technique, which involves contact with the cornea and can result in corneal epithelial injury, infection, and patient discomfort. Error due to corneal indentation, which can lead to axial lengths 0.1 to 0.3 mm shorter than those measured by the immersion technique, is also a major disadvantage of the applanation ultrasound method [2].
To overcome this limitation, a partial coherence interferometer (PCI), which is based on the principle similar to that of optical coherence tomography, was introduced. Axial length measured by this method was comparable to that of other methods in precision and repeatability. Especially considering that the method is of the non-applanation type, it has the advantage of giving the patient less discomfort and has a low interobserver error [3,4,5,6].
Commonly used PCIs in the clinical setting include the IOL Master (Carl Zeiss Meditiec, Jena, Germany) and Lenstar (Haag Steit AG, Koeniz, Switzerland). Recently, the new PCI AL-scan (Nidek, Gamagori, Japan) has been introduced and increases precision by using a 3-dimentional ocular tracking technique. By using PCI and scheimpflug imaging techniques, AL-scan made it possible to measure axial length, corneal refractive power, anterior chamber depth, central corneal thickness, white-to-white distance, and pupil size in a single sitting based on those values and automatically calculates the appropriate IOL power to be used in cataract surgery by onboard software.
According to previous studies, it is known that the IOL Master and Lenstar show greater or similar accuracy compared to the conventional ultrasound techniques [3,7,8]. However, studies on the newly launched PCI, AL-scan, are lacking. Therefore, in this study we used AL-scan to measure axial length and refraction. With the obtained values, IOL power is calculated to look for the degree of error compared with the predicted value preoperatively and analyze the accuracy compared to values obtained from ultrasound.
Materials and Methods
Medical records of 76 patients (104 eyes) who underwent phacoemulsification and posterior chamber IOL (AcrySof IQ SN60WF; Alcon, Forth Worth, TX, USA) implantation from January 2013 to June 2013 were retrospectively reviewed. The operation was the temporal clear corneal incision technique. Patients had follow up of more than 1 month.
Those with factors known to inf luence visual acuity were excluded such as history of intraocular operation, inflammation, retinopathy, and others. Cases of posterior capsule rupture during cataract surgery and those requiring sutures at the corneal incision site were excluded. Furthermore, patients with axial length that could not be measured by the AL-scan were not included in the statistical process.
In the AL-scan group, axial length, anterior chamber depth, and corneal refractive power were measured by the AL-scan. In the ultrasound group, corneal refractive power was measured by auto kerato refractometer (KR-1; Topcon, Tokyo, Japan), and applanation ultrasound (Echoscan US-4000, Nidek) was used to obtain anterior chamber depth and axial length.
IOL power was calculated using the SRK-T formula and the A-constant was maintained at 118.7, a value provided by the manufacturer. The predicted refraction value targeted emmetropia and myopia on patient request. Automatic refraction examination was done at the 1-month follow-up after the operation to obtain the actual postoperative refraction value.
Mean numerical error (MNE) was achieved based on the difference between actual refraction value and desired refraction value using the SRK-T formula. We assessed the result as hyperopic if the value was negative and myopic when positive. Also, with the absolute value of actual difference and by averaging it, mean absolute error (MAE) could be calculated to evaluate the precision of the IOL power calculation. Differences between the ultrasound group and AL-scan group were compared and analyzed by MNE and MAE.
It has been reported that biometry measurements by applanation ultrasound technique and by PCI technique vary by axial length. Generally the measurement attained from the PCI is higher [6,9] so if the reverse result is drawn in the actual clinic setting the reliability of the measurement must be questioned. Also, Lee et al. [10] reported that postoperative refraction predicted by using the SRK-T formula is generally precise but refraction error had a relationship with the axial length. Based on the axial length of 24.4 mm, which is the value used to compensate axial length in the formula, axial lengths shorter than 24.4 mm tend to have hyperopic shift as eyes get shorter, and vice versa. Therefore, in this study we separated the group of patients with longer axial length measurements by ultrasound and AL-scan and analyzed the results drawn from those groups. Also, patients were divided into two groups by axial length of 24.4 mm, which was measured by ultrasound.
Differences of axial length measured by ultrasound and PCI were compared and analyzed using the paired t-test. Comparison of predictive error between those two methods was analyzed by absolute value using Wilcoxon rank test. A p-value of less than 0.05 indicated statistical significance.
Results
A total of 75 patients and 104 eyes were included in this study. The mean age was 67.4 ± 9.6 years. A total of 6 eyes failed to have axial length measured by the AL-scan (5.77%) and were excluded from the statistic analysis.
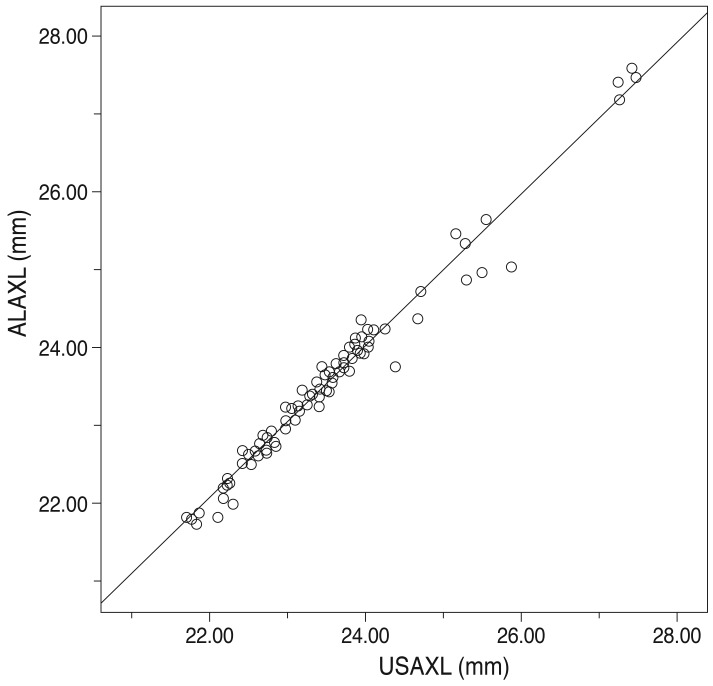
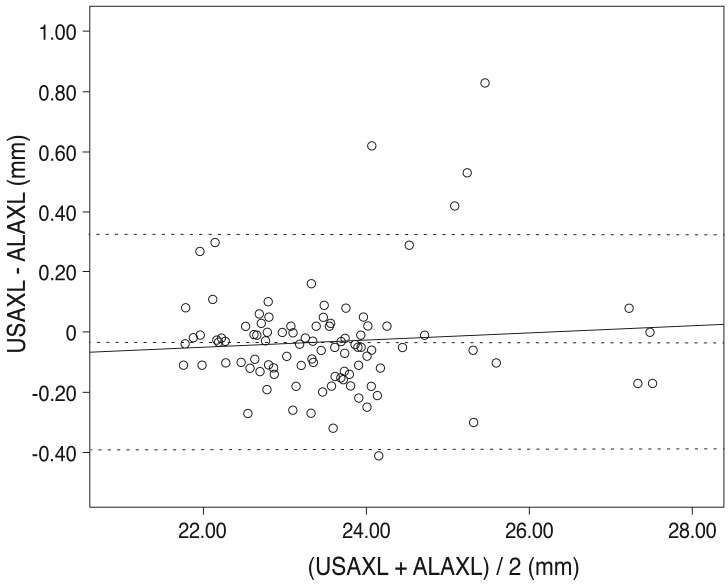
Axial length measurement by the two methods showed a statistically significant relationship (r = 0.976, p < 0.01) (Fig. 1). The dotted lines are the line of equality for the corresponding two methods. The Bland-Altman plot was examined to evaluate the agreement between the two methods (Fig. 2). Most values are along the dotted line (±1.96 standard deviation [SD], -0.3919 to 0.3251), meaning the methods were comparable. Furthermore, the slope of the regression line being 0.012 represents low error between the two methods and high reliability.
Fig. 1.
Scatter plots to compare means for axial length measured with ultrasound and AL-scan. Axial length measurement by two methods showed a statistically significant relationship. USAXL = axial length measured with applanation ultrasound; ALAXL = axial length measured with AL-scan. r = 0.976, p < 0.01.
Fig. 2.
Bland-Altman plots for assessing agreement of pairs of two methods. y = 0.012 X - 0.317; mean = -0.0334 ; mean +2 SD = 0.32508 ; mean -2 SD = -0.39188. The solid line represents the average mean difference (-0.0334) and the dotted line represents the 95 percentile confidence interval. The slope of regression line is 0.012 and represents low error between two methods and high reliability. In the scatter plot, most values are on the dotted line (±1.96 SD, -0.3919 to 0.3251), suggesting good comparability. SD = standard deviation; USAXL = axial length measured with applanation ultrasound; ALAXL = axial length measured with AL-scan.
Mean axial length measured preoperatively by ultrasound was 23.53 ± 1.17 mm, and AL-scan measurements were 0.03 mm longer than that of the ultrasound group (23.56 ± 1.15 mm). However, there was no significant difference in this finding (p = 0.638). Anterior chamber depth measured by the two methods was 3.17 ± 0.34 mm and 3.14 ± 0.39 mm, with the ultrasound group having the lower value, but the difference was not significant (p = 0.764) (Table 1).
Table 1.
Refractive results: comparison of the ultrasound group and the AL-scan group (all eyes, n = 98)
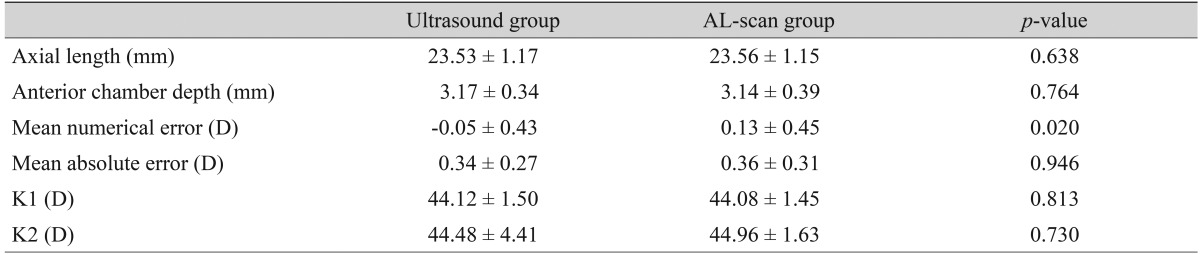
Values are presented as mean ± standard deviation.
D = diopter; K = corneal refractive power (keratometric diopter).
Post operative MNE in the ultrasound group was slightly hyperopic by -0.05 ± 0.43 D. In addition the AL-scan group had a tendency to be myopic by 0.13 ± 0.45 D, which was a statistically significant difference (p = 0.02). MAE was 0.34 ± 0.27 D in the ultrasound group and 0.36 ± 0.31 D in the AL-scan group, which showed no significant difference (p = 0.946) in precision in predicting post-operative refraction (Table 1). There was no statistically significant difference in corneal refractive power between the two groups as group in 73.5% of cases, and 75.5% of cases in the AL-scan group. Furthermore, MAE less than 1 D was 99% and 97% each, and less than 1.5 D was 100% and 99% each, respectively (Table 2).
Table 2.
Percentage of cases predicted to within ±0.50 D, ±1.00 D, and ±1.50 D of each group
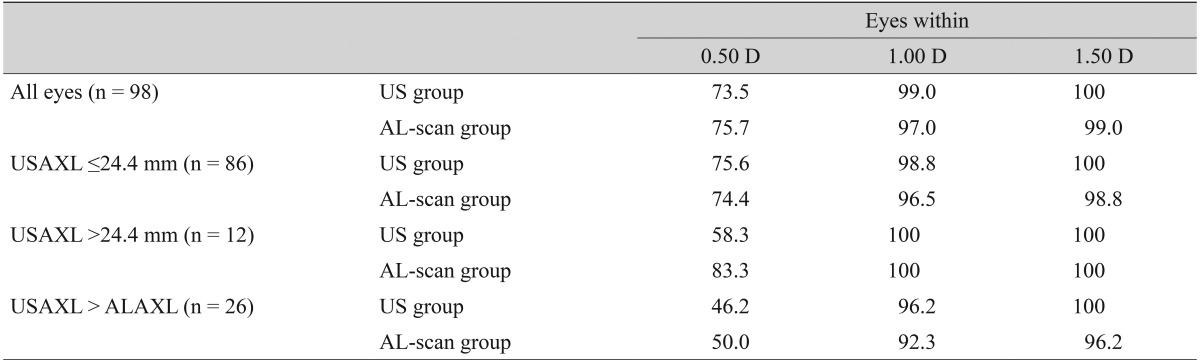
Values are presented as %.
D = diopter; US = ultrasound; USAXL = axial length measured with applanation ultrasound; ALAXL = axial length measured with AL-scan.
The group with an axial length shorter than 24.4 mm included a total of 86 eyes, mean axial length measured by applanation ultrasound method and AL-scan was 23.18 ± 0.65 mm and 23.23 ± 0.68 mm, respectively and neither finding had a significant difference (p = 0.551) (Table 3). There were 12 eyes with an axial length longer than 24.4 mm and mean axial length measured by each method was 25.84 ± 1.21 mm (ultrasound group) and 25.95 ± 1.08 mm (AL-scan group), which also showed no significant difference (p = 0.751) (Table 4). However, the difference between the two methods was 0.11 mm, which was relatively higher than the group with an axial length of less than 24.4 mm.
Table 3.
Refractive results: comparison of the ultrasound group and the AL-scan group (axial length ≤24.4 mm, n = 86)
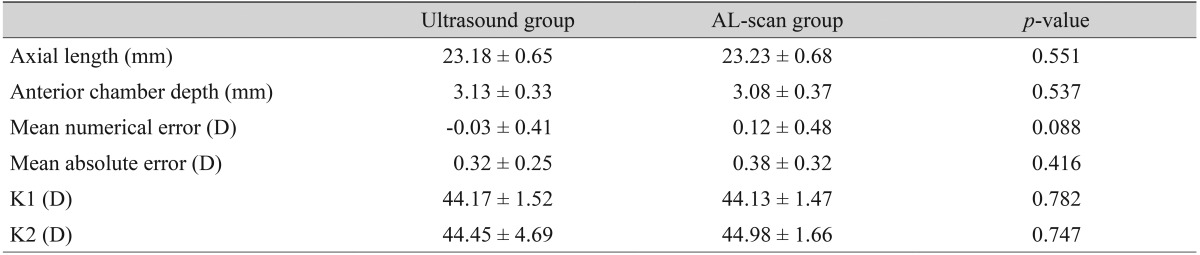
Values are presented as mean ± standard deviation.
D = diopter; K = corneal refractive power (keratometric diopter).
Table 4.
Refractive results: comparison of the ultrasound group and the AL-scan group (axial length >24.4 mm, n = 12)
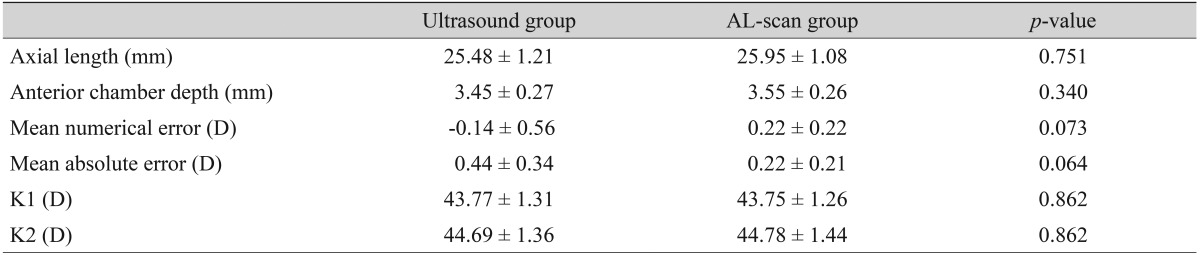
Values are presented as mean ± standard deviation.
D = diopter; K = corneal refractive power (keratometric diopter).
In the case of an axial length less than 24.4 mm, MNE was -0.03 ± 0.41 D in the ultrasound group and MAE was 0.32 ± 0.25 D. In the AL-scan group, it was 0.12 ± 0.48 D, 0.38 ± 0.32 D each, which showed no significant difference in precision between two groups (p = 0.088, 0.416) (Table 3). In the case of an axial length longer than 24.4 mm, MNE was -0.14 ± 0.56 D in the ultrasound group and MAE was 0.44 ± 0.34 D. In the AL-scan group, it was 0.22 ± 0.22 D, 0.22 ± 0.21 D each, which has shown no significant difference in precision between two groups (p = 0.076, 0.064) (Table 4). However, comparing the results of the axial length shorter than 24.4 mm group (MAE, 0.06), MAE was relatively higher in axial length longer than 24.4 mm group by 0.22 D. Also, by looking at the result distribution of each method, when the axial length is shorter than 24.4 mm, the distribution between each method was even. On the other hand, in the group with an axial length longer than 24.4 mm, the percentage of patients with an MAE lower than 0.5 D was 83.3% in AL-scan group, which was higher than the ultrasound group (58.3%) (Table 2).
The axial length of 21 eyes measured by the ultrasound method was longer than that measured by the AL-scan. In this case, MAE values of the ultrasound and AL-scan groups were 0.49 ± 0.33 and 0.53 ± 0.38 D, respectively, and there was no significant difference in precision between the two groups (p = 0.876) (Table 5). However, both groups had higher MAE than any of the other cases, and also rated the MAE lower than 0.5 D was shown to be lower by 46.2% and 50%, respectively (Table 2).
Table 5.
Refractive results: comparison of the ultrasound group and the AL-scan group (ultrasound axial length > AL-scan axial length, n = 26)
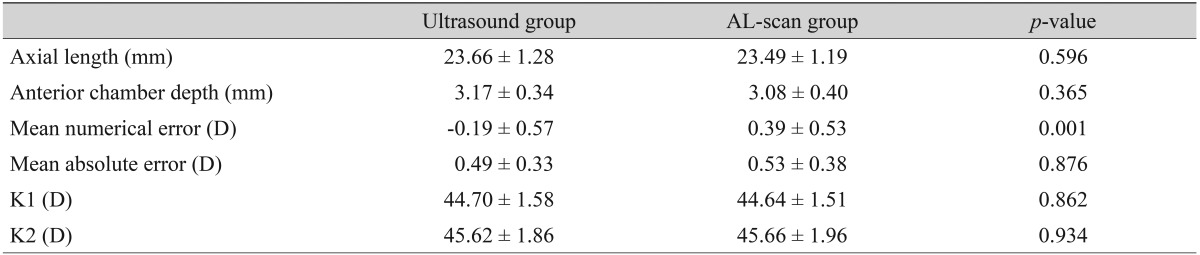
Values are presented as mean ± standard deviation.
D = diopter; K = corneal refractive power (keratometric diopter).
Discussion
Since PCI principle-based IOL Master was introduced in 1992, many studies have been launched to compare this new technology to the conventional applanation ultrasound method. Generally PCI is capable of measuring axial lengths 0.1 to 0.5 mm longer than applanation ultrasound [11,12]. In this study using AL-scan which is also based on the PCI principle, total patients had longer axial length than that measured by the ultrasound method. This study showed that there was no statistically significant difference between these two groups, but a strong relationship was identified. This result is thought to be from the different measurement technique. PCI and ultrasound have different refraction planes and also, in the case of applanation ultrasound, the degree of corneal indentation differs depending on the skill of the examiner [13,14].
By reviewing the previous studies regarding prediction of postoperative refraction, there are many studies reporting PCI benefit over the ultrasound method [12,15]. Conversely, there are studies reporting similar precision between those two methods [16]. This study of the AL-scan also has shown no significant difference in MAE between the two groups using each method. However, the study revealed that MAE in the AL-scan group had the tendency of becoming statistically significantly myopic compared to the ultrasound group. We used the A constant offered by the manufacturer but studies revealed a need for adjusting the A-constant [17].
Furthermore, to compare precision in predicting postoperative refraction by the axial length, patients were divided into two groups based on axial length above and below 24.4 mm. Analysis performed by these groups revealed no significant difference in precision between the two groups. However, in the axial length over 24.4 mm group, MAE difference between the ultrasound group and AL-scan group was larger and the percentage of patients showing a desired refraction of +0.5 D was higher in the AL-scan group. There is controversy regarding the relevance of refraction prediction error by axial length using PCI, so further studies will be necessary [9,16].
The axial length of 26 eyes was longer using the ultrasound method than the AL-scan method, and there was no significant difference between the two groups using each method. However, MAE was higher than other cases in both the ultrasound and AL-scan group. Postoperative target refraction of +0.5 D was less than 50% in both groups. Theoretically, ultrasound measures sound wave refracted from the internal limiting membrane while PCI measures the light refracted from the retinal pigment epithelium, which results in a difference of 130 µm. When considering not only the theoretical background but also the results of this study, systemic error may have played an important role when axial length was longer with the ultrasound technique. Such systemic error includes examiner error, patient error, and possible error when using the AL-scan. So, in this case, remeasurement is required.
PCI is a simple method and is more comfortable for the patient compared to the ultrasound method. It is also a noncontact method, and thus risk for infection is lower. However, in cases of severe cataracts, posterior capsular cataract, and difficult fixation, PCI cannot measure axial length with an accuracy greater than 10% to 20% [13]. In our study, of the 104 eyes, six eyes (5.77%) could not be measured by the AL-scan. According to the manufacturer, AL-scan can measure eyes with dense cataracts, as advanced measurement algorithms enhance the signal-tonoise ratio by decreasing noise and boosting the signal. However, further study is warranted if AL-scan has higher success rates in measuring axial length.
Here, we compared the precision in predicting postoperative refraction between AL-scan and applanation ultrasound. Eventually, IOL calculations made with the AL-scan were nearly similar in predicting postoperative refraction compared to that of using the applanation ultrasound, but it may be more precise in predicting postoperative refraction when the axial length is longer than 24.4 mm. Except in the case of opacity of the media, which makes it more difficult to obtain measurements by the AL-scan, this technique could be a useful biometry in cataract surgery.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Olsen T. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 1992;18:125–129. doi: 10.1016/s0886-3350(13)80917-0. [DOI] [PubMed] [Google Scholar]
- 2.Giers U, Epple C. Comparison of A-scan device accuracy. J Cataract Refract Surg. 1990;16:235–242. doi: 10.1016/s0886-3350(13)80737-7. [DOI] [PubMed] [Google Scholar]
- 3.Findl O, Drexler W, Menapace R, et al. Improved prediction of intraocular lens power using partial coherence interferometry. J Cataract Refract Surg. 2001;27:861–867. doi: 10.1016/s0886-3350(00)00699-4. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth J, Fekete O, Pesztenlehrer N. Optical and ultrasound measurement of axial length and anterior chamber depth for intraocular lens power calculation. J Cataract Refract Surg. 2003;29:85–88. doi: 10.1016/s0886-3350(02)01500-6. [DOI] [PubMed] [Google Scholar]
- 5.Findl O, Drexler W, Menapace R, et al. High precision biometry of pseudophakic eyes using partial coherence interferometry. J Cataract Refract Surg. 1998;24:1087–1093. doi: 10.1016/s0886-3350(98)80102-8. [DOI] [PubMed] [Google Scholar]
- 6.Rajan MS, Keilhorn I, Bell JA. Partial coherence laser interferometry vs conventional ultrasound biometry in intraocular lens power calculations. Eye (Lond) 2002;16:552–556. doi: 10.1038/sj.eye.6700157. [DOI] [PubMed] [Google Scholar]
- 7.Cruysberg LP, Doors M, Verbakel F, et al. Evaluation of the Lenstar LS 900 non-contact biometer. Br J Ophthalmol. 2010;94:106–110. doi: 10.1136/bjo.2009.161729. [DOI] [PubMed] [Google Scholar]
- 8.Buckhurst PJ, Wolffsohn JS, Shah S, et al. A new optical low coherence reflectometry device for ocular biometry in cataract patients. Br J Ophthalmol. 2009;93:949–953. doi: 10.1136/bjo.2008.156554. [DOI] [PubMed] [Google Scholar]
- 9.Hasemeyer S, Hugger P, Jonas JB. Preoperative biometry of cataractous eyes using partial coherence laser interferometry. Graefes Arch Clin Exp Ophthalmol. 2003;241:251–252. doi: 10.1007/s00417-002-0531-6. [DOI] [PubMed] [Google Scholar]
- 10.Lee MK, Hwang KY, Kim MS. Effects of axial length and vitrectomy on refractive error after cataract surgery using SRK/T formula. J Korean Ophthalmol Soc. 2013;54:257–264. [Google Scholar]
- 11.Drexler W, Findl O, Menapace R, et al. Partial coherence interferometry: a novel approach to biometry in cataract surgery. Am J Ophthalmol. 1998;126:524–534. doi: 10.1016/s0002-9394(98)00113-5. [DOI] [PubMed] [Google Scholar]
- 12.Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238:765–773. doi: 10.1007/s004170000188. [DOI] [PubMed] [Google Scholar]
- 13.Tehrani M, Krummenauer F, Blom E, Dick HB. Evaluation of the practicality of optical biometry and applanation ultrasound in 253 eyes. J Cataract Refract Surg. 2003;29:741–746. doi: 10.1016/s0886-3350(02)01740-6. [DOI] [PubMed] [Google Scholar]
- 14.Packer M, Fine IH, Hoffman RS, et al. Immersion A-scan compared with partial coherence interferometry: outcomes analysis. J Cataract Refract Surg. 2002;28:239–242. doi: 10.1016/s0886-3350(01)01259-7. [DOI] [PubMed] [Google Scholar]
- 15.Kiss B, Findl O, Menapace R, et al. Refractive outcome of cataract surgery using partial coherence interferometry and ultrasound biometry: clinical feasibility study of a commercial prototype II. J Cataract Refract Surg. 2002;28:230–234. doi: 10.1016/s0886-3350(01)01274-3. [DOI] [PubMed] [Google Scholar]
- 16.Song BY, Yang KJ, Yoon KC. Accuracy of partial coherence interferometry in intraocular lens power calculation. J Korean Ophthalmol Soc. 2005;46:775–780. [Google Scholar]
- 17.Madge SN, Khong CH, Lamont M, et al. Optimization of biometry for intraocular lens implantation using the Zeiss IOLMaster. Acta Ophthalmol Scand. 2005;83:436–438. doi: 10.1111/j.1395-3907.2005.00486.x. [DOI] [PubMed] [Google Scholar]