Abstract
Purpose
To describe the clinical course of congenital aniridia and to evaluate prognostic factors for visual outcome after long-term follow-up.
Methods
The medical records of 120 eyes from 60 patients with congenital aniridia were retrospectively reviewed. The prevalence and clinical course of ophthalmic characteristics, systemic disease, refractive errors, and visual acuity were assessed. Prognostic factors for final visual outcomes were analyzed.
Results
Aniridic keratopathy developed in 82 (69%) of 119 eyes. Macular hypoplasia was observed in 70 eyes of 35 patients (91%). Cataract was observed in 63 of 120 eyes (53%). Nystagmus was present in 41 patients (68% of 60 patients) at the initial visit but decreased in five patients (8% of 60 patients). Ocular hypertension was detected in 19 eyes (20% of 93 eyes), six (32% of 19 eyes) of which developed secondarily after cataract surgery. The mean changes in spherical equivalent and astigmatism during the follow-up period were -1.10 and 1.53 diopter, respectively. The mean final visual acuity was 1.028 logarithm of minimal angle of resolution. Nystagmus and ocular hypertension were identified as prognostic factors for poor visual outcome.
Conclusions
Identification of nystagmus and ocular hypertension was important to predict final visual outcome. Based on the high rate of secondary ocular hypertension after cataract surgery, careful management is needed.
Keywords: Aniridia, Eye abnormalities, Prognosis
Congenital aniridia is a rare congenital disorder characterized by varying degrees of hypoplasia of the iris. It develops due to a mutation in the PAX6 gene, which is located on chromosome 11p [1,2,3]. Although the prevalence of aniridia was reported to range from 1:64,000 to 1:96,000 [4,5], one study found a greater prevalence (1:47,000) in a cohort of patients younger than 20 years [6]. In two-thirds of aniridia cases, the condition is inherited as an autosomal dominant disorder, whereas it occurs sporadically in one-third of cases [4,7]. Since aniridia is a panocular disorder, visual impairment in aniridia results from various ocular structural abnormalities including corneal opacity, cataract, glaucoma, and macular hypoplasia. Although several articles have been published that discuss various surgical techniques for managing aniridia [8,9,10] or describe cross-sectional findings with small patient groups [11], little information is known about the clinical characteristics or visual outcomes of aniridia after long-term follow-up. In this study, we describe the ophthalmic and systemic features of congenital aniridia over time, including those of patients who underwent ocular surgery. In addition, we identified prognostic factors for final visual outcome.
Materials and Methods
This study was approved by the Seoul National University Hospital Institutional Review Board and was conducted in accordance with the Declaration of Helsinki ethical principles for medical research. Detailed medical records from all patients who were diagnosed with aniridia at Seoul National University Children's Hospital between 1990 and 2010 were retrospectively reviewed. All patients were diagnosed and examined by the same ophthalmologist (YSY) and were referred to pediatricians for systemic evaluations.
The initial and final statuses of the cornea, lens, fundus, intraocular pressure (IOP), nystagmus, strabismus, and refractive errors as determined using cycloplegic refraction were compared. Fundus examination was performed with an indirect ophthalmoscope at the initial visit, and ultrasonography was used in cases in which the fundus was not visible. Macular hypoplasia was diagnosed when there was no macular depression or macular reflex during fundus examination. IOP was recorded with the TonoPen (Mentor, Norwell, MA, USA), and ocular hypertension was diagnosed if the IOP was continuously elevated over 21 mmHg and in patients for whom IOP-lowering eye drops had been prescribed. Cycloplegic refraction was performed via manual retinoscopy 30 minutes after a series of three drops of 1% cyclopentolate, which were administered five minutes apart. When lens opacity was considered prohibitive of visual improvement, cataracts were removed. Posterior capsulotomy and anterior vitrectomy were performed in every surgery. Intraocular lenses were inserted considering ocular size and patient age.
To identify changes in refractive errors, we analyzed refractive errors in phakic patients only, comparing the results to those who were initially assessed at age 5 years or younger and who were followed for at least three years. We compared the initial and final refractive errors in these patients. For the evaluation of long-term visual outcomes, we identified the patients who underwent follow-up for five or more years after the initial assessment. The final visual acuities were measured using the Snellen visual acuity chart. Various ocular and systemic status characteristics were analyzed in order to identify prognostic factors for final visual outcome. We used only the right eyes of patients in order to exclude inter-eye correlation during evaluation of refractive error changes and prognostic factors for final visual outcome.
Statistical methods
We used SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) for all statistical analyses. All of the tests were two-tailed, and significance was set at 0.05. Refractive error changes measured during the follow-up period were compared with paired t-test. A correlation analysis was performed to evaluate the associations of variables and potential prognostic factors with final visual outcome and assessments of light perception and no light perception. In the data analysis, light perception was assigned a logarithm of minimal angle of resolution (logMAR) value of 2.7, and no light perception was assigned a logMAR value of 3.0 [12].
Results
Patients
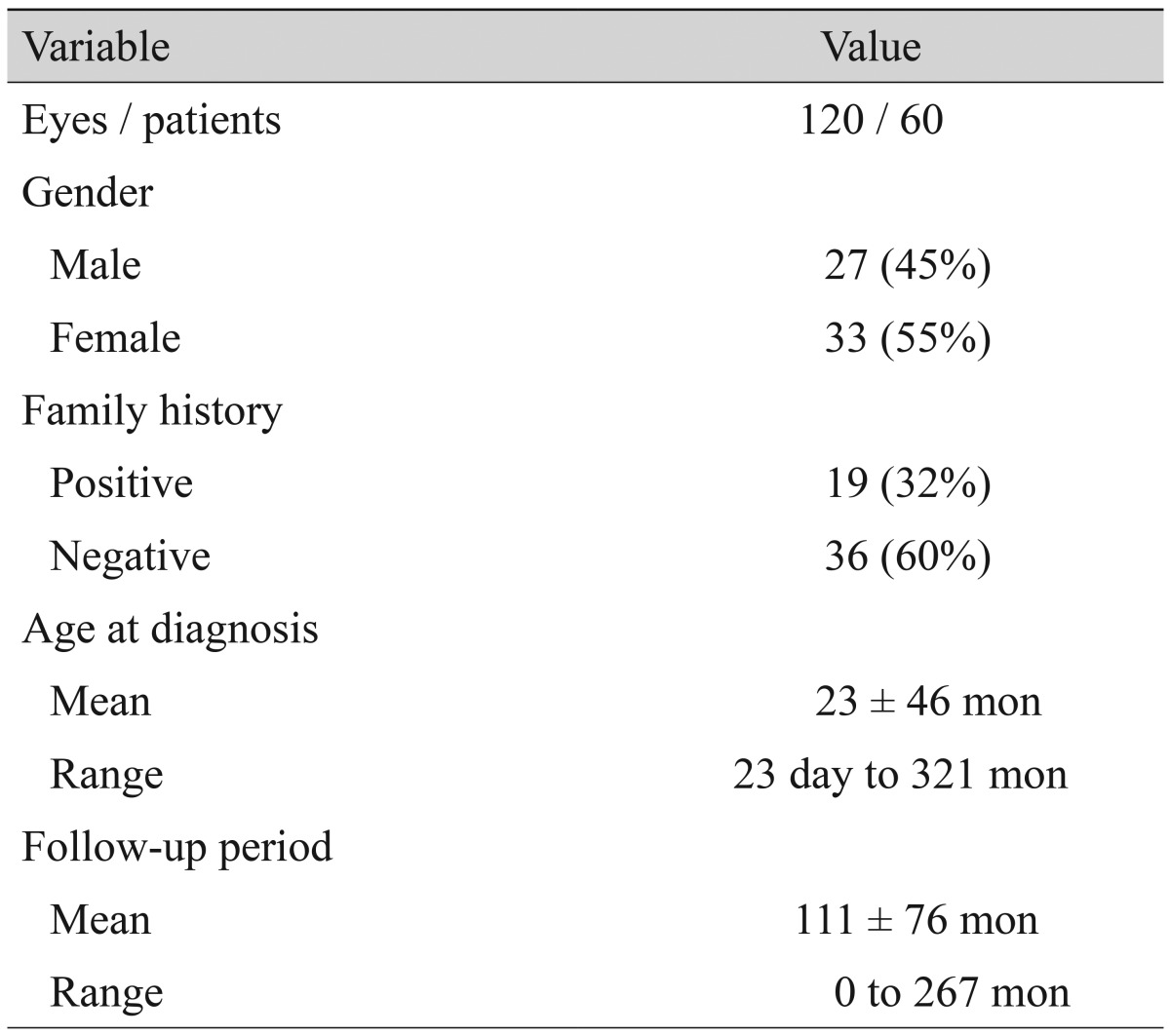
In total, 120 eyes from 60 patients were enrolled in this study. Of the study population, 19 patients (32%) had family histories of aniridia, and 36 patients (60%) did not. The remaining five patients (8%) had no information regarding a family history of aniridia. The mean patient age at the initial visit was 23 months, and 85% of the patients were aged <3 years. Detailed patient demographics are summarized in Table 1.
Table 1.
Demographic characteristics of patients with congenital aniridia
Ophthalmic characteristics
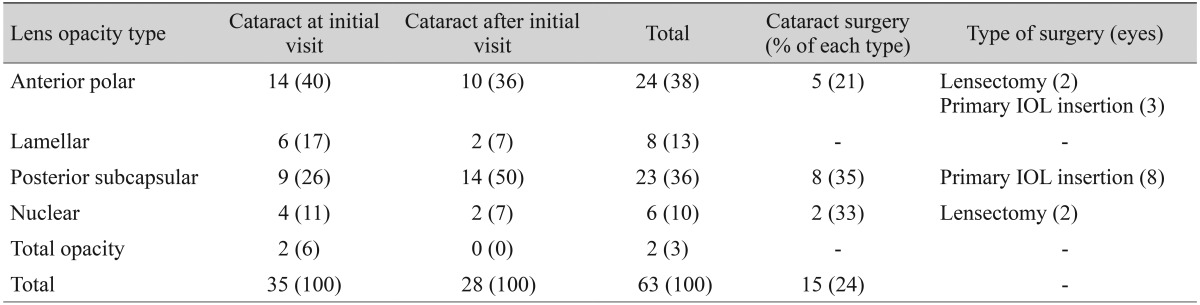
Six eyes from three patients (5%) had other congenital ophthalmic anomalies: Peters' anomaly (four eyes, two patients) and microphthalmia with sclerocornea (two eyes, one patient). Corneal opacities were documented in 82 eyes from 41 patients (69%) during the follow-up period. Cataracts were observed in 63 eyes total (53%); 35 eyes (56% of cataracts) had cataracts at the initial visit, and 28 eyes (44% of cataracts) developed cataracts during the follow-up period (Table 2). Cataract surgery was performed in 15 eyes from 11 patients (24% of cataracts), and mean age at surgery was 31 months (range, 6 to 66 months). Fundus examinations with an indirect ophthalmoscope were possible in 78 eyes from 39 patients (65%). Among these patients, macular hypoplasia was observed in 70 eyes of 35 patients (91%). However, only seven eyes had normally developed maculae on inspection with an indirect ophthalmoscope. Two eyes with normal maculae had 20 / 20 vision, and one eye had a visual acuity of 20 / 60. The remaining four eyes were lost to follow-up after the initial visit. Nystagmus was present in 41 of 60 patients (68%) at the initial visit. However, at the final visit, nystagmus was decreased in five patients (8%). IOP was measured in 93 eyes from 47 patients, and ocular hypertension developed in 19 eyes (20% of IOP checked). Eight eyes (42% of ocular hypertension) had ocular hypertension at the initial presentation, and 11 eyes (58% of ocular hypertension) developed ocular hypertension during the follow-up period. Six eyes (40% of total cataract surgery) developed secondary ocular hypertension af ter cataract surgeries (two eyes af ter phacoemulsification and intraocular lens insertion, and four eyes after lensectomy). Trabeculectomies were performed for four eyes of two patients, and one eye received multiple trabeculectomies but ultimately received an Ahmed valve implantation. Multiple cyclophotocoagulation treatments were performed for one eye in one patient.
Table 2.
Dominant types of lens opacities in congenital aniridia, and the chronology of their appearance during the follow-up period
Values are presented as number (%).
IOL = intraocular lens.
Refractive errors
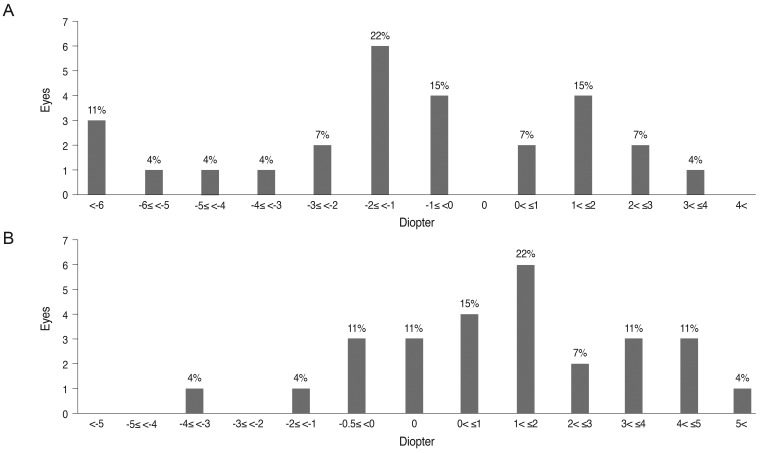
Refractive error was assessed in 27 eyes from 27 patients who were initially assessed at age five years or younger and who were followed for at least three years. The mean age of initial and final cycloplegic refraction was 37 and 141 months of age, respectively. The mean change in spherical equivalent over the course of the follow-up period was -1.10 diopter (D), and the mean change in astigmatism was 1.53 D. Overall, 18 eyes (67%) experienced myopic shifts, and nine eyes (33%) experienced hyperopic shifts (Fig. 1). Moreover, 19 eyes (70%) experienced increased astigmatism. Nine eyes (33%) showed increases in astigmatism of more than 2.0 D. The mean changes in spherical equivalent and astigmatism were small, but the range of change was wide.
Fig. 1.
Changes in spherical equivalent (A) and astigmatism (B) between the initial and final exams in congenital aniridia.
Visual outcomes and prognostic factors
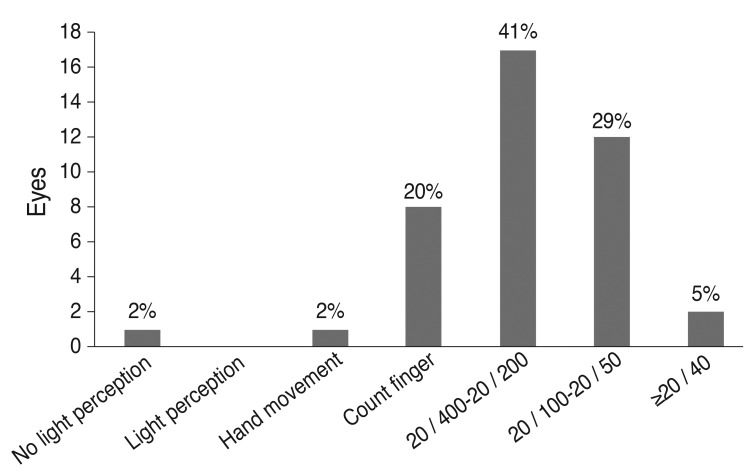
Visual outcomes were assessed in 41 eyes from 41 patients. The average final visual acuity was 1.028 ± 0.611 logMAR (20 / 213). The mean age at which final visual acuity was measured was 158 months (range, 60 to 270 months), and the mean follow-up period before final visual acuity assessment was 143 months (range, 60 to 267 months). The distribution of final visual acuities is presented in Fig. 2. A correlation analysis was performed to determine whether ocular or systemic variables were correlated with final visual outcome (Table 3). The results of the correlation analysis showed that the presence of nystagmus at either the initial or final visit and the development of ocular hypertension significantly influenced the final visual outcome.
Fig. 2.
Distribution of final visual acuity in congenital aniridia.
Table 3.
Correlations between clinical characteristics and final visual outcome (logarithm of minimal angle of resolution)
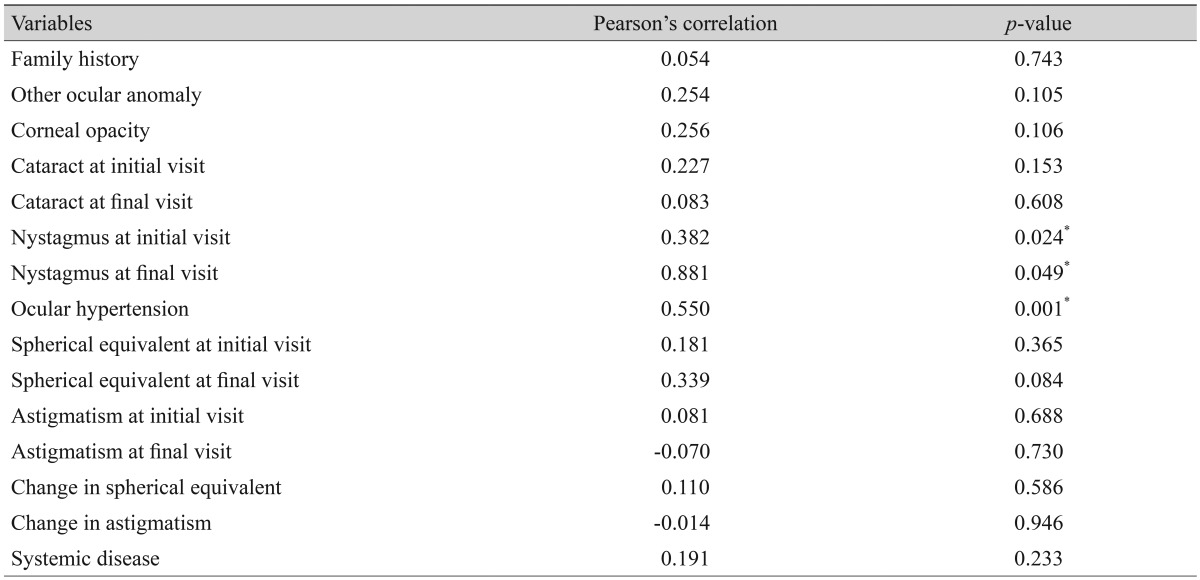
*Statistical significance.
Systemic characteristics
Four patients (7%) who participated in this study developed Wilms' tumors. On average, these patients were diagnosed at 38.3 months of age (range, 12 to 48 months). Among those four patients with Wilms' tumors, three were diagnosed with Wilms' tumors, aniridia, genitourinary abnormalities, and mental retardation (WAGR) syndrome. Three patients (5%) had Gillespie syndrome (aniridia, cerebellar ataxia, and mental retardation). Other associated systemic anomalies included cardiopulmonary anomalies (four patients), urogenital anomalies (two patients), a gastrointestinal anomaly (one patient), and a central nervous system anomaly (one patient). Three patients had multiple systemic anomalies.
Discussion
Aniridia is a rare congenital anomaly in which iris abnormalities are the prominent feature. However, since other ocular structures are also affected, visual outcomes are typically poor. Among the systemic anomalies that occur in conjunction with aniridia, some appear at birth, while some develop during early childhood [11]. Therefore, it is important to understand the clinical course of aniridia and its visual outcome.
Cataracts occur in 50% to 85% of aniridia patients and are known to develop during the first two decades of life [4]. In this study, 63 eyes (53%) had cataracts, 56% of which were discovered during initial evaluations; 44% developed during the follow-up period. During the initial visit, anterior polar opacities were more common. However, the lens opacities that developed during the follow-up period were predominantly of the posterior subcapsular type (Table 2). Thus, determination of the type and presence of lens opacity in an aniridia patient is valuable for predicting cataract progression. We performed cataract extraction surgeries in 15 eyes from 11 patients (24% of cataracts). Among the patients who received primary intraocular lens implantation (average age, 13 years; range, 5 to 16 years), only four eyes of two patients achieved improvements of more than two lines of Snellen visual acuity; two eyes had the anterior polar type of opacity and two eyes had the posterior subcapsular type. We are not certain if the time of occurrence or type of lens opacity influenced visual outcome. However, we assumed that it was important to establish the presence of lens opacity, the type of opacity, and the timing of its development in order to appropriately manage and plan for cataract operations.
Macular hypoplasia is a common condition in aniridia [4]. PAX6 is a protein expressed in differentiating cells of various ocular structures, including the retina, throughout development [13]. Therefore, the PAX6 gene mutation is thought to be responsible for the macular hypoplasia that is associated with aniridia and other diseases [14]. Although the reported incidence of macular hypoplasia ranges from 10.7% to 54.5% [11,15], we observed macular hypoplasia in 91% of our aniridia patients. This discrepancy may be related to the vague definition of macular hypoplasia and to the lack of a standardized diagnostic method between studies. In both the current and previous studies, macular hypoplasia was diagnosed based on the absence of a macular depression and on reflex during direct fundus examination. However, a recent study reported that optical coherence tomography (OCT) was a useful tool for diagnosing macular hypoplasia in children with aniridia [16]. The study found that macular thickness was significantly increased in aniridia patients, which suggests that OCT could eventually be used as a standardized method to evaluate macular development in aniridia. However, this is not an easy technique to use in diagnosing infants or young children.
The cause of nystagmus in aniridia is not well established. However, it is thought to be either the result of poor development of central vision due to foveal hypoplasia [17,18] or the result of a developmental defect in the neural control of eye movements [19]. In the present study, nystagmus was identified in 68% of aniridia patients at the initial examination. However, the incidence of nystagmus decreased to 22% by the final examination. We hypothesized that the prevalence of nystagmus decreased due to visual development and maturation of the central nervous system.
Glaucoma in congenital aniridia is known to develop in 6 to 75% of patients during the adolescent or early adolescent years due to progressive change in the structure of the angle [4,11,20]. During the first few years of life, the trabecular meshwork appears open and is not covered by iris tissue. As patients age, the extension of the iris stroma covers the filtration area, leading to the development of glaucoma. Ocular hypertension developed in 19 eyes (20% of IOPs measured) in this study. Six eyes (32% of those with ocular hypertension, 40% of those who received cataract surgery) developed secondary ocular hypertension after intraocular surgeries. Park et al. [21] also reported that deterioration of glaucoma was the main postoperative problem and was observed in 50% of patients after cataract surgery. Therefore, it is important to prepare for and manage secondary ocular hypertension after cataract surgery.
Among the various factors, nystagmus and ocular hypertension had statistically significant correlations with final visual outcome. In patients that developed ocular hypertension or had nystagmus at the initial examination or the follow-up examination, the final visual outcome was poor. Therefore, nystagmus may be either a cause or a result of a poor visual outcome; however, it may still be an indicator of poor visual outcome. In addition, ocular hypertension was identified as another significant risk factor for poor visual outcome. The mean final visual acuities of the eyes with and without ocular hypertension were 1.645 ± 0.691 logMAR (20 / 880) and 0.890 ± 0.442 logMAR (20 / 155), respectively (independent sample t-test, p = 0.003). Taylor et al. [22] also reported that aniridia with pediatric glaucoma was associated with poor visual outcome. They found that secondary ocular hypertension occurring after ocular surgeries accounted for 32% of total ocular hypertension cases, and ocular hypertension developed in 60% of eyes after cataract surgery. Therefore, to optimize final visual outcome, it is important to consider ocular hypertension and appropriate management after cataract surgery in patients with aniridia.
This study has several limitations. Due to the retrospective design of this study, although keratopathy is a well known cause of visual disturbance in aniridia [23], we were unable to describe or grade the defects of the cornea and iris in detail. We did not screen for the PAX6 mutation in most patients. Therefore, we were not able to evaluate for associations between the PAX6 mutation and structural and accompanying congenital abnormalities. Additionally, we used the term ocular hypertension instead of glaucoma because we could not identify glaucomatous change in the optic disc. Thus, we cannot directly compare the exact incidence of glaucoma in aniridia in our study with the results of previous studies. Our final limitation is that we did not analyze the macular structure using OCT. We observed macular hypoplasia in 91% of eyes. However, the structural difference may influence the final visual outcome.
Nevertheless, this study has several valuable contributions. To the best of our knowledge, no prior studies have described the general clinical course of congenital aniridia over a long-term follow-up period with a large number of patients, particularly younger patients. Therefore, we were able describe the changes in various ophthalmic features in aniridia that occurred during the developmental period. In addition, this is the first study that analyzed the prognostic factors for visual outcome. Nystagmus and ocular hypertension were identified as poor prognostic factors for final visual outcome. If cataract surgery was performed, IOP monitoring is important since there was a high rate of secondary ocular hypertension after cataract operations.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ton CC, Hirvonen H, Miwa H, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- 2.Glaser T, Walton DS, Maas RL. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet. 1992;2:232–239. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- 3.Churchill A, Booth A. Genetics of aniridia and anterior segment dysgenesis. Br J Ophthalmol. 1996;80:669–673. doi: 10.1136/bjo.80.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson LB, Spaeth GL, Nowinski TS, et al. Aniridia: a review. Surv Ophthalmol. 1984;28:621–642. doi: 10.1016/0039-6257(84)90184-x. [DOI] [PubMed] [Google Scholar]
- 5.Eden U, Iggman D, Riise R, Tornqvist K. Epidemiology of aniridia in Sweden and Norway. Acta Ophthalmol. 2008;86:727–729. doi: 10.1111/j.1755-3768.2008.01309.x. [DOI] [PubMed] [Google Scholar]
- 6.Eden U, Beijar C, Riise R, Tornqvist K. Aniridia among children and teenagers in Sweden and Norway. Acta Ophthalmol. 2008;86:730–734. doi: 10.1111/j.1755-3768.2008.01310.x. [DOI] [PubMed] [Google Scholar]
- 7.Mannens M, Bleeker-Wagemakers EM, Bliek J, et al. Autosomal dominant aniridia linked to the chromosome 11p13 markers catalase and D11S151 in a large Dutch family. Cytogenet Cell Genet. 1989;52:32–36. doi: 10.1159/000132834. [DOI] [PubMed] [Google Scholar]
- 8.Neuhann IM, Neuhann TF. Cataract surgery and aniridia. Curr Opin Ophthalmol. 2010;21:60–64. doi: 10.1097/ICU.0b013e328333ea49. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Khan R, O'Keefe M. Aniridia: current pathology and management. Acta Ophthalmol. 2008;86:708–715. doi: 10.1111/j.1755-3768.2008.01427.x. [DOI] [PubMed] [Google Scholar]
- 10.De la Paz MF, Alvarez de Toledo J, Barraquer RI, Barraquer J. Long-term visual prognosis of corneal and ocular surface surgery in patients with congenital aniridia. Acta Ophthalmol. 2008;86:735–740. doi: 10.1111/j.1755-3768.2008.01293.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee H, Meyers K, Lanigan B, O'Keefe M. Complications and visual prognosis in children with aniridia. J Pediatr Ophthalmol Strabismus. 2010;47:205–210. doi: 10.3928/01913913-20090818-07. [DOI] [PubMed] [Google Scholar]
- 12.Schulze-Bonsel K, Feltgen N, Burau H, et al. Visual acuities "hand motion" and "counting fingers" can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47:1236–1240. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 13.Nishina S, Kohsaka S, Yamaguchi Y, et al. PAX6 expression in the developing human eye. Br J Ophthalmol. 1999;83:723–727. doi: 10.1136/bjo.83.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azuma N, Nishina S, Yanagisawa H, et al. PAX6 missense mutation in isolated foveal hypoplasia. Nat Genet. 1996;13:141–142. doi: 10.1038/ng0696-141. [DOI] [PubMed] [Google Scholar]
- 15.McCulley TJ, Mayer K, Dahr SS, et al. Aniridia and optic nerve hypoplasia. Eye (Lond) 2005;19:762–764. doi: 10.1038/sj.eye.6701642. [DOI] [PubMed] [Google Scholar]
- 16.Holmstrom G, Eriksson U, Hellgren K, Larsson E. Optical coherence tomography is helpful in the diagnosis of foveal hypoplasia. Acta Ophthalmol. 2010;88:439–442. doi: 10.1111/j.1755-3768.2009.01533.x. [DOI] [PubMed] [Google Scholar]
- 17.Grove JH, Shaw MW, Bourque G. A family study of aniridia. Arch Ophthalmol. 1961;65:81–94. doi: 10.1001/archopht.1961.01840020083016. [DOI] [PubMed] [Google Scholar]
- 18.Elsas FJ, Maumenee IH, Kenyon KR, Yoder F. Familial aniridia with preserved ocular function. Am J Ophthalmol. 1977;83:718–724. doi: 10.1016/0002-9394(77)90139-8. [DOI] [PubMed] [Google Scholar]
- 19.Sonoda S, Isashiki Y, Tabata Y, et al. A novel PAX6 gene mutation (P118R) in a family with congenital nystagmus associated with a variant form of aniridia. Graefes Arch Clin Exp Ophthalmol. 2000;238:552–558. doi: 10.1007/s004170000124. [DOI] [PubMed] [Google Scholar]
- 20.Grant WM, Walton DS. Progressive changes in the angle in congenital aniridia, with development of glaucoma. Trans Am Ophthalmol Soc. 1974;72:207–228. [PMC free article] [PubMed] [Google Scholar]
- 21.Park SH, Park YG, Lee MY, Kim MS. Clinical features of Korean patients with congenital aniridia. Korean J Ophthalmol. 2010;24:291–296. doi: 10.3341/kjo.2010.24.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor RH, Ainsworth JR, Evans AR, Levin AV. The epidemiology of pediatric glaucoma: the Toronto experience. J AAPOS. 1999;3:308–315. doi: 10.1016/s1091-8531(99)70028-5. [DOI] [PubMed] [Google Scholar]
- 23.Eden U, Riise R, Tornqvist K. Corneal involvement in congenital aniridia. Cornea. 2010;29:1096–1102. doi: 10.1097/ICO.0b013e3181d20493. [DOI] [PubMed] [Google Scholar]