Abstract
The risk of developing colorectal cancer increases in patients with inflammatory bowel disease (IBD) and a growing body of evidence shows the critical role of interleukin (IL-6) in this process. IL-6 is both a pro- and anti-inflammatory cytokine whose effects are mediated through activation of STAT3. Recent studies have also demonstrated that IL-6 trans-signaling through its soluble receptor occurs in IBD and cancer. IL-6 trans-signaling therefore is emerging as an attractive approach to diminish the inflammatory signals in conditions of chronic inflammation. The purpose of cancer chemoprevention is to either delay the onset or progression from precancerous lesions. Natural compounds because of their low toxicity render themselves excellent candidates that can be administered over the lifetime of an individual. With the focus of managing IBD over a long time and preventing onset of colitis-associated cancer, we believe that there should be increased research focus on identifying chemopreventive compounds that can render themselves to long term use possibly for the lifetime of predisposed individuals. Here, we review the role of IL-6 signaling in IBD and colitis-associated cancer and underscore the importance of searching for natural compounds that would target the IL-6 trans-signaling pathway as a way to diminish chronic inflammatory conditions in the gastrointestinal tract and possibly hamper the progression to colon cancer. We propose that effective screening and identification of natural chemopreventive compounds that target IL-6 trans-signaling has important implications for the development of optimal strategies against cancer development triggered by inflammation.
Keywords: Chemoprevention, Chemopreventive compounds, Chronic inflammation, Colitis-associated cancer, Colorectal cancer, Crohn’s disease, Inflammatory bowel disease, Inflammatory cytokines, Interleukin-6, IL-6 signaling, IL-6 trans-signaling, Natural chemopreventive compounds, Natural compounds, Soluble IL-6 receptor, STAT3, Ulcerative colitis
1. Introduction
Inflammation, a normal physiological response to tissue injury or infection, is now recognized as a critical component of tumor progression in many types of malignancies including colorectal cancer [1–4]. Colorectal cancer is one of the most common fatal malignancies in the world. It is the third most frequently diagnosed cancer in both men and women, and was the second leading cause of cancer deaths in the United States [5]. Of great concern from the report is the finding that there was an increase in incidence in men and women under 50 years of age.
Various studies have continued to show evidence that inflammation increases the risk of developing colon cancer [6–9]. Inflammatory bowel Disease (IBD) is characterized by recurring inflammation of the gastrointestinal tract and it is a collective term for Crohn’s disease (CD) and ulcerative colitis (UC) [10]. Both diseases are characterized by an abnormal response of the immune system leading to a chronically inflamed environment. There is significant infiltration of immune cells that produce a variety of cytokines and chemokines to propagate the inflammatory response, increase proliferation, differentiation and inhibit apoptosis [1, 2, 8, 11]. In its most advanced stage, this environment can lead to development of colitis-associated cancer [12, 13]. Indeed there is a recognized increase in risk for colorectal cancer in patients with inflammatory bowel disease [6, 14–17]. On the other hand, there is also evidence showing that reducing inflammation might play a role in reducing progression from inflammation to cancer. For instance long-term use of anti-inflammatory drugs reduces colon cancer risk by 40–50% [1].
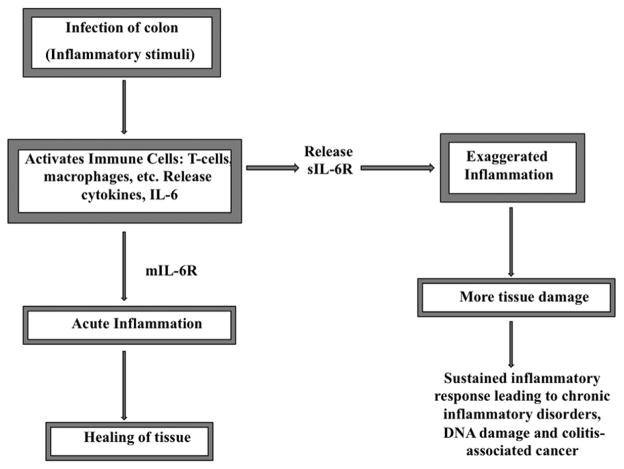
An estimated 1.4 million persons in the United States suffer from IBD (http://www.cdc.gov/ibd/). Nearly 30% of these are diagnosed during their childhood years (http://www.ccfa.org/advocacy/IBDResearchEnhancementAct). Currently, there is no medical cure for IBD and therefore the patient requires a lifetime of care. More research is therefore needed to help us better understand the causes of uncontrolled inflammation and the immunopathogenesis of IBD. Currently, advocacy around the United States has led to enactment of the Inflammatory Bowel Disease Research Enhancement Act. In the meantime, there is an urgent need to use our current understanding and recent advances in the understanding of IBD, to develop therapeutic or preventive strategies that will halt the disease and hamper its progression to cancer. The advancement from inflammation to cancer is a process that could be interrupted by developing strategies that target various pathways and processes, therefore managing the disease and hindering it from becoming lethal. This review focuses on prospects of chemoprevention of colitis-associated cancer by targeting interleukin-6 (IL-6), as trans-signaling by IL-6 is now believed to play a fundamental role in the development and maintenance of IBD and in the progression of this inflammation to colon cancer [18, 19] (Figure 1).
Figure 1.
IL-6 magnifies the inflammatory response through its soluble receptor. Release of the sIL-6R acts to amplify the inflammatory response by making cells lacking mIL-6R responsive to IL-6. The resulting sustained inflammatory response paves way for the progression from acute to chronic inflammation and could ultimately lead to colitis-associated cancer.
2. Inflammation to colon cancer
Colonic inflammation may pave the way for colitis-associated cancer [12]. Inflammation is usually in response to tissue injury or presence of foreign invaders, in which case the body initiates an inflammatory response, designed to heal the afflicted tissue (Figure 1). The review of Coussens and Werb [1] is an excellent source for details of this complex process characterized by a multifactorial network of chemical signals required to initiate and maintain a host response. The normal inflammatory process (acute) is self-limiting and the pathological (chronic) inflammatory conditions result from dysregulation of any of the factors critical in triggering and maintaining the inflammatory response [1]. The abnormal sustained inflammatory response in chronic inflammatory conditions may start as a response to tissue injury or the presence of foreign invaders and in some cases it is merely an autoimmune process. Research is not conclusive on what triggers the abnormal inflammatory response but there is evidence for various factors [20]. Genetic contribution is considered an important factor [21]; for instance a susceptibility gene nucleotide-binding oligomerisation domain 2/caspase recruitment domain 15 (NOD2/CARD15) that was identified as a risk factor [22, 23] is reported to account for about 20% of the genetic predisposition to CD [24]. More susceptibility genes including, DLG5, OCTN1 and 2, TLR4, CARD4 (NODI) IL23R, IRGM, PTGER4, ATG16L1, HLA, IBD5 have been identified through whole genome association studies, genome-wide linkage studies, fine-mapping as well as candidate gene studies [24–30]. Other factors include, but not limited to, defects in the mucosal barrier, environmental factors including smoking, geography, sanitation, hygiene, work environment as well as pathogenic microorganisms, changes in the gastrointestinal microbiota and any factor that could cause alterations in the function of the immune system [31–33]. However, the greatest relative risk of IBD is found among first-degree relatives (http://www.cdc.gov/ibd/). The relative risk of IBD to siblings compared to general population risk ranges from 30 to 40 for CD and from 10 to 20 for UC [34].
Inflammatory Bowel Diseases are associated with T-cell activation [35] and consequently a lot of research has focused on these cells. T cells play an important role in short-term effector immunity as well as in directing other immune cells. An imbalanced mucosal T cell response has been identified in both CD and UC [36]. Research shows that there are persistently elevated levels of activated T cells in the mucosa of CD and UC patients [37]. CD4+ effector T cells associated with CD and UC are categorized as T helper type 1 (TH1) and T helper type 2 (TH2) and a recently identified category of T cells (TH-17 cells) that produce interleukin 17 and are highly pro-inflammatory [38]. CD is regarded as a Thl-mediated inflammatory disorder since lamina propria cells from patients with CD overproduce cytokines associated with a Thl response [35, 39]. On the other hand UC is regarded as a Th2-like disease since cells from patients with UC overproduce cytokines associated with the Th2 response [35, 39]. Activated T-cells are resistant to apoptosis, produce cytokines, activate macrophages, which once activated, produce additional inflammatory growth factors and cytokines [1, 40].
Cytokines contribute to amplifying and sustaining the ongoing mucosal inflammation and therefore play a central role in modulating inflammation [20, 40–42]. Indeed the profile of cytokines persisting at an inflammatory site is important in the development of chronic disease [1, 43]. Cytokines profoundly affect endothelial, epithelial and mesenchymal cells in the local microenvironment. They mediate their effects through binding to specific membrane bound receptors expressed on target cells [41]. The body can also mitigate by inducing an inflammatory response that includes producing anti-inflammatory cytokines or using the soluble forms of the receptors for the proinflammatory cytokines as cytokine scavengers, thereby quenching cytokine signaling. However, interleukin 6 (IL-6) is an exception as its soluble receptor does not quench but rather promotes cytokine signaling in a process called trans-signaling [19, 41]. To further underscore the importance of IL-6 among the pro-inflammatory cytokines, recent evidence suggests that the development and perpetuation of IBD relies, in part, on IL-6 trans-signaling [18].
3. IL-6 and Regulation of its Expression
Various names have been used for IL-6 because of its multiple biological activities [44–46]. IL-6 has been shown to function as a hepatocyte-stimulating factor to induce acute phase reactions, and B-cell stimulatory factor to induce antibody production, in addition to inducing T-cell growth and cytotoxic T-cell differentiation. IL-6 is pleiotropic [45–51]. The term Interleukin-6 was assigned once it was clear that the various activities of differently named molecules were linked to the same gene on chromosome [7, 46].
Human IL-6 consists of 184 amino acids with two potential N-glycosylation sites and four cysteine residues [47]. IL-6 is secreted as a heterogeneous set of proteins with molecular mass ranging from 19 to 70 kDa. IL-6 is glycosylated and/or phosphorylated in post-translational modifications that may be tissue-specific and may also influence the biologic activity of IL-6 [52, 53]. The predominant isoforms have mass of 23 to 30 kDa. The 23–25-kDa species are O-glycosylated and that the 28- to 30-kDa species are both O- and N-glycosylated [52, 54]
IL-6 is produced by many cell types including, lymphoid cells, such as T cells, B cells, and non-lymphoid cells, monocytes, fibroblasts, endothelial cells, epithelial cells, several kinds of tumor cells as well as cells of the adipose tissue [45, 47, 51]. IL-6 has pleiotropic effects on various target cells although some of its activities are also mediated by other cytokines [45]. The expression of IL-6 under normal physiologic conditions is tightly controlled [19]. Expression of IL-6 is highly inducible in response to a variety of signals. These include antigenic stimulation with lipopolysaccharide (LPS) and other bacterial products, viral infection, agents that activate protein kinase C, agents that increase intracellular cAMP, various cytokines such as interleukin 1 (IL-1), platelet-derived growth factor (PDGF), tumor necrosis factor (TNF) and in conditions characterized by inflammation [47, 55]. Glucocorticoids, on the other hand, downregulate IL6 gene expression, thereby providing a negative feedback pathway on the inflammatory response [47, 55]. Promoter polymorphism is another factor that not only affects the expression of the IL-6 gene but may also influence susceptibility to chronic disorders involving IL-6 activity [56–60]. For instance, colon cancer risk is associated with an interaction between rs 1800795 and rs 1800796 IL6 polymorphisms and the use of aspirin /NSAIDs [61].
4. IL-6 and Colitis-associated Colon cancer
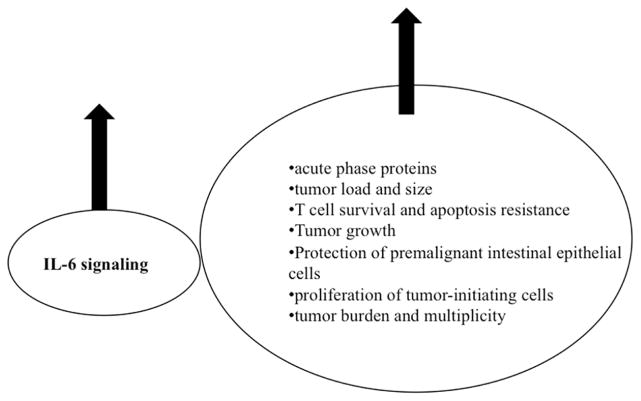
IL-6, a major modulator of inflammation [62], is essential for inflammation-associated carcinogenesis [63]. An increase in IL-6 is associated with an increase in biomarkers of inflammation severity [42, 46] and cancer progression (Figure 2). IL-6 trans-signaling sets IL-6 apart from other pro-inflammatory cytokines, which explains accumulating evidence about its potential pathological role in IBD and the development of colitis associated cancer. There is evidence for continuous stimulation of IL-6 production in IBD and levels of IL-6 and the soluble IL-6 receptor (sIL-6R) are elevated in both serum and intestinal tissue from patients with IBD [18, 20, 64–66]. The increased serum concentrations of IL-6 and sIL-6R have been correlated to clinical activity of the disease. IL-6 levels are also increased in the serum of patients suffering from colon cancer [67–69] and IL-6 levels correlate with tumor load and size in colorectal cancer. In vitro IL-6 stimulates growth of primary and metastatic human colon carcinoma cells [70]. IL-6, along with TGFβ, is also important in differentiation of TH17 cells from naive precursors [38, 71]. TH17 cells induce severe autoimmunity, which might explain the inconsistencies seen in the Thl/Th2 theory in the development of IBD [39] and other autoimmune diseases such as rheumatoid arthritis [71]. IL6 contributes to enhanced T cell survival and resistance to apoptosis in IBD [36]. Consequently, a humanized anti-IL-6R monoclonal antibody induces intestinal T cell apoptosis in patients with active CD [72, 73].
Figure 2.
Increase in IL-6 is associated with increase in biomarkers of inflammation severity and cancer progression. An increase in IL-6 signaling is associated with poor prognosis.
Inhibition of IL-6 signaling suppresses the growth of colon cancer [6, 74]. The proliferative and survival effects of IL-6 are largely mediated by the transcription factor STAT3, which is constitutively activated in colon cancer [75]. In addition, IL-6 is a critical tumor promoter during early colitis-associated colon tumorigenesis [8]. IL-6 protects normal and premalignant intestinal epithelial cells (IECs) from apoptosis, in addition to enhancing proliferation of tumor-initiating cells. Exogenous administration of IL-6 to mice during tumor initiation results in increased tumor burden and multiplicity, while administration during the late stages of colitis associated cancer growth increases tumor burden [8]. TGF-β, a negative regulator of mucosal inflammation in the intestine, works in part through down-regulation of IL-6 induced tyrosine phosphorylation of STAT1 and STAT3 [6, 76]. TGF-β receptor II is also frequently mutated in intestinal epithelial cells of patients with colon cancer [77]. IL-6 is continuing to attract attention due to its influence on tumor-initiating cells in various cancers. In cases of a disfunctional TGF-β pathway, the ability of cancer stem cells (CSCs) to give rise to human hepatocellular cancer is attributable to IL-6 signaling [78, 79]. IL-6, secreted by mesenchymal stem cells (MSCs), increases the number of colorectal tumor initiating cells and promotes tumor formation [80]. Cells, including embryonic, cancer and mesenchymal stem cells which do not express the membrane bound IL-6R are only responsive to IL-6 in the presence of sIL-6R [81, 82]. The ability of IL-6 to induce differentiation of neural stem cells is attributed to sIL-6R [83]. Cancer stem cells are critical for the initiation, propagation, treatment resistance and metastasis of multiple cancers including colon cancer. Therefore, the potential role of IL-6 in CSC propagation makes IL-6 status a significant predictor of poor prognosis for cancer patients. However, IL-6 mediated STAT3 signaling also plays a protective role in IBD [18]. For instance, severity of DSS-induced mucosal inflammation is increased in both IL-6 knockout mice and in mice lacking STAT3 in intestinal epithelial cells while the number of tumors is reduced [6, 7]. In addition, increased IL-6 levels in the gut mucosa counteracts some of the injurious effects of sepsis and endotoxemia [84, 85, 86 55]. Classic IL-6 signaling through the membrane bound IL-6 receptor (Figure 1) is responsible for the anti-inflammatory and regenerative response of intestinal cells to the stressor, while the IL-6 trans-signaling through the sIL-6R is responsible for the pro-inflammatory properties of IL-6, promoting apoptotic resistance of T cells hence leading to progression of the disease [18,87].
5. IL-6 signaling
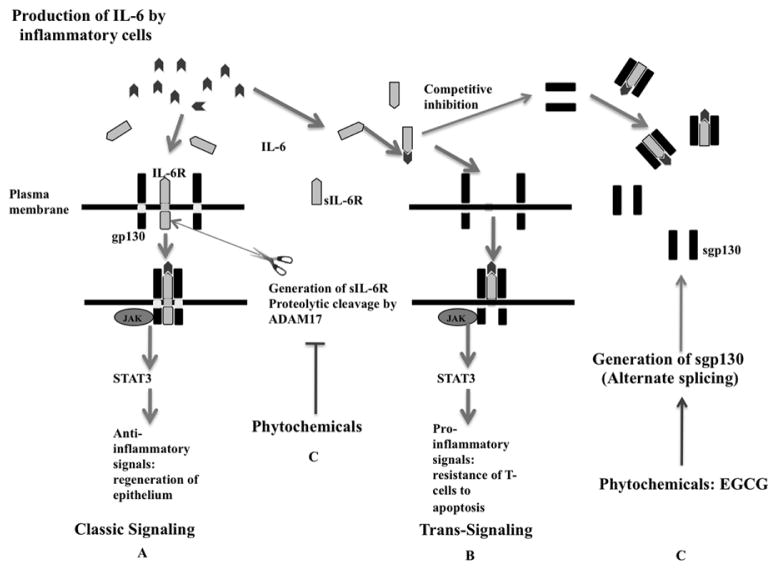
IL-6 is a member of a cytokine superfamily whose signaling mechanism requires the 130-kDa signal transducing protein, gp130 [48]. Binding of IL-6 to its cognate receptor (IL-6R) is not sufficient to transduce a signal and requires interaction with the membrane bound gp130 to induce signal transduction [45, 46, 48]. There are two types of IL-6 signaling, classic signaling and trans-signaling (Figure 4). In the classic signaling, IL-6 binds to its membrane bound IL-6R (mIL-6R, 80-kDa). The expression of mIL-6R is mainly confined to hepatocytes, neutrophils, monocytes, macrophages as well as some lymphocytes [82], while gp130, is widely expressed on many cell types. In trans-signaling IL-6 binds to the naturally occurring soluble form of the IL-6 receptor (sIL-6R, 50-kDa) forming a complex (IL-6/sIL-6R). IL-6/sIL-6R associates with gp130 to induce signal transduction in cells that lack the membrane-bound IL-6R and would not ordinarily respond to IL-6 [6, 46, 71, 82, 88, 89]. Association of IL-6/IL-6R (IL-6/sIL-6R) with gp130 results in activation of Janus kinases (JAKs). The JAKs then phoshorylate gp130 leading to recruitment and subsequent phosphorylation of STAT3 and other molecules as well as activation of various pathways including SHP-2/Ras, and PI3k/Akt [13, 45, 49, 90]. gp130 is shared by several other cytokine receptors including those of IL-11, leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), Oncostatin M (OSM), cardiotrophin-1 (IL-6-type cytokines). Therefore, the pleiotropic effects of IL-6 on target cells are also mediated by other cytokines partly explaining the molecular mechanisms of redundancy in cytokine functions [45, 48].
Figure 4.
Chemoprevention colitis-associated cancer. Exposing predisposed individuals to safe natural compounds that target IL-6 trans-signaling over their lifetime has the potential to hamper progression from acute to chronic inflammation and to delay onset of colitis and associated cancer.
Most soluble cytokine receptors (sCRs) prevent cytokines from binding to their membrane receptors and thereby inhibit cytokine signaling [91]. However, in addition to IL-6, two other cytokines, IL-11 and ciliary neurotrophic factor, in the IL-6 superfamily of cytokines display some trans-signaling activity [41, 89, 92]. The IL-11 receptor (IL-11R) shows many structural and functional similarities with IL-6R [93]. IL-11 signaling through interacting with IL-11R induces dimerization of membrane bound gp130 and subsequent signal transduction. Similarly the IL-11 through its soluble receptor (sIL-11R) is able to associate with gp130 on cells that don’t express mIL-11R [94]. Both IL-6 and IL-11 induce gp130-mediated STAT3 activation during colitis-associated cancer [7], suggesting the need to focus on IL-6 trans-signaling. Soluble IL-6R acts as an agonist, always amplifying IL-6 signaling, while sIL-11R can act both as an agonist and an antagonist [94]. For instance, sIL-11 antagonizes IL-11 signaling in cells expressing the membrane bound IL-11R [94]. In addition, IL-11 binds to sIL-HR with a low affinity and the concentration of IL-11 required for signaling through the sIL-11R is greater than that required for cells expressing membrane bound IL-11R [94]. This implies that classic IL-11 signaling is favored over IL-11 trans-signaling. Hence IL-11 trans-signaling may not amplify IL-11 signaling as seen with IL-6 trans-signaling. The role of IL-11 in IBD is not very clear. It is implicated in linking inflammation to cancer [7, 95]. On the other hand, its role might be to stimulate intestinal epithelial cells, which leads to STAT3 phosphorylation and increased regeneration of the epithelial cells [18]. Moreover there is no strong evidence for the existence of a naturally occurring sIL-11R at least not to the extent of sIL-6R [95]. T cells don’t express membrane-bound IL-11R but do express membrane bound IL-6R, which can act as a source of sIL-6R to cause continuous IL-6 signaling in intestinal epithelial cells [18]. Also, IL-6 binds to sIL-6R with the same affinity as the membrane bound receptor [96], and to date, no antagonistic activity has been reported for soluble IL-6R.
6. Role of IL-6 Trans-signaling in IBD and Colitis-associated Cancer
The role played by the IL-6 trans-signaling pathway in conditions of chronic inflammation is now becoming clearer. IL-6 and its soluble receptor (sIL-6R) are present at very high levels in both serum and intestinal tissue from IBD patients [18, 65, 66, 97].
There is strong evidence showing the importance of IL-6 trans-signaling in IBD and colitis associated colon cancer. TGF-β receptor II is frequently mutated in intestinal epithelial cells of patients with colon cancer [77], leading to dysfunctional TGF-β signaling. The effect of TGF-β, a negative regulator of mucosal inflammation, is modulated in part by IL-6 trans-signaling. In a AOM/DSS model, mice with impaired TGF-β signaling, having a mutated TGF-β receptor, show larger tumors and higher IL-6 expression than the wild type whereas TGF-β over-expressing mice show smaller tumors and lower IL-6 expression than their wild type counterparts [74]. In this case, TGF-β signaling in infiltrating T cells suppress IL-6-mediated STAT-3 activation in tumor cells thereby preventing tumor progression in vivo [74]. Important to note is that these tumor cells do not express the membrane bound IL-6R and are therefore dependent on IL-6 trans-signaling via the sIL-6R [74]. This implies that TGF-β production in tumor infiltrating T lymphocytes suppresses tumor growth by inhibition of IL-6 trans-signaling in an AOM/DSS model. This underscores the importance of IL-6 trans-signaling in inflammation derived colon cancer.
IL-6 trans-signaling in colonic epithelial cells plays a crucial role in the development of colitis-associated cancer in a murine model of colitis-associated premalignant cancer (CApC) and chronic colitis (CC) [9]. Epithelial cells of CC and CApC show increased levels of membrane-bound gp130. Moreover, sgp130 fusion protein, a specific IL-6 trans-signaling inhibitor, suppressed colitis associated premalignant cancer, demonstrating that IL-6 trans-signaling in epithelial cells plays a crucial role in the development of colitis-associated cancer [9].
IL-6 trans-signaling plays a major role in increasing the resistance of mucosal T cells against apoptosis in Crohn’s disease [98]. Treatment with sgp130 fusion protein, specific for sIL-6R suppresses TNBS-induced colitis by inducing apoptosis of lamina propria T cells [98]. Furthermore, blockade of IL-6 trans signaling in mucosal T cells isolated from colonic specimens of patients with Crohn’s disease causes T-cell apoptosis, indicating that the IL-6 trans-signaling mediates the resistance of T cells to apoptosis in Crohn’s disease [98]. It is evident that defective T cell apoptosis mediated by IL-6 trans-signaling contributes to the perpetuation of chronic intestinal inflammation [98–101]. Use of anti-IL-6R monoclonal antibody, which targets both classic and trans-signaling, in various murine models of colitis suppresses established colitis by inducing lamina propria T cell apoptosis [42, 102]. Both classic and trans-signaling induce activation of STAT3 (Figure 4), which mediates anti-inflammatory as well as proinflammatory signals [18]. Activation of STAT3 mediates resistance against apoptosis through induction of anti-apoptotic genes such as Bc12 and BclXL [36]. STAT3 activation therefore is a critical point in regulating the anti-apoptotic and pro-inflammatory effects in pathological inflammatory conditions, but it is not an attractive therapeutic target. Nonspecific targeting of STAT3 in IBD would not be useful because of blocking the necessary anti-inflammatory mechanisms as well [7, 36]. Moreover, deletion of STAT3 in intestinal epithelial cells (IEC) increases susceptibility to DSS and subsequent mucosal inflammation [7]. In fact, IL-6 knockout mice also develop inflammation even though at less severity compared to the inflammation in the mice with STAT3 depleted IECs [7], indicating that IL-6 is not the only STAT3-inducing protector [18]. Therefore, STAT3 plays a critical role in shaping the inflammatory response elicited by pro-inflammatory signals and anti-inflammatory signals [7]. However, over activation of STAT3 as in the case of IL-6 trans-signaling has the potential of fueling gastrointestinal inflammation and eventual carcinogenesis.
The role of IL-6 signaling in IBD and colitis-associated cancer is both protective and harmful, a double-edged sword (Figure 3A, B). Classic IL-6 signaling through the mIL-6R activates STAT3 leading to intestinal epithelial cell proliferation and inhibition of epithelial cell apoptosis thus playing a therapeutic role leading to regeneration of intestinal epithelial cells after damage from stressors [18]. Whereas, IL-6 trans-signaling through sIL-6R activates STAT3 leading to inhibition of T-cell apoptosis resulting in persistently high levels of activated T-cells which continue to produce factors like cytokines that contribute to amplifying and sustaining the ongoing mucosal inflammation [18]. In its most serious case, this environment paves way to colitis-associated cancer.
Figure 3.
Selective inhibition of IL-6 trans-signaling. (A) Classic IL-6 signaling. IL-6 binds to its mIL-6R causing dimeralization of gp130 and subsequent signal transduction leading to activation of STAT3. (B) IL-6 trans-signaling. IL-6 binds it sIL-6R generated from mIL-6R. The IL-6/sIL-6R associates with gp130 on cells that lack mIL-6R and lead to signal transduction. (C) Inhibition of IL-6 trans-signaling. Anti-sIL-6R natural compounds can target the sIL-6 receptor at the point of production, by inhibiting proteolytic cleavage of the mIL-6Ror by inhibiting signaling. sgp130 binds IL6/sIL-6R, preventing association of the same complex with membrane bound sgp130, thus inhibiting IL-6 trans-signaling in a more or less competitive inhibition mechanism. The natural compound EGCG increases sgp130, a mechanism that would down-regulate IL-6 signaling.
IL-6 signaling has beneficial effects of IL-6 [43] with regenerative effects in mucosal inflammation resulting from its stimulation of proliferative pathways and maintenance of normal homeostasis, dependent on signals via the mIL-6R [19]. IL-6 trans-signaling via the sIL-6R, is the main driving force of chronic inflammation and associated pathophysiological inflammatory conditions [103].
Undoubtedly the expression IL-6 is important for host defense although we should also consider the redundancy in the IL-6 family of cytokines. However, it is clear that the agonistic sIL-6 receptor that serves to potentiate IL-6 activity through the process of trans-signaling is of major concern in the prognosis of inflammatory conditions like IBD and colitis associated cancer. IL-6 trans-signaling fuels on-going inflammation allowing progression from acute to chronic inflammation. IL-6 trans-signaling therefore emerges as an important target for dealing with IBD and curtailing progression to colitis-associated cancer. With all that we know and continue to understand about the complex and pleiotropic nature of this cytokine, it is clear that global blockade of IL-6 as a chemopreventive strategy would not be without unpredictable results [52, 92]. This makes specific targeting of IL-6 trans-signaling emerge as a promising attractive strategy to combat IL-6 related pathological conditions. Moreover, in light of the recent interest in the effect of IL-6 on the growth and differentiation of tumor initiating cells or cancer stem cells, targeting IL-6 trans-signaling holds promise since these cells mostly do not express mIL-6R and would therefore only respond to IL-6 through the sIL-6R. This implies an exciting possibility of inhibiting proliferation of colorectal tumor initiating cells by specifically inhibiting IL-6 trans-signaling. With the knowledge that a significant number of colorectal cancer deaths are due to metastases that are resistant to conventional therapy [104], strategies that would target cancer stem cells are greatly needed.
7. Blockade of IL-6 Trans-Signaling
As mentioned above, inhibiting IL-6 trans-signaling has significant therapeutic and preventive value for colitis-associated cancers. Previously, Tocilizumab, a humanized antibody against the IL-6R was developed and approved by the FDA for use in patients with rheumatoid arthritis [105]. Not only has this agent showed good results with treatment of rheumatoid arthritis, but has also presented with promising results in colon cancer clinical trials [72, 106]. However, the cost of the drug and lack of easy administration makes this a somewhat prohibitive drug [107]. More importantly, the drug inhibits not only trans-signaling but also the classic IL-6 signaling [87]. Various approaches including fusion proteins, peptides and down-stream targets have been discussed in literature as promising candidates for blockade of IL-6 signaling [107]. These however would be more suited for therapeutic rather than preventive purposes. A naturally occurring soluble gp130 (sgp130) is a selective inhibitor of IL-6 trans-signaling that does not interfere with IL-6 bound to the membrane bound IL-6R [108]. The sgp130 which exists in the circulation at relatively high concentrations [109] has been used effectively to block IL-6 trans-signaling in animal models [9, 82, 89, 110]. sgp130 binds to the IL-6/sIL-6R complex in the circulation, and specifically inhibit IL-6 trans-signaling without affecting classic-signaling via the mIL-6R [109]. At present, there is a fusion protein (sgp130Fc) developed by fusing the extracellular portion of gp130 to the Fc region of human IgG1 [109]. This protein has more than 10-fold higher inhibitory potential compared to the monomeric natural occurring sgp130 and it has shown beneficial therapeutic effects in many mouse models of human diseases including antigen-induced arthritis, inflammatory bowel disease, and colitis associated colon cancer [109]. An optimized variant of the fusion protein sgp130Fc is also being considered for clinical evaluation [69]. The success of this molecule holds great promise for anti-IL-6 therapy in the treatment of colitis and colitis-associated cancer. However, whether the sgp130Fc molecule lends its self to long-term use as an anti-inflammatory to deter chronic inflammation associated with IBD and delay or hinder onset of colitis-associated cancer remains to be determined.
8. Chemoprevention of colitis-associated cancer by targeting sIL-6R
Given that colorectal cancer is the third most frequently diagnosed cancer in both men and women, and the second leading cause of cancer deaths in the United States, and that its incidence has increased in men and women that are under 50 years of age, we desperately need a strategy for preventing this disease. We also know that many individuals live with IBD, and are at a high risk for colorectal cancer. In addition, about one third of the people living with IBD in the United States are diagnosed during their childhood years (http://www.ccfa.org/advocacy/IBDResearchEnhancementAct). As mentioned above, trans-signaling through the soluble receptor (sIL-6R) acts to amplify the activity of IL-6 thus promoting transition from acute to chronic inflammation and eventual carcinogenesis [18]. Therefore, IL-6 trans-signaling is emerging as an attractive target that could serve to diminish the inflammatory signal in IBD, consequently hampering the progression from inflammation to cancer. It is also important to note that over activity of IL-6 signaling is a more regular phenomenon, and therefore cannot be addressed by a chemotherapeutic approach alone but highlights the importance of including a chemopreventive approach as well.
Chemoprevention seeks to cause delay in onset of cancer or progression from precancerous lesions as an alternative to treatment of cancer after clinical symptoms have appeared, with a goal to live without cancer for as long as possible [111]. This implies exposure to safe chemopreventive agents possibly over the lifetime of an individual predisposed to IBD. “Prevention of colorectal cancer by administration of chemopreventive agents is one of the most promising options for IBD patients who are at increased risks of the disease” [112, 113]. Chemopreventive agents can be taken as supplements or by modulation of diet [111]. In either case, identification of substances capable of affording protection or modulating the onset and severity of chronic inflammatory disorders is important. Given that IL-6 trans-signaling through the sIL-6R is key to the progression to tumorigenesis, identifying safe inhibitors that target the IL-6 soluble receptor would be beneficial in modulating inflammation and lead to better management of IBD. It is, however, difficult to ensure that a compound will not have any toxicity, especially when used for an extended period of time [111]. Hence, naturally occurring compounds appear to be excellent candidates for long-term use. Through epidemiological and experimental studies, various phytochemicals present in foods and plants have been reported to have anti-inflammatory activity. However, the challenge facing use of natural compounds is that mechanisms underlying the chemopreventive potential of these compounds are still elusive and many clinical trials have not provided the success seen in experimental models. In spite of this, chemoprevention remains an attractive concept in colorectal cancer prevention [114, 115]. It is also possible that the synergistic involvement of the various phytochemicals found in the natural products is responsible for the efficacy. In addition, genetic polymorphisms may modify the responses to specific bioactive phytochemicals. For instance, IL-6 genotypes may influence susceptibility to chronic disorders involving IL-6 activity [56–59, 116] and also influence individual response to chemopreventive regimens. Chemoprevention still faces many challenges, however these should not discourage research dedicated to this important area. Further studies are required to discover phytochemicals that target the sIL-6R either individually or synergistically with other compounds. Chemoprevention holds the promise of preventing, hampering, reversing or at least delaying the onset of cancer (Figure 4).
9. Principles of targeting sIL-6R with natural compounds
The sIL-6 receptor can be targeted at the point of production or by inhibiting signaling (Figure 3C). The sIL-6R is produced by two mechanisms, by alternative splicing of mRNA transcripts or by proteolytic cleavage (ectodomain shedding) of the membrane bound IL-6R [96]. Alternative splicing generates 10% of the sIL-6R and the remaining is generated by proteolytic cleavage [19]. Proteolytic cleavage leading to the release of the ectodomain of the IL-6R is catalyzed by zinc metalloproteases of the ADAM (A Disintegrin And Metalloproteinase) family, including TNF-α converting enzyme (TACE) also called ADAM17 and ADAM10 [19, 74, 96, 117–119]. ADAM17, the main enzyme responsible for sIL-6R shedding is elevated in several cancers [19]. ADAM17 is also responsible for proteolytic cleavage of the pro-inflammatory cytokine Tumor Necrosis Factor-α (TNF-α) leading to the release of the biologically active cytokine. In addition, ADAM17 is involved in shedding ligands of the EGFR that are important in mammalian development [120]. ADAM17 plays a critical role in development and regeneration of epithelial tissues and hypomorphic ADAM17 knockout mice show an increased susceptibility to inflammation in the DSS colitis model of inflammatory bowel disease [103, 121]. In colorectal cancer, ADAM17 increases resistance to chemotherapy, due to increased shedding of growth factors and consequently leading to activation of growth factor receptor-mediated pro-survival response [104, 122]. Since in addition to orchestrating inflammatory responses ADAM17 plays an important role in regulating cell growth [19, 103, 121, 123], global targeting of ADAM17 as a therapeutic or chemopreventive strategy is not a viable alternative. Exploiting the therapeutic potential of ADAM17 will depend on our understanding of how its activity is regulated and how specific organs and cells can be targeted to inactivate or activate the enzyme [124]. Therefore it is important to understand the mechanisms that lead to release of sIL-6R by ADAM17.
ADAM-mediated shedding is regulated by various regulatory pathways. Phorbol ester activates shedding by ADAM17 by affecting the activity of protein disulfide isomerase (PDI) [125]. PDI maintains ADAM17 in an inactive closed state until PMA stimulation generates reactive oxygen species (ROS) leading to an altered redox environment, which leads to inactivation of PDI. Inactivated PDI allows ADAM17 to adopt an open active conformation. This finding and further understanding of the mechanisms of ADAMs will be critical in helping us identify strategies that would regulate activity of ADAM17 and regulate its shedding of sIL-6R in cases of over activity of IL-6 signaling. Other stimuli of IL-6R cleavage include bacterial toxins, bacterial metalloproteinases and apoptosis [103].
Due to the important physiological activities of ADAM17 there are the potential pitfalls with suppressing the production of the sIL-6R by targeting ADAM17. However, this underscores the importance of using natural compounds, which usually have mild mechanisms of action especially considering prolonged use. Identification of natural compounds that would regulate over activity of ADAM17 would be important in dampening the inflammatory response and lead to better management of IBD. Candidate compounds may be the anti-inflammatory compounds that also possess the ability to inhibit metalloproteinases. But probably the most ideal approach would be to discover compounds that specifically inhibit sIL-6R shedding activity by ADAM17 without inhibiting the actual enzyme. Tissue inhibitors of metalloproteinases-3 (TIMP-3), a factor that inhibits most matrix metalloproteinases, is a native inhibitor of ADAM-17 [124, 126]. The development of ADAM17 (TACE) inhibitors has faced many challenges including musculoskeletal and hepatotoxicity side effects in clinical trials, however there are many promising TACE inhibitors in the preclinical studies [127, 128]. Whether these compounds would lend themselves to long-term use remains to be determined. Identification of natural compounds that modulate the activity of ADAM17 will be beneficial in advancing this field. TIMP3 is upregulated during the quiescent phase of CD [129], implying that natural compounds that increase expression of TIMP3 may improve the management of CD.
To date, however, the most promising compounds in targeting IL-6 trans-signaling will be those that specifically target the sIL-6R, for instance anti-inflammatory compounds that have the ability to increase the levels of soluble gp130 receptor. The IL-6/sIL-6R complex has equal affinity for membrane bound and soluble gp130 and IL-6 trans-signaling can be inhibited by a molar excess of sgp130 [89]. Administration of sgp130 to patients would serve to supplement the existing circulating levels of sgp130 and lead to specific blockade of IL-6 trans-signaling, leaving classic signaling intact [89]. Identification of natural compounds that can increase the circulating levels of sgp130 will greatly benefit the management of IBD over the long term.
Currently, only one study has shown a natural compound that holds promise in inhibiting IL-6 trans-signaling by enhancing production of soluble gp130 [130]. (−)-Epigallocatechin gallate (EGCG), an anti-inflammatory compound found in green tea, inhibits IL-1β–induced IL-6 production and trans-signaling in rheumatoid athritis synovial fibroblasts by inducing alternative splicing of gp130 mRNA, resulting in enhanced sgp130 production [130]. EGCG also inhibits IL-6/sIL-6R–induced matrix metalloproteinase-2 activity in RA synovial fibroblasts and in joint homogenates, possibly via up-regulation of sgp130 synthesis. In addition, there is a marked decrease in membrane-bound gp130 protein expression in the joint homogenates of the EGCG-treated group. In contrast, EGCG increases gp130/IL-6R mRNA ratio by 2-fold, possibly suggesting a possible mechanism of sgp130 activation by EGCG 130. These results open up hope for a new research focus that searches for natural compounds that target IL-6 trans-signaling. Based on the above results, a high possibility exists that already known natural anti-inflammatory compounds may also target the sIL-6R as one of their mechanisms. Therefore, effective screening of these compounds has important implications for the development of optimal strategies against chronic inflammation and cancer development triggered by inflammation.
10. Promising Natural anti- inflammatory compounds
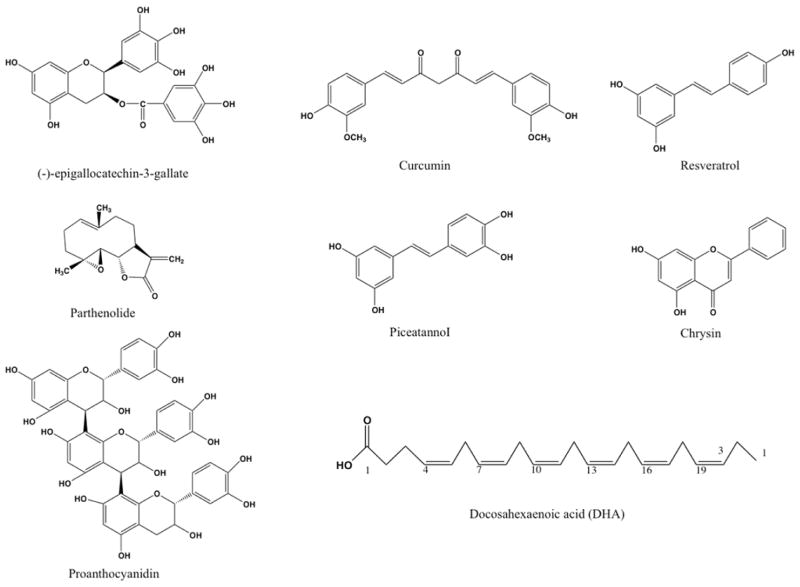
The search for natural anti-inflammatory compounds that hold promise for targeting IL-6 trans-signaling can start with screening those compounds with molecular targets downstream of IL-6. One such target could be STAT3. Due to the consititutive activation of STAT3 in most cancers including colon cancer, identification of pharmacologically safe agents that can block STAT3 activation may be important in suppressing of tumorigenesis [131]. Natural compounds that suppress STAT3 activation include epigallocatechin-3-gallate, curcumin, resveratrol, curcurbitacin, indirubin, piceatannol, parthenolide, flavopiridol, and magnolol [131]. However, studies have not been conclusive on how these agents suppress STAT3 activation, and additional studies are required to fully understand the mode of action of these compounds [131]. Here, we propose that these compounds could have an effect on IL-6 trans-signaling, a key pathway in activating STAT3 in cells that do not express mIL-6R. Some of the promising compounds are briefly described in the following sections and their structures shown in Figure 5.
Figure 5.
Chemical structures of some natural compounds that may have activity against IL-6 trans-signaling. Most of these compounds suppress activation of STAT3.
10.1 Epigallo-catechin-3-gallate (EGCG)
Green tea contains four main catechins including (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin-3-gallate, and the major component (−)-epigallocatechin-3-gallate (EGCG) accounting for about 59% [132]. EGCG possess antioxidant and anti-inflammatory properties. Its anti-inflammatory properties demonstrated in cell culture and animal models include, regulating the expression of cytokines, chemokines, MMPs, aggrecanase, reactive oxygen species (ROS), nitric oxide (NO), COX-2, and PGE2 [132]. However, studies on the effects of EGCG on colitis-associated tumorigenesis, have not been consistent, with some reporting EGCG as beneficial in colitis associated animal models and others finding no beneficial effects. In a TNBS (2,4,6-trinitrobenzene sulfonic acid) model of colitis EGCG improved acute experimental colitis by the suppression of mast cells and macrophage activities [133]. Other beneficial effects of EGCG in the TNBS mouse model of colitis, are attributed to a significant reduction of NF-κB and AP-1 activation [134]. EGCG also ameliorates rats colitis induced by acetic acid by inhibiting the production of TNF-α, IFN-gamma and nuclear factor-κB (NF-κB) p65 [135]. In an AOM/DDS experimental model, EGCG and polyphenol E suppressed the multiplicity and volume of colonic neoplasms thereby attenuating inflammation-related mouse colon carcinogenesis. This was also accompanied by reduced levels of inflammatory cytokines including IL-6 in the colonic mucosa [136]. However, in another AOM/DSS model, dietary EGCG (0.1% and 0.3%) did not inhibit colon carcinogenesis [137]. Instead, 0.3% of EGCG enhanced rectal bleeding and carcinogenesis while 0.5% EGCG caused rectal bleeding, enhanced inflammation, and loss of body weight. Furthermore, there are inconsistencies for EGCG’s effect on STAT3 activation [132]. In various cell lines, including mammary carcinoma cell lines, cervical carcinoma cell line and hepatocarcinoma cell line EGCG inhibited activation of STAT1 without affecting STAT3 [138]. EGCG inhibits STAT3 activation in human gingival fibroblasts [139], in keloid fibroblasts [140] and in gastric cancer [141]. These inconsistencies probably imply that the effects of EGCG may depend on cell type and the concentration of EGCG. Hence, more research is needed to establish the effects of EGCG on STAT3 activation, whose overactivity in pathological conditions is mediated by IL-6 trans-signaling through the sIL-6R. In rheumatoid athritis synovial fibroblasts and in a rat adjuvant-induced arthritis model EGCG suppresses IL-6 trans-signaling by enhancing soluble gp130 production [130]. More studies are needed to clarify the role of EGCG in suppression of STAT3 activity mediated by IL-6 trans-signaling.
10.2 Curcumin
Curcumin, the principal component of turmeric has anti-inflammatory properties and beneficial effects in IBD [142]. In two clinical trials, curcumin improved patient symptoms when used in conjunction with conventional medications for UC [142]. Curcumin inhibits the activation of various transcription factors that play a key role in inflammation, including NF-κB, activated protein-1 (AP-1) and signal transducer and activator of transcription (STAT) proteins. [143, 144]. In cancer cells, curcumin downregulates the expression of key pro-inflammatory proteins and cytokines including, cyclooxygenase-2 (COX-2), interleukin-1β (IL-1β), IL-6 and TNF-α. [142]. In a recent case study, a patient with ulcerative colitis (UC) refractory to mesalamine and multiple courses of steroids achieved clinical and endoscopic remission with oral curcumin [145]. The colonoscopy results of the patient at the end of the study showed ulceration and biopsies consistent with chronic inactive UC, showing that curcumin indeed holds promise for management of IBD [145]. However, the mechanism of action for curcumin in treating colitis is not fully understood [145] and unraveling these mechanisms is important in developing optimal strategies using chemopreventive phytochemicals. Here we propose that curcumin might play a role in suppressing IL-6 trans-signaling in IBD. Since there are no studies that have addressed this yet, we discuss some preliminary evidence from other studies that might implicate a role for curcumin in IL-6 trans-signaling- mediated inflammatory disorders including IBD that might include cross-talk between various pathways.
IL-6 trans-signaling via the sIL-6R is the primary mode of signaling that elicits the potent pro-inflammatory actions of IL-6 during LPS/TLR4-driven endotoxic shock [146]. IL-6 trans-signaling via STAT3, activates downstream of TLR4 in response to LPS, feeds back into the Mal/NF-κB pathway to specifically modulate TLR4/LPS-driven IL-6 production and therefore the inflammatory response. IL-6 trans-signaling via STAT3 is a critical modulator of LPS-driven pro-inflammatory responses through cross-talk regulation of the TLR4/Mal signaling pathway, and implicates cross-talk between JAK/STAT and TLR pathways as a mechanism that regulates the severity of the host inflammatory response [146]. Whereas the anti-inflammatory compound, curcumin, has the ability to inhibit activation of pathogen recognition receptors (PRRs), including TLR4, and subsequent PRR-mediated inflammation [147]. Curcumin and other compounds e.g helenalin, and cinnamaldehyde with a, β-unsaturated carbonyl groups and sulforaphane with an isothiocyanate group inhibit TLR4 activation by interfering with cysteine residue-mediated receptor dimerization [147]. Combining the model whereby IL-6 trans-signaling via STAT3 modulates LPS/TLR4 driven pro-inflammatory response [146] with the finding that curcumin inhibits TLR4 activation shows a possible mechanism that could result in decreased inflammation in IBD by curcumin. Indeed curcumin suppresses inflammation in a TNBS experimental colitis model through inhibition of TLR-4 receptor [148]. In addition, curcumin inhibits activation of STAT3 [149, 150]. In the axis that IL-6 trans-signaling leading to activation of STAT3, is the key pathway implicated in the maintenance of chronic inflammatory conditions, we propose that curcumin might inhibit STAT3 activation by inhibiting IL-6 trans-signaling. In addition, we believe that cross-talk between IL-6/sIL-6R mediated JAK/STAT3 pathway and other pathways for example those involving TLR4 might play a role in curcumin’s ability to repress inflammation in colitis models. Studies in this area have potential to improve our knowledge of how a promising and widely research anti-inflammatory compound curcumin might be used in management of IBD and prevention of colitis associated cancer.
10.3 Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene), found in grapes and grapes products, berries and other fruits, vegetables, legumes, and weeds has shown anti-inflammatory properties [151–154] in mice and rat colitis models. In a TNBS rat model, resveratrol reduces COX-2 and the NF-κB p65 protein expression, attenuates the damage score and corrects the disturbances in morphology associated with injury and stimulates apoptosis in colonic cells [155]. In a rat model of DSS-induced colitis, resveratrol protects the colonic mucosa architecture, reduces body weight loss, diminishes the induced anemia and reduces systemic inflammation markers, colonic mucosa prostaglandin E2, cycloxygenase-2, prostaglandin E synthase and nitric oxide levels [156]. Resveratrol also suppresses DSS-induced colitis in mice by inducing silent mating type information regulation-1 and down-regulating NF-κB [157]. In addition, resveratrol reduces tumor incidence and tumor multiplicity in an AOM/DSS mouse model [158, 159]. Resveratrol also reduces prostaglandin E synthase-1 (PGES-1), cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) proteins expression, via downregulation of p38, a mitogen-activated protein kinases (MAPK) signal pathway in a mouse DSS induced colitis model [160]. Resveratrol’s effects on colitis include anti-oxidant effects [161]. The beneficial effects of resveratrol in colitis and colitis-associated tumorigenesis are mediated through several mechanisms. These mechanisms might include cross talk between different pathways including anti-oxidant pathways, MAPK pathways and IL-6/sIL-6R mediated JAK/STAT3 pathways.
Resveratrol suppresses activation of STAT3 in various models, including a mouse model of DSS-induced colitis [162]. In multiple myeloma cells, resveratrol inhibits both the constitutive and the IL-6-induced activation of STAT3 [163]. In addition, resveratrol suppresses STAT3 activation in human leukemia cells [153]. Activation of STAT3 plays an important role in regulating the self-renewal of glioblastoma multiforme (GBM) tumor initiating cells (TIC) and in enhancing their resistance to radiation therapies [164]. On the other hand, treatment with resveratrol suppresses activation of STAT3, inhibits anti-apoptotic activity, suppresses self-renewal and enhances the sensitivity of GBM-TIC to radiotherapies. IL-6 induced STAT3 activity is gaining attention in the role it plays in tumor-initiating cells, cells that don’t express the membrane bound IL-6 receptor and would therefore depend on IL-6 trans-signaling through the sIL-6R. For these reasons, resveratrol could be a candidate compound worthy of further investigation, especially related IL-6 trans-signaling. This is especially true because IL-6 trans-signaling in colorectal cancer is partly dependent on TGF-β signaling, which is commonly altered in colorectal cancers [6, 165]. Indeed, resveratrol treatment of SW480 colon cancer cells decreases the levels of several oncogenic microRNAs targeting genes that are effectors of the TGF-β signaling pathway, while increasing the levels of miR-663, a tumor-suppressor microRNA that targets TGFβ1 transcripts [165]. Also, while up-regulating several components of the TGF-β signaling pathway such as TGF-β receptors type I (TGFβR1) and type II (TGFβR2), resveratrol decreases the transcriptional activity of SMADs, the main effectors of the canonical TGFβ pathway [165]. The ability of resveratrol to regulate the behavior of the TGF-β signaling pathway opens the possibility of using resveratrol in colon cancers where this pathway is impaired or works to favor metastasis. This implies that cross talk between the IL-6/sIL-6R dependent JAK/STAT3 pathway and TGF-β/SMAD signaling pathway may play a role in resveratrol’s ability to control inflammation in IBD. Resveratrol is recognized as one of the more promising natural molecules in the prevention and treatment of chronic inflammatory disease and autoimmune disorders [157, 159]. However more research is needed to understand the mechanism of resveratrol-modulated suppression of STAT3 activation.
10.4 Piceatannol
The plant polyphenol piceatannol (3,4,3′, 5′-tetrahydroxy-trans-stilbene) found in grapes, Rheum undulatum, rhubarb, and sugar cane is structurally related to resveratrol (3,5,4′-trihydroxy-trans-stilbene) and it also possesses antioxidant, anti-inflammatory, and chemopreventive properties [162, 166]. Piceatannol is a protein kinase inhibitor that modifies multiple cellular targets, exerting immunosuppressive and antitumorigenic activities in several cell lines [166]. It inhibits TNF-induced NF-κB activation in myeloid cells, lymphocyte and epithelial cells [167], and suppresses the expression of COX-2 in human breast epithelial cells, [168] and in human mammary epithelial cells [169]. Piceatannol also suppresses LPS-induced inflammation in murine and in vitro models [170–172]. In addition, piceatannol selectively inhibits the tyrosine phosphorylation in STAT3 in human T and B cells 173. In a DSS-induced colitis model, piceatannol treatment suppresses the expression of phophorylated colonic STAT-3, thereby protecting the mucosa [166]. A significant reduction in colonic myeloperoxidase (MPO) activity, a decrease in production of inflammatory mediators such as nitric oxide (NO), prostaglandin (PG) E2, and pro-inflammatory cytokines are also associated with the beneficial effects of piceatannoI on DSS-induced colitis [166]. In addition to suppression of iNOS expression, piceatannoI suppresses DSS-induced inflammatory injury by suppressing activation of NF-κB, ERK and STAT3 [162]. STAT3 is the most strongly tyrosine phosphorylated STAT member in dextran sulfate sodium (DSS)-induced colitis in mice as well as in human ulcerative colitis and Crohn’s disease [174]. STAT3, whose phosphorylation correlates well to the severity of colitis, is mainly activated by IL-6–related cytokines [174]. Given these results, further evaluation the potential for piceatannol as an agent for the prevention and/or treatment of inflammatory bowel diseases is needed and understanding the mechanisms behind its ability to modulate STAT3 activity will be very beneficial. Again, we hypothesize that inhibition of IL-6 trans-signaling may play a role in the ability of piceatannol to attenuate inflammation in IBD.
10.5 Parthenolide
Parthenolide is a naturally occurring sesqueterpene lactone of the plant feverfew (Tanacetum parthenium), a member of the sunflower family [175, 176]. Feverfew, also called Bachelor’s Buttons, is a perennial European herb used for centuries in folk remedies for migraine headaches, arthritis, and fever [177], and its leaves are rich in parthenolide. Its anti-inflammatory activities are partly explained by ability to inhibit NF-κB activation and IL-1/TNF-α-induced signaling, blocking expression of pro-inflammatory cytokines including IL-6 and inhibiting IL-6-type cytokine signaling [176]. Parthenolide has been shown to block STAT3 phosphorylation on Tyr705 thereby preventing STAT3 dimerization necessary for its nuclear translocation [176]. In addition, parthenolide has shown promise in treatment of various cancers via inhibition STAT3 [175]. Moreover, parthenolide inhibits IL-6/sIL-6R induced phosphorylation of ERK1/2 and STAT3 in fibroblast-like synoviocytes from RA patients (RA-FLS), [178] and articular chondrocytes [179]. In these models, parthenolide inhibits IL-6 trans-signaling, through yet unknown mechanisms that might include cross talk between ERK1/2 and STAT3 pathways. In cardiotrophin-1-treated rat cardiomyocytes, ERK1/2 inhibits the phosphorylation of STAT3 [180], implying a crosstalk between these mechanisms. There are some inconsistencies regarding the effect of parthenolide on colitis. In a zebrafish larvae model of TNBS-induced colitis, parthenolide downregulates TNF-a expression without rescuing the disease changes observed in vivo or on histological analysis [181]. Together, these data suggest that more studies are needed to evaluate the role of parthenolide in IL-6/sIL-6 induced STAT3 activation in colitis models and the mechanisms involved in inhibition of IL-6 trans-signaling.
10.6 Chrysin
Chrysin (5,7-dihydroxyflavone) is a natural flavonoid found in many plant extracts, honey, and propolis [182]. Its anti-inflammatory properties are attributed in part to inhibition of NF-κB activation and suppression of pro-inflammatory cytokines and COX-2 expression [182–185]. In vivo, chrysin alleviates the symptoms of DSS-induced colitis, improves colitis DAI scores and reduces the production of various inflammatory mediators, such as NO, PGE2, inflammatory cytokines, and chemokines [182]. Not much more research has been done to determine the effect of chrysin on inflammatory diseases including IBD. However studies in other models imply a possible role for chrysin in regulating the IL-6 trans-signaling pathway. Chrysin suppresses IL-6-induced angiogenesis through modulation of the sIL-6R/gp130/JAK1/STAT3/VEGF signaling pathway [186]. In this model, chrysin suppresses IL-6-induced angiogenesis in vitro (HUVECs) and in vivo, down-regulates gp130 expression, sIL-6R, and phosphorylated JAK1 and STAT3 [186]. Human umbilical vein endothelial cells (HUVECs) do not express the mIL-6R and would therefore respond to IL-6 via IL-6 trans-signaling through the sIL-6R. These results suggest that chrysin may hold promise in alleviating IL-6/IL-6R dependent pathological conditions including IBD. More research needs to be done to determine its mechanism of inhibiting of STAT3 activation induced by IL-6 trans-signaling.
10.7 Proanthocyanidins
Proanthocyanidins from grape seed extracts (GSPE) are phenolic compounds that have strong anti-inflammatory properties [187, 188]. Grape seed proanthocyanidin extract (GSPE) consist of a combination of ingredients with 15% (+)-catechin, (−)-epicatechin; 80% (−)-epicatechin 3-O-gallate, dimmers, trimers, tetramers and their gallates; 5% pentamers, hexamers, heptamers and their gallates [189]. Proanthocyanidins from grape seeds are believed to affect the colonic mucosa directly since they are not absorbed in the stomach or small intestine but reach the colon where possibly beneficial metabolites are formed by the colonic microflora [187, 190]. GSPE exert protective effects in the recurrent phase of TNBS twice-induced colitis of rats [187]. The ability to suppress mucosal inflammation in the colon is attributed to the inhibition of NF-κB signal transduction pathways as evidenced by reduced the levels of pIKKα/β, pIκBα and NF-κB in GSPE treated rats [187]. In addition, GSPE treatment suppresses production of oxygen free radicals and infiltration of inflammatory cells. Moreover, GSPE exerts a beneficial anti-inflammatory effect in the acute phase of TNBS-induced colitis in rats by inhibiting inflammatory cell infiltration and oxidation damage, promoting damaged tissue repair to improve colonic oxidative stress, decreasing production of proinflammatory cytokines IL-1β, and increasing production of anti-inflammatory cytokines IL-2 and IL-4 [191]. GSPE treatment also facilitates recovery of pathologic changes in the colon after induction of recurrent colitis and reduces malonyldialdehyde and nitric oxide levels in serum and colon tissues of colitis rats [192]. GSPE exerts a protective effect on recurrent colitis in rats by modifying the inflammatory response, inhibiting inflammatory cell infiltration and oxidation damage, promoting damaged tissue repair to improve colonic oxidative stress, and inhibiting colonic iNOS activity to reduce the production of nitric oxide. [192]. GSPE seem to hold some promise in treating human ulcerative colitis [187] even though the mechanism by which GSPE regulates the immune response is still unclear. Most work on the ant-inflammatory effects of GSPE has not looked at the role of STAT3 activation. In an IL-17-dependent arthritis model, GSPE suppresses activation of STAT3 in Th17 cells thereby regulating the differentiation of human Th17 cells [189]. Studies evaluating the effect of GSPE on IL-6 trans-signaling and STAT3 will therefore be beneficial.
10.8 Omega-3 fatty acids
Omega-3 (n-3) fatty acids have anti-inflammatory and immune-modulating effects that have been demonstrated in various disorders including inflammatory bowel disorders [193]. These essential fatty acids, which are abundant in fish oil, are also present in various plant sources including flaxseed. Docosahexaenoic acid (DHA), an n-3 PUFA, alleviates DSS-induced colitis in mice, partly by suppressing the expression of inflammatory cytokines (IL-1β), CD14 antigen and TNF receptor superfamily member 1b, membrane remodeling genes (matrix metalloproteinase-3, -10 and -13) and acute phase proteins (S100 calcium-binding protein A8) [194]. In TNBS-induced colitis rat model, an n-3 fatty-acid-enriched diet improves the expression of inflammatory mediators in the colon and colitis compared with the control group [195]. The effect of the n-3 fatty acids is attributed to suppression of the production of pro-inflammatory cytokines in the colon. A diet rich in α-linolenic acid, an n-3 fatty acid, reduces oxidative stress and inflammation in a TNBS-induced colitis rat model by suppressing NF-κB activation [196]. The anti-inflammatory mechanisms are also attributed to inhibition of nitric oxide [197]. Even though the effect of n-3 fatty acids on STAT3 activation has not been evaluated in IBD, the possibility exists that the effects are modulated at least in part by preventing STAT3 activation [198]. In fact n-3 fatty acids have been shown to inhibit STAT3 phosphorylation thereby suppressing proliferation of chemoresistant pancreatic cancer cells [199]. Hence the possibility exists that n-3 fatty acids may also target IL-6 trans-signaling. In animal studies, n-3 fatty acids have shown great promise in alleviating symptoms of IBD, however, inconsistencies have also been reported. For instance, meta-analyses of randomized controlled trials of n-3 revealed a lack of clinical benefit of n-3 in maintaining remission in IBD [200]. These results imply that additional studies are needed to better understand the mechanisms of n-3 fatty acids in alleviating inflammatory conditions. It is also possible that n-3 compounds could have a better effect when used in combination with other natural compounds. In a DSS/AOM mouse model dietary fish oil and curcumin combined to modulate colonic cytokinetics, and gene expression thereby regulating mucosal homeostasis and the resolution of chronic inflammation in the colon [201]. Further understanding of the mechanisms of n-3 fatty acids whether individually or in combination, for example a possible effect on IL-6 trans-signaling, will be important in determining how these compounds may be beneficial in alleviating symptoms of patients leading to better management of IBD.
11. Conclusion and Future Directions
The risk of developing colorectal cancer increases in patients with inflammatory bowel disease (IBD), a collective term for Crohn’s disease and ulcerative colitis. Various factors including pro-inflammatory cytokines have been demonstrated to play a crucial role in the pathogenesis of IBD. Among the cytokines, there is strong evidence for the role played by IL-6, whose effects are mediated through activation of STAT3. Unlike other pro-inflammatory cytokines, the IL-6 soluble receptor (sIL-6R) promotes IL-6 signaling instead of quenching it. This trans-signaling acts to amplify the inflammatory response consequently allowing a significant role for IL-6 in the maintenance of chronic inflammation and pathogenesis of colitis-associated cancer. The protective role of IL-6 is through classic IL-6 signaling via the membrane-bound IL-6R. Recent evidence points to IL-6 trans-signaling via the sIL-6R, as the main driving force of chronic inflammation and associated pathophysiological inflammatory conditions. This opens up a new possibility of alleviating excessive inflammation in IBD by specifically targeting IL-6 trans-signaling while leaving the beneficial classic signaling intact. With the knowledge that there is a high number of people living with IBD and that about one third are diagnosed during their childhood years we need a chemopreventive approach possibly over the life time of predisposed individuals. This requires administration of safe agents for the purpose of delaying the onset or progression from precancerous lesions. Chemopreventive compounds that target IL-6 trans-signaling have the ability to reduce excessive IL-6 signaling in IBD and hamper progression to colitis associated colon cancer.
Currently, a fusion protein sgp130Fc, based on soluble gp130, a naturally occurring selective inhibitor of IL-6 trans-signaling is being considered for clinical evaluation. This holds great promise for therapeutic purposes but it is not clear whether it can be used as a preventive agent. Long-term effects of using such a molecule have not been pursued. Given the need to focus on managing IBD over an extended time course, identification of natural compounds that safely target IL-6 trans-signaling could offer great promise. In this review, we have presented some such compounds that affect IL-6 activity. Although these compounds are known to be anti-inflammatory, their mechanism of action remains unidentified. We propose that including the IL-6 trans-signaling axis in the on-going research with anti-inflammatory natural compounds has the potential to unravel mechanisms that may lead to the closer understanding of how they can be optimally used for the management of IBD and prevention of colitis associated cancer. Compounds that suppress STAT3 activation may potentially affect IL-6 trans-signaling, which allows cells that don’t express the mIL-6R to respond to IL-6 through sIL-6R. We therefore suggest an increased research focus on screening promising compounds for their ability to target IL-6 trans-signaling.
Acknowledgments
The author’s work is supported by a National Cancer Institute grant CA109269 (CM, SA) and CA135559 (SA).
ABBREVIATIONS
- IL-6
Interleukin 6
- IBD
Inflammatory bowel disease
- STAT3
Signal transducer and activator of transcription 3
- CD
Crohn’s disease
- UC
Ulcerative colitis
- NOD2(CARD15)
Nucleotide-binding oligomerisation domain 2 (Caspase recruitment domain 15)
- DLG5
Discs large homolog 5
- OCTN1
Organic cation transporter 1
- TLR4
Toll-like receptor 4
- CARD4(NOD1)
Caspase recruitment domain 4 (Nucleotide-binding oligomerisation domain 1)
- IL23R
Interleukin 23 Receptor
- IRGM
Immunity-related GTPase family M
- PTGER4
Prostaglandin receptor EP4
- ATG16L1
Autophagy related protein 16-like 1
- HLA
Human leukocyte antigen
- IBD5
Inflammatory bowel disease 5
- CD4+
Cluster of differentiation 4
- TH1
T helper type 1
- TH2
T helper type 2
- TH-17
T helper type 17
- LPS
Lipopolysaccharide
- cAMP
Cyclic adenosine monophosphate
- IL-1
Interleukin 1
- PDGF
Platelet-derived growth factor
- TNFα
Tumor necrosis factor α
- NSAIDs
Nonsteroidal anti-inflammatory drugs
- sIL-6R
Soluble IL-6 receptor
- TGFβ
Transforming growth factor β
- IECs
Intestinal epithelial cells
- STAT1
Signal transducer and activator of transcription 1
- CSCs
Cancer stem cells
- MSCs
Mesencymal stem cells
- DSS
Dextran sulphate sodium
- mIL-6R
Membrane bound IL-6R
- JAKs
Janus kinases
- IL-11
Interleukin 11
- LIF
leukemia inhibitory factor
- CNTF
Ciliary neurotrophic factor
- OSM
Oncostatin M
- sCRs
Cytokine receptors
- IL-11R
IL-11 receptor
- mIL-11R
membrane bound IL-11 receptor
- AOM/DSS
Azoxymethane/dextran sodium sulphate
- CApC
Colitis-associated premalignant cancer
- CC
Chronic colitis
- sgp130
Soluble gp130
- TNBS
2,4,6-trinitrobenzenesulfonic acid
- sgp130Fc
Soluble gp130 fusion protein
- ADAM
A Disintegrin And Metalloproteinase
- TACE
TNF-α converting enzyme
- ADAM 17
A Disintegrin And Metalloproteinase 17
- ADAM 10
A Disintegrin And Metalloproteinase 10
- EGFR
Epidermal growth factor receptor
- PDI
Protein disulfide isomerase
- PMA
Phorbol myristic acid
- ROS
Reactive oxygen species
- TIMP-3
Tissue inhibitors of metalloproteinases-3
- EGCG
(−)-Epigallocatechin gallate
- NO
Nitric oxide
- COX-2
Cyclooxygenase 2
- PGE2
Prostaglandin E2
- NF-κB
Nuclear factor-κB
- AP-1
Activator protein 1
- IFN
Interferons
- Mai/NF-kB
MyD88-adapter-like/ NF-kB
- PRRs
Pathogen recognition receptors
- PGES-1
Prostaglandin E synthase-1
- iNOS
Inducible nitric oxide synthase
- MAPK
Mitogen-activated protein kinases
- GBM
Glioblastoma multiforme
- TIC
Tumor initiating cells
- MPO
Myeloperoxidase
- ERK
Extracellular-signal-regulated kinases
- RA-FLS
Rheumatoid arthritis fibroblast-like synoviocytes
- HUVECs
Human umbilical vein endothelial cells
- GSPE
Grape seed proanthocyanidin extract
- n-3
Omega-3
- DHA
Docosahexaenoic acid
- PUFA
Polyunsaturated fatty acids
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 3.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15(2):79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4(4):221–33. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975–2006; featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker C, Fantini MC, Wirtz S, Nikolaev A, Lehr HA, Galle PR, Rose-John S, Neurath MF. IL-6 signaling promotes tumor growth in colorectal cancer. Cell Cycle. 2005;4(2):217–20. [PubMed] [Google Scholar]
- 7.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, Matthews V, Schmid RM, Kirchner T, Arkan MC, Ernst M, Greten FR. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15(2):91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15(2):103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto S, Hara T, Mitsuyama K, Yamamoto M, Tsuruta O, Sata M, Scheller J, Rose-John S, Kado S, Takada T. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol. 2010;184(3):1543–51. doi: 10.4049/jimmunol.0801217. [DOI] [PubMed] [Google Scholar]
- 10.Herrinton LJ, Liu L, Lewis JD, Griffin PM, Allison J. Incidence and prevalence of inflammatory bowel disease in a Northern California managed care organization, 1996–2002. Am J Gastroenterol. 2008;103(8):1998–2006. doi: 10.1111/j.1572-0241.2008.01960.x. [DOI] [PubMed] [Google Scholar]
- 11.Foley KF, Kao P. Biomarkers for inflammatory bowel disease. Clin Lab Sci. 2007;20(2):84–8. [PubMed] [Google Scholar]
- 12.Eaden J. Review article: the data supporting a role for aminosalicylates in the chemoprevention of colorectal cancer in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 2):15–21. doi: 10.1046/j.1365-2036.18.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 13.Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell. 2008;13(1):7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 14.Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103(5):1444–51. doi: 10.1016/0016-5085(92)91163-x. [DOI] [PubMed] [Google Scholar]
- 15.Brentnall TA, Rubin CE, Crispin DA, Stevens A, Batchelor RH, Haggitt RC, Bronner MP, Evans JP, McCahill LE, Bilir N, et al. A germline substitution in the human MSH2 gene is associated with high-grade dysplasia and cancer in ulcerative colitis. Gastroenterology. 1995;109(1):151–5. doi: 10.1016/0016-5085(95)90280-5. [DOI] [PubMed] [Google Scholar]
- 16.Freeman HJ. Colorectal cancer risk in Crohn’s disease. World J Gastroenterol. 2008;14(12):1810–1. doi: 10.3748/wjg.14.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(Suppl 2):1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 18.Rose-John S, Mitsuyama K, Matsumoto S, Thaiss WM, Scheller J. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr Pharm Des. 2009;15(18):2095–103. doi: 10.2174/138161209788489140. [DOI] [PubMed] [Google Scholar]
- 19.Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble Interleukin 6 receptor: generation and role in inflammation and cancer. Eur J Cell Biol. 2011;90(6–7):484–94. doi: 10.1016/j.ejcb.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Bosani M, Ardizzone S, Porro GB. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics. 2009;3:77–97. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Mathew CG, Lewis CM. Genetics of inflammatory bowel disease: progress and prospects. Hum Mol Genet. 2004;13(Spec No 1):R161–8. doi: 10.1093/hmg/ddh079. [DOI] [PubMed] [Google Scholar]
- 22.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nunez G, Cho JH. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411(6837):603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 23.Hugot JP, Zouali H, Lesage S. Lessons to be learned from the NOD2 gene in Crohn’s disease. Eur J Gastroenterol Hepatol. 2003;15(6):593–7. doi: 10.1097/00042737-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Henckaerts L, Figueroa C, Vermeire S, Sans M. The role of genetics in inflammatory bowel disease. Curr Drug Targets. 2008;9(5):361–8. doi: 10.2174/138945008784221161. [DOI] [PubMed] [Google Scholar]
- 25.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314(5804):1461–3. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40(8):955–62. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher SA, Tremelling M, Anderson CA, Gwilliam R, Bumpstead S, Prescott NJ, Nimmo ER, Massey D, Berzuini C, Johnson C, Barrett JC, Cummings FR, Drummond H, Lees CW, Onnie CM, Hanson CE, Blaszczyk K, Inouye M, Ewels P, Ravindrarajah R, Keniry A, Hunt S, Carter M, Watkins N, Ouwehand W, Lewis CM, Cardon L, Lobo A, Forbes A, Sanderson J, Jewell DP, Mansfield JC, Deloukas P, Mathew CG, Parkes M, Satsangi J. Genetic determinants of ulcerative colitis include the ECM1 locus and five loci implicated in Crohn’s disease. Nat Genet. 2008;40(6):710–2. doi: 10.1038/ng.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(7):1090–100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 29.Vermeire S, Rutgeerts P. Current status of genetics research in inflammatory bowel disease. Genes Immun. 2005;6(8):637–45. doi: 10.1038/sj.gene.6364257. [DOI] [PubMed] [Google Scholar]
- 30.Cucchiara S, Latiano A, Palmieri O, Staiano AM, D’Inca R, Guariso G, Vieni G, Rutigliano V, Borrelli O, Valvano MR, Annese V. Role of CARD15, DLG5 and OCTN genes polymorphisms in children with inflammatory bowel diseases. World J Gastroenterol. 2007;13(8):1221–9. doi: 10.3748/wjg.v13.i8.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369(9573):1627–40. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Sundquist J, Sundquist K. Educational level and occupation as risk factors for inflammatory bowel diseases: A nationwide study based on hospitalizations in Sweden. Inflamm Bowel Dis. 2009;15(4):608–15. doi: 10.1002/ibd.20815. [DOI] [PubMed] [Google Scholar]
- 33.Shanahan F, Bernstein CN. The evolving epidemiology of inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25(4):301–5. doi: 10.1097/MOG.0b013e32832b12ef. [DOI] [PubMed] [Google Scholar]
- 34.Satsangi J, Jewell DP, Bell JI. The genetics of inflammatory bowel disease. Gut. 1997;40(5):572–4. doi: 10.1136/gut.40.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plevy SE, Targan SR. Future therapeutic approaches for inflammatory bowel diseases. Gastroenterology. 2011;140(6):1838–46. doi: 10.1053/j.gastro.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mudter J, Neurath MF. Apoptosis of T cells and the control of inflammatory bowel disease: therapeutic implications. Gut. 2007;56(2):293–303. doi: 10.1136/gut.2005.090464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sturm A, de Souza HS, Fiocchi C. Mucosal T cell proliferation and apoptosis in inflammatory bowel disease. Curr Drug Targets. 2008;9(5):381–7. doi: 10.2174/138945008784221198. [DOI] [PubMed] [Google Scholar]
- 38.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8(4):345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 39.Liu ZJ, Yadav PK, Su JL, Wang JS, Fei K. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2009;15(46):5784–8. doi: 10.3748/wjg.15.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monteleone G, Caprioli F. T-cell-directed therapies in inflammatory bowel diseases. Clin Sci (Lond) 2010;118(12):707–15. doi: 10.1042/CS20100027. [DOI] [PubMed] [Google Scholar]
- 41.Gustot T, Lemmers A, Louis E, Nicaise C, Quertinmont E, Belaiche J, Roland S, Van Gossum A, Deviere J, Franchimont D. Profile of soluble cytokine receptors in Crohn’s disease. Gut. 2005;54(4):488–95. doi: 10.1136/gut.2004.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito H. Anti-interleukin-6 therapy for Crohn’s disease. Curr Pharm Des. 2003;9(4):295–305. doi: 10.2174/1381612033391900. [DOI] [PubMed] [Google Scholar]
- 43.Kopf M, Bachmann MF, Marsland BJ. Averting inflammation by targeting the cytokine environment. Nat Rev Drug Discov. 2010;9(9):703–18. doi: 10.1038/nrd2805. [DOI] [PubMed] [Google Scholar]
- 44.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4(Suppl 3):S233–42. doi: 10.1186/ar565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choy E. Inhibiting interleukin-6 in rheumatoid arthritis. Curr Rheumatol Rep. 2008;10(5):413–7. doi: 10.1007/s11926-008-0066-x. [DOI] [PubMed] [Google Scholar]
- 47.Kishimoto T. The biology of interleukin-6. Blood. 1989;74(1):1–10. [PubMed] [Google Scholar]
- 48.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 49.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 50.Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9(13):4653–65. [PMC free article] [PubMed] [Google Scholar]
- 51.Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 52.Biffl WL, Moore EE, Moore FA, Peterson VM. Interleukin-6 in the injured patient. Marker of injury or mediator of inflammation? Ann Surg. 1996;224(5):647–64. doi: 10.1097/00000658-199611000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parekh RB, Dwek RA, Rademacher TW, Opdenakker G, Van Damme J. Glycosylation of interleukin-6 purified from normal human blood mononuclear cells. Eur J Biochem. 1992;203(1–2):135–41. doi: 10.1111/j.1432-1033.1992.tb19838.x. [DOI] [PubMed] [Google Scholar]
- 54.Santhanam U, Ghrayeb J, Sehgal PB, May LT. Post-translational modifications of human interleukin-6. Arch Biochem Biophys. 1989;274(1):161–70. doi: 10.1016/0003-9861(89)90427-x. [DOI] [PubMed] [Google Scholar]
- 55.Hershko DD, Robb BW, Luo G, Hasselgren PO. Multiple transcription factors regulating the IL-6 gene are activated by cAMP in cultured Caco-2 cells. Am J Physiol Regul Integr Comp Physiol. 2002;283(5):R1140–8. doi: 10.1152/ajpregu.00161.2002. [DOI] [PubMed] [Google Scholar]
- 56.Ferrari SL, Ahn-Luong L, Garnero P, Humphries SE, Greenspan SL. Two promoter polymorphisms regulating interleukin-6 gene expression are associated with circulating levels of C-reactive protein and markers of bone resorption in postmenopausal women. J Clin Endocrinol Metab. 2003;88(1):255–9. doi: 10.1210/jc.2002-020092. [DOI] [PubMed] [Google Scholar]
- 57.Litovkin KV, Domenyuk VP, Bubnov VV, Zaporozhan VN. Interleukin-6 -174G/C polymorphism in breast cancer and uterine leiomyoma patients: a population-based case control study. Exp Oncol. 2007;29(4):295–8. [PubMed] [Google Scholar]
- 58.DeMichele A, Martin AM, Mick R, Gor P, Wray L, Klein-Cabral M, Athanasiadis G, Colligan T, Stadtmauer E, Weber B. Interleukin-6 -174G-->C polymorphism is associated with improved outcome in high-risk breast cancer. Cancer Res. 2003;63(22):8051–6. [PubMed] [Google Scholar]
- 59.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275(24):18138–44. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 60.Yeh KY, Li YY, Hsieh LL, Chen JR, Tang RP. The -174 G/C polymorphism in interleukin-6 (IL-6) promoter region is associated with serum IL-6 and carcinoembryonic antigen levels in patients with colorectal cancers in Taiwan. J Clin Immunol. 2010;30(1):53–9. doi: 10.1007/s10875-009-9324-6. [DOI] [PubMed] [Google Scholar]
- 61.Slattery ML, Wolff RK, Herrick JS, Caan BJ, Potter JD. IL6 genotypes and colon and rectal cancer. Cancer Causes Control. 2007;18(10):1095–105. doi: 10.1007/s10552-007-9049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, Faggioni R, Luini W, van Hinsbergh V, Sozzani S, Bussolino F, Poli V, Ciliberto G, Mantovani A. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6(3):315–25. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- 63.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14(3):109–19. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Reinisch W, Gasche C, Tillinger W, Wyatt J, Lichtenberger C, Willheim M, Dejaco C, Waldhor T, Bakos S, Vogelsang H, Gangl A, Lochs H. Clinical relevance of serum interleukin-6 in Crohn’s disease: single point measurements, therapy monitoring, and prediction of clinical relapse. Am J Gastroenterol. 1999;94(8):2156–64. doi: 10.1111/j.1572-0241.1999.01288.x. [DOI] [PubMed] [Google Scholar]
- 65.Gross V, Andus T, Caesar I, Roth M, Scholmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn’s disease. Gastroenterology. 1992;102(2):514–9. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- 66.Mitsuyama K, Toyonaga A, Sasaki E, Ishida O, Ikeda H, Tsuruta O, Harada K, Tateishi H, Nishiyama T, Tanikawa K. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut. 1995;36(1):45–9. doi: 10.1136/gut.36.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83(4):222–6. doi: 10.1002/jso.10269. [DOI] [PubMed] [Google Scholar]
- 68.Galizia G, Orditura M, Romano C, Lieto E, Castellano P, Pelosio L, Imperatore V, Catalano G, Pignatelli C, De Vita F. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol. 2002;102(2):169–78. doi: 10.1006/clim.2001.5163. [DOI] [PubMed] [Google Scholar]
- 69.Rose-John S, Waetzig GH, Scheller J, Grotzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11(5):613–24. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- 70.Schneider MR, Hoeflich A, Fischer JR, Wolf E, Sordat B, Lahm H. Interleukin-6 stimulates clonogenic growth of primary and metastatic human colon carcinoma cells. Cancer Lett. 2000;151(1):31–8. doi: 10.1016/s0304-3835(99)00401-2. [DOI] [PubMed] [Google Scholar]
- 71.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130(1):27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ito H. Treatment of Crohn’s disease with anti-IL-6 receptor antibody. J Gastroenterol. 2005;40(Suppl 16):32–4. doi: 10.1007/BF02990576. [DOI] [PubMed] [Google Scholar]
- 73.Atreya R, Atreya I, Neurath MF. Novel signal transduction pathways: analysis of STAT-3 and Rac-1 signaling in inflammatory bowel disease. Ann N Y Acad Sci. 2006;1072:98–113. doi: 10.1196/annals.1326.001. [DOI] [PubMed] [Google Scholar]
- 74.Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Galle PR, Blessing M, Rose-John S, Neurath MF. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21(4):491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 75.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 76.Walia B, Wang L, Merlin D, Sitaraman SV. TGF-beta down-regulates IL-6 signaling in intestinal epithelial cells: critical role of SMAD-2. Faseb J. 2003;17(14):2130–2. doi: 10.1096/fj.02-1211fje. [DOI] [PubMed] [Google Scholar]
- 77.Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD, Neumann A, Brattain MG, Chang J, Kim SJ, Kinzler KW, Vogelstein B, Willson JK, Markowitz S. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59(2):320–4. [PubMed] [Google Scholar]
- 78.Tang Y, Kitisin K, Jogunoori W, Li C, Deng CX, Mueller SC, Ressom HW, Rashid A, He AR, Mendelson JS, Jessup JM, Shetty K, Zasloff M, Mishra B, Reddy EP, Johnson L, Mishra L. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci U S A. 2008;105(7):2445–50. doi: 10.1073/pnas.0705395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shackel NA, McCaughan GW, Warner FJ. Hepatocellular carcinoma development requires hepatic stem cells with altered transforming growth factor and interleukin-6 signaling. Hepatology. 2008;47(6):2134–6. doi: 10.1002/hep.22369. [DOI] [PubMed] [Google Scholar]
- 80.Tsai KS, Yang SH, Lei YP, Tsai CC, Chen HW, Hsu CY, Chen LL, Wang HW, Miller SA, Chiou SH, Hung MC, Hung SC. Mesenchymal Stem Cells Promote Formation of Colorectal Tumors in Mice. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 81.Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63(5):321–9. doi: 10.1111/j.1365-3083.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- 82.Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res. 2005;25(5):241–53. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- 83.Islam O, Gong X, Rose-John S, Heese K. Interleukin-6 and neural stem cells: more than gliogenesis. Mol Biol Cell. 2009;20(1):188–99. doi: 10.1091/mbc.E08-05-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parikh AA, Moon MR, Kane CD, Salzman AL, Fischer JE, Hasselgren PO. Interleukin-6 production in human intestinal epithelial cells increases in association with the heat shock response. J Surg Res. 1998;77(1):40–4. doi: 10.1006/jsre.1998.5332. [DOI] [PubMed] [Google Scholar]
- 85.Wang Q, Sun X, Pritts TA, Wong HR, Hasselgren PO. Induction of the stress response increases interleukin-6 production in the intestinal mucosa of endotoxaemic mice. Clin Sci (Lond) 2000;99(6):489–96. [PubMed] [Google Scholar]
- 86.Wang Q, Wang JJ, Boyce S, Fischer JE, Hasselgren PO. Endotoxemia and IL-1 beta stimulate mucosal IL-6 production in different parts of the gastrointestinal tract. J Surg Res. 1998;76(1):27–31. doi: 10.1006/jsre.1998.5288. [DOI] [PubMed] [Google Scholar]
- 87.Rose-John S, Mitsuyama K, Matsumoto S, Thaiss WM, Scheller J. Interleukin-6 trans-signaling and colonic cancer associated with inflammatory bowel disease. Curr Pharm Des. 2009;15(18):2095–103. doi: 10.2174/138161209788489140. [DOI] [PubMed] [Google Scholar]
- 88.Jones SA, Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim Biophys Acta. 2002;1592(3):251–63. doi: 10.1016/s0167-4889(02)00319-1. [DOI] [PubMed] [Google Scholar]
- 89.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80(2):227–36. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 90.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80(2):227–36. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 92.Heaney ML, Golde DW. Soluble receptors in human disease. J Leukoc Biol. 1998;64(2):135–46. doi: 10.1002/jlb.64.2.135. [DOI] [PubMed] [Google Scholar]
- 93.Hilton DJ, Hilton AA, Raicevic A, Rakar S, Harrison-Smith M, Gough NM, Begley CG, Metcalf D, Nicola NA, Willson TA. Cloning of a murine IL-11 receptor alpha-chain; requirement for gp130 for high affinity binding and signal transduction. Embo J. 1994;13(20):4765–75. doi: 10.1002/j.1460-2075.1994.tb06802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Curtis DJ, Hilton DJ, Roberts B, Murray L, Nicola N, Begley CG. Recombinant soluble interleukin-11 (IL-11) receptor alpha-chain can act as an IL-11 antagonist. Blood. 1997;90(11):4403–12. [PubMed] [Google Scholar]
- 95.Putoczki T, Ernst M. More than a sidekick: the IL-6 family cytokine IL-11 links inflammation to cancer. J Leukoc Biol. 2010;88(6):1109–17. doi: 10.1189/jlb.0410226. [DOI] [PubMed] [Google Scholar]
- 96.Levine SJ. Mechanisms of soluble cytokine receptor generation. J Immunol. 2004;173(9):5343–8. doi: 10.4049/jimmunol.173.9.5343. [DOI] [PubMed] [Google Scholar]
- 97.Mitsuyama K, Tomiyasu N, Suzuki A, Takaki K, Takedatsu H, Masuda J, Yamasaki H, Matsumoto S, Tsuruta O, Toyonaga A, Sata M. A form of circulating interleukin-6 receptor component soluble gp130 as a potential interleukin-6 inhibitor in inflammatory bowel disease. Clin Exp Immunol. 2006;143(1):125–31. doi: 10.1111/j.1365-2249.2005.02960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schurmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6(5):583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 99.van den Brande J, Hommes DW, Peppelenbosch MP. Infliximab induced T lymphocyte apoptosis in Crohn’s disease. J Rheumatol Suppl. 2005;74:26–30. [PubMed] [Google Scholar]
- 100.Peppelenbosch MP, van Deventer SJ. T cell apoptosis and inflammatory bowel disease. Gut. 2004;53(11):1556–8. doi: 10.1136/gut.2004.040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Itoh J, de La Motte C, Strong SA, Levine AD, Fiocchi C. Decreased Bax expression by mucosal T cells favours resistance to apoptosis in Crohn’s disease. Gut. 2001;49(1):35–41. doi: 10.1136/gut.49.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ito H, Hirotani T, Yamamoto M, Ogawa H, Kishimoto T. Anti-IL-6 receptor monoclonal antibody inhibits leukocyte recruitment and promotes T-cell apoptosis in a murine model of Crohn’s disease. J Gastroenterol. 2002;37(Suppl 14):56–61. doi: 10.1007/BF03326415. [DOI] [PubMed] [Google Scholar]
- 103.Chalaris A, Gewiese J, Paliga K, Fleig L, Schneede A, Krieger K, Rose-John S, Scheller J. ADAM17-mediated shedding of the IL6R induces cleavage of the membrane stub by gamma-secretase. Biochim Biophys Acta. 2010;1803(2):234–45. doi: 10.1016/j.bbamcr.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 104.Lee AM, Diasio RB. ADAM-17: a target to increase chemotherapeutic efficacy in colorectal cancer? Clin Cancer Res. 2010;16(13):3319–21. doi: 10.1158/1078-0432.CCR-10-1059. [DOI] [PubMed] [Google Scholar]
- 105.Nishimoto N, Nakahara H, Yoshio-Hoshino N, Mima T. Successful treatment of a patient with Takayasu arteritis using a humanized anti-interleukin-6 receptor antibody. Arthritis Rheum. 2008;58(4):1197–200. doi: 10.1002/art.23373. [DOI] [PubMed] [Google Scholar]
- 106.Ito H. Novel therapy for Crohn’s disease targeting IL-6 signalling. Expert Opin Ther Targets. 2004;8(4):287–94. doi: 10.1517/14728222.8.4.287. [DOI] [PubMed] [Google Scholar]
- 107.Adachi Y, Yoshio-Hoshino N, Nishimoto N. The blockade of IL-6 signaling in rational drug design. Curr Pharm Des. 2008;14(12):1217–24. doi: 10.2174/138161208784246072. [DOI] [PubMed] [Google Scholar]
- 108.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268(1):160–7. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 109.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 110.Rose-John S, Neurath MF. IL-6 trans-signaling: the heat is on. Immunity. 2004;20(1):2–4. doi: 10.1016/s1074-7613(04)00003-2. [DOI] [PubMed] [Google Scholar]
- 111.Tsuda H, Ohshima Y, Nomoto H, Fujita K, Matsuda E, Iigo M, Takasuka N, Moore MA. Cancer prevention by natural compounds. Drug Metab Pharmacokinet. 2004;19(4):245–63. doi: 10.2133/dmpk.19.245. [DOI] [PubMed] [Google Scholar]
- 112.Cheng Y, Desreumaux P. 5-aminosalicylic acid is an attractive candidate agent for chemoprevention of colon cancer in patients with inflammatory bowel disease. World J Gastroenterol. 2005;11(3):309–14. doi: 10.3748/wjg.v11.i3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: changes, causes and management strategies. World J Gastroenterol. 2008;14(25):3937–47. doi: 10.3748/wjg.14.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.O’Morain C, Qasim A. Concept of chemoprevention in colorectal cancer. World J Gastrointest Oncol. 2009;1(1):21–5. doi: 10.4251/wjgo.v1.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Courtney ED, Melville DM, Leicester RJ. Review article: chemoprevention of colorectal cancer. Aliment Pharmacol Ther. 2004;19(1):1–24. doi: 10.1046/j.1365-2036.2003.01806.x. [DOI] [PubMed] [Google Scholar]
- 116.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102(7):1369–76. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abel S, Hundhausen C, Mentlein R, Schulte A, Berkhout TA, Broadway N, Hartmann D, Sedlacek R, Dietrich S, Muetze B, Schuster B, Kallen KJ, Saftig P, Rose-John S, Ludwig A. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172(10):6362–72. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- 118.Matthews V, Schuster B, Schutze S, Bussmeyer I, Ludwig A, Hundhausen C, Sadowski T, Saftig P, Hartmann D, Kallen KJ, Rose-John S. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE) J Biol Chem. 2003;278(40):38829–39. doi: 10.1074/jbc.M210584200. [DOI] [PubMed] [Google Scholar]
- 119.Garbers C, Janner N, Chalaris A, Moss ML, Floss DM, Meyer D, Koch-Nolte F, Rose-John S, Scheller J. Species specificity of ADAM10 and ADAM17 proteins in interleukin-6 (IL-6) trans-signaling and novel role of ADAM10 in inducible IL-6 receptor shedding. J Biol Chem. 2011;286(17):14804–11. doi: 10.1074/jbc.M111.229393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA. An essential role for ectodomain shedding in mammalian development. Science. 1998;282(5392):1281–4. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 121.Chalaris A, Adam N, Sina C, Rosenstiel P, Lehmann-Koch J, Schirmacher P, Hartmann D, Cichy J, Gavrilova O, Schreiber S, Jostock T, Matthews V, Hasler R, Becker C, Neurath MF, Reiss K, Saftig P, Scheller J, Rose-John S. Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med. 2010;207(8):1617–24. doi: 10.1084/jem.20092366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kyula JN, Van Schaeybroeck S, Doherty J, Fenning CS, Longley DB, Johnston PG. Chemotherapy-induced activation of ADAM-17: a novel mechanism of drug resistance in colorectal cancer. Clin Cancer Res. 2010;16(13):3378–89. doi: 10.1158/1078-0432.CCR-10-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bass R, Edwards DR. ADAMs and protein disulfide isomerase: the key to regulated cell-surface protein ectodomain shedding? Biochem J. 2010;428(3):e3–5. doi: 10.1042/BJ20100568. [DOI] [PubMed] [Google Scholar]
- 124.Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45(2):146–69. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Willems SH, Tape CJ, Stanley PL, Taylor NA, Mills IG, Neal DE, McCafferty J, Murphy G. Thiol isomerases negatively regulate the cellular shedding activity of ADAM17. Biochem J. 2010;428(3):439–50. doi: 10.1042/BJ20100179. [DOI] [PubMed] [Google Scholar]
- 126.Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, Shelley C, Hutton M, Knauper V, Docherty AJ, Murphy G. TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett. 1998;435(1):39–44. doi: 10.1016/s0014-5793(98)01031-x. [DOI] [PubMed] [Google Scholar]
- 127.Moss ML, Sklair-Tavron L, Nudelman R. Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2008;4(6):300–9. doi: 10.1038/ncprheum0797. [DOI] [PubMed] [Google Scholar]
- 128.DasGupta S, Murumkar PR, Giridhar R, Yadav MR. Current perspective of TACE inhibitors: a review. Bioorg Med Chem. 2009;17(2):444–59. doi: 10.1016/j.bmc.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 129.Cesaro A, Abakar-Mahamat A, Brest P, Lassalle S, Selva E, Filippi J, Hebuterne X, Hugot JP, Doglio A, Galland F, Naquet P, Vouret-Craviari V, Mograbi B, Hofman PM. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1332–43. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- 130.Ahmed S, Marotte H, Kwan K, Ruth JH, Campbell PL, Rabquer BJ, Pakozdi A, Koch AE. Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production. Proc Natl Acad Sci U S A. 2008;105(38):14692–7. doi: 10.1073/pnas.0802675105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–69. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 132.Singh R, Akhtar N, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate: inflammation and arthritis, [corrected] Life Sci. 2010;86(25–26):907–18. doi: 10.1016/j.lfs.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mochizuki M, Hasegawa N. (−)-Epigallocatechin-3-gallate reduces experimental colon injury in rats by regulating macrophage and mast cell. Phytother Res. 2010;24(Suppl 1):S120–2. doi: 10.1002/ptr.2862. [DOI] [PubMed] [Google Scholar]
- 134.Abboud PA, Hake PW, Burroughs TJ, Odoms K, O’Connor M, Mangeshkar P, Wong HR, Zingarelli B. Therapeutic effect of epigallocatechin-3-gallate in a mouse model of colitis. Eur J Pharmacol. 2008;579(1–3):411–7. doi: 10.1016/j.ejphar.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 135.Ran ZH, Chen C, Xiao SD. Epigallocatechin-3-gallate ameliorates rats colitis induced by acetic acid. Biomed Pharmacother. 2008;62(3):189–96. doi: 10.1016/j.biopha.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 136.Shirakami Y, Shimizu M, Tsurumi H, Hara Y, Tanaka T, Moriwaki H. EGCG and Polyphenon E attenuate inflammation-related mouse colon carcinogenesis induced by AOM plus DDS. Mol Med Report. 2008;1(3):355–61. [PubMed] [Google Scholar]
- 137.Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol Res. 2011;64(2):113–22. doi: 10.1016/j.phrs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 138.Tedeschi E, Suzuki H, Menegazzi M. Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann N Y Acad Sci. 2002;973:435–7. doi: 10.1111/j.1749-6632.2002.tb04678.x. [DOI] [PubMed] [Google Scholar]
- 139.Hosokawa Y, Hosokawa I, Ozaki K, Nakanishi T, Nakae H, Matsuo T. Catechins inhibit CXCL10 production from oncostatin M-stimulated human gingival fibroblasts. J Nutr Biochem. 2010;21(7):659–64. doi: 10.1016/j.jnutbio.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 140.Park G, Yoon BS, Moon JH, Kim B, Jun EK, Oh S, Kim H, Song HJ, Noh JY, Oh C, You S. Green tea polyphenol epigallocatechin-3-gallate suppresses collagen production and proliferation in keloid fibroblasts via inhibition of the STAT3-signaling pathway. J Invest Dermatol. 2008;128(10):2429–41. doi: 10.1038/jid.2008.103. [DOI] [PubMed] [Google Scholar]
- 141.Zhu BH, Chen HY, Zhan WH, Wang CY, Cai SR, Wang Z, Zhang CH, He YL. (−)-Epigallocatechin-3-gallate inhibits VEGF expression induced by IL-6 via Stat3 in gastric cancer. World J Gastroenterol. 2011;17(18):2315–25. doi: 10.3748/wjg.v17.i18.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Taylor RA, Leonard MC. Curcumin for inflammatory bowel disease: a review of human studies. Altern Med Rev. 2011;16(2):152–6. [PubMed] [Google Scholar]
- 143.Glienke W, Maute L, Wicht J, Bergmann L. Curcumin inhibits constitutive STAT3 phosphorylation in human pancreatic cancer cell lines and downregulation of survivin/BIRC5 gene expression. Cancer Invest. 2010;28(2):166–71. doi: 10.3109/07357900903287006. [DOI] [PubMed] [Google Scholar]
- 144.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171(7):3863–71. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 145.Lahiff C, Moss AC. Curcumin for clinical and endoscopic remission in ulcerative colitis. Inflamm Bowel Dis. 2011;17(7):E66. doi: 10.1002/ibd.21710. [DOI] [PubMed] [Google Scholar]
- 146.Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PJ, Mansell A, Jenkins BJ. IL-6 trans-signaling modulates TLR4-dependent inflammatory responses via STAT3. J Immunol. 2011;186(2):1199–208. doi: 10.4049/jimmunol.1002971. [DOI] [PubMed] [Google Scholar]
- 147.Zhao L, Lee JY, Hwang DH. Inhibition of pattern recognition receptor-mediated inflammation by bioactive phytochemicals. Nutr Rev. 2011;69(6):310–20. doi: 10.1111/j.1753-4887.2011.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lubbad A, Oriowo MA, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. 2009;322(1–2):127–35. doi: 10.1007/s11010-008-9949-4. [DOI] [PubMed] [Google Scholar]
- 149.Tomida M, Ohtake H, Yokota T, Kobayashi Y, Kurosumi M. Stat3 up-regulates expression of nicotinamide N-methyltransferase in human cancer cells. J Cancer Res Clin Oncol. 2008;134(5):551–9. doi: 10.1007/s00432-007-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin L, Liu Y, Li H, Li PK, Fuchs J, Shibata H, Iwabuchi Y, Lin J. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer. 2011 doi: 10.1038/bjc.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 152.Larrosa M, Tome-Carneiro J, Yanez-Gascon MJ, Alcantara D, Selma MV, Beltran D, Garcia-Conesa MT, Urban C, Lucas R, Tomas-Barberan F, Morales JC, Espin JC. Preventive oral treatment with resveratrol pro-prodrugs drastically reduce colon inflammation in rodents. J Med Chem. 2010;53(20):7365–76. doi: 10.1021/jm1007006. [DOI] [PubMed] [Google Scholar]
- 153.Li T, Wang W, Chen H, Ye L. Evaluation of anti-leukemia effect of resveratrol by modulating STAT3 signaling. Int Immunopharmacol. 2010;10(1):18–25. doi: 10.1016/j.intimp.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 154.Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB. Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci. 2011;1215:150–60. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martin AR, Villegas I, Sanchez-Hidalgo M, de la Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol. 2006;147(8):873–85. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Larrosa M, Yanez-Gascon MJ, Selma MV, Gonzalez-Sarrias A, Toti S, Ceron JJ, Tomas-Barberan F, Dolara P, Espin JC. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J Agric Food Chem. 2009;57(6):2211–20. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- 157.Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, Nagarkatti PS. Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332(3):829–39. doi: 10.1124/jpet.109.160838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hofseth LJ, Singh UP, Singh NP, Nagarkatti M, Nagarkatti PS. Taming the beast within: resveratrol suppresses colitis and prevents colon cancer. Aging (Albany NY) 2010;2(4):183–4. doi: 10.18632/aging.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cui X, Jin Y, Hofseth AB, Pena E, Habiger J, Chumanevich A, Poudyal D, Nagarkatti M, Nagarkatti PS, Singh UP, Hofseth LJ. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res (Phila) 2010;3(4):549–59. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sanchez-Fidalgo S, Cardeno A, Villegas I, Talero E, de la Lastra CA. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur J Pharmacol. 2010;633(1–3):78–84. doi: 10.1016/j.ejphar.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 161.Yao J, Wang JY, Liu L, Li YX, Xun AY, Zeng WS, Jia CH, Wei XX, Feng JL, Zhao L, Wang LS. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch Med Res. 2010;41(4):288–94. doi: 10.1016/j.arcmed.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 162.Youn J, Lee JS, Na HK, Kundu JK, Surh YJ. Resveratrol and piceatannol inhibit iNOS expression and NF-kappaB activation in dextran sulfate sodium-induced mouse colitis. Nutr Cancer. 2009;61(6):847–54. doi: 10.1080/01635580903285072. [DOI] [PubMed] [Google Scholar]
- 163.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109(6):2293–302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 164.Yang YP, Chang YL, Huang PI, Chiou GY, Tseng LM, Chiou SH, Chen MH, Chen MT, Shih YH, Chang CH, Hsu CC, Ma HI, Wang CT, Tsai LL, Yu CC, Chang CJ. Resveratrol suppresses tumorigenicity and enhances radiosensitivity in primary glioblastoma tumor initiating cells by inhibiting the STAT3 axis. J Cell Physiol. 2011 doi: 10.1002/jcp.22806. [DOI] [PubMed] [Google Scholar]
- 165.Tili E, Michaille JJ, Alder H, Volinia S, Delmas D, Latruffe N, Croce CM. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFbeta signaling pathway in SW480 cells. Biochem Pharmacol. 2010;80(12):2057–65. doi: 10.1016/j.bcp.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim YH, Kwon HS, Kim DH, Cho HJ, Lee HS, Jun JG, Park JH, Kim JK. Piceatannol, a stilbene present in grapes, attenuates dextran sulfate sodium-induced colitis. Int Immunopharmacol. 2008;8(12):1695–702. doi: 10.1016/j.intimp.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 167.Ashikawa K, Majumdar S, Banerjee S, Bharti AC, Shishodia S, Aggarwal BB. Piceatannol inhibits TNF-induced NF-kappaB activation and NF-kappaB-mediated gene expression through suppression of IkappaBalpha kinase and p65 phosphorylation. J Immunol. 2002;169(11):6490–7. doi: 10.4049/jimmunol.169.11.6490. [DOI] [PubMed] [Google Scholar]
- 168.Son PS, Park SA, Na HK, Jue DM, Kim S, Surh YJ. Piceatannol, a catechol-type polyphenol, inhibits phorbol ester-induced NF-{kappa}B activation and cyclooxygenase-2 expression in human breast epithelial cells: cysteine 179 of IKK{beta} as a potential target. Carcinogenesis. 2010;31(8):1442–9. doi: 10.1093/carcin/bgq099. [DOI] [PubMed] [Google Scholar]
- 169.Liu D, Kim DH, Park JM, Na HK, Surh YJ. Piceatannol inhibits phorbol ester-induced NF-kappa B activation and COX-2 expression in cultured human mammary epithelial cells. Nutr Cancer. 2009;61(6):855–63. doi: 10.1080/01635580903285080. [DOI] [PubMed] [Google Scholar]
- 170.Jin CY, Moon DO, Lee KJ, Kim MO, Lee JD, Choi YH, Park YM, Kim GY. Piceatannol attenuates lipopolysaccharide-induced NF-kappaB activation and NF-kappaB-related proinflammatory mediators in BV2 microglia. Pharmacol Res. 2006;54(6):461–7. doi: 10.1016/j.phrs.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 171.Djoko B, Chiou RY, Shee JJ, Liu YW. Characterization of immunological activities of peanut stilbenoids, arachidin-1, piceatannol, and resveratrol on lipopolysaccharide-induced inflammation of RAW 264. 7 macrophages. J Agric Food Chem. 2007;55(6):2376–83. doi: 10.1021/jf062741a. [DOI] [PubMed] [Google Scholar]
- 172.Dang O, Navarro L, David M. Inhibition of lipopolysaccharide-induced interferon regulatory factor 3 activation and protection from septic shock by hydroxystilbenes. Shock. 2004;21(5):470–5. doi: 10.1097/01.shk.0000123513.13212.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Su L, David M. Distinct mechanisms of STAT phosphorylation via the interferon-alpha/beta receptor. Selective inhibition of STAT3 and STAT5 by piceatannol. J Biol Chem. 2000;275(17):12661–6. doi: 10.1074/jbc.275.17.12661. [DOI] [PubMed] [Google Scholar]
- 174.Suzuki A, Hanada T, Mitsuyama K, Yoshida T, Kamizono S, Hoshino T, Kubo M, Yamashita A, Okabe M, Takeda K, Akira S, Matsumoto S, Toyonaga A, Sata M, Yoshimura A. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193(4):471–81. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Skoumal R, Toth M, Serpi R, Rysa J, Leskinen H, Ulvila J, Saiho T, Aro J, Ruskoaho H, Szokodi I, Kerkela R. Parthenolide inhibits STAT3 signaling and attenuates angiotensin II-induced left ventricular hypertrophy via modulation of fibroblast activity. J Mol Cell Cardiol. 2011;50(4):634–41. doi: 10.1016/j.yjmcc.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 176.Sobota R, Szwed M, Kasza A, Bugno M, Kordula T. Parthenolide inhibits activation of signal transducers and activators of transcription (STATs) induced by cytokines of the IL-6 family. Biochem Biophys Res Commun. 2000;267(1):329–33. doi: 10.1006/bbrc.1999.1948. [DOI] [PubMed] [Google Scholar]
- 177.Kurdi M, Booz GW. Evidence that IL-6-type cytokine signaling in cardiomyocytes is inhibited by oxidative stress: parthenolide targets JAK1 activation by generating ROS. J Cell Physiol. 2007;212(2):424–31. doi: 10.1002/jcp.21033. [DOI] [PubMed] [Google Scholar]
- 178.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 2008;47(11):1635–40. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 179.Legendre F, Dudhia J, Pujol JP, Bogdanowicz P. JAK/STAT but not ERK1/ERK2 pathway mediates interleukin (IL)-6/soluble IL-6R down-regulation of Type II collagen, aggrecan core, and link protein transcription in articular chondrocytes. Association with a down-regulation of SOX9 expression. J Biol Chem. 2003;278(5):2903–12. doi: 10.1074/jbc.M110773200. [DOI] [PubMed] [Google Scholar]
- 180.Tian ZJ, Cui W, Li YJ, Hao YM, Du J, Liu F, Zhang H, Zu XG, Liu SY, Chen L, An W. Different contributions of STAT3, ERK1/2, and PI3-K signaling to cardiomyocyte hypertrophy by cardiotrophin-1. Acta Pharmacol Sin. 2004;25(9):1157–64. [PubMed] [Google Scholar]
- 181.Fleming A, Jankowski J, Goldsmith P. In vivo analysis of gut function and disease changes in a zebrafish larvae model of inflammatory bowel disease: a feasibility study. Inflamm Bowel Dis. 2010;16(7):1162–72. doi: 10.1002/ibd.21200. [DOI] [PubMed] [Google Scholar]
- 182.Shin EK, Kwon HS, Kim YH, Shin HK, Kim JK. Chrysin, a natural flavone, improves murine inflammatory bowel diseases. Biochem Biophys Res Commun. 2009;381(4):502–7. doi: 10.1016/j.bbrc.2009.02.071. [DOI] [PubMed] [Google Scholar]
- 183.Romier B, Van De Walle J, During A, Larondelle Y, Schneider YJ. Modulation of signalling nuclear factor-kappaB activation pathway by polyphenols in human intestinal Caco-2 cells. Br J Nutr. 2008;100(3):542–51. doi: 10.1017/S0007114508966666. [DOI] [PubMed] [Google Scholar]
- 184.Woo KJ, Jeong YJ, Inoue H, Park JW, Kwon TK. Chrysin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression through the inhibition of nuclear factor for IL-6 (NF-IL6) DNA-binding activity. FEBS Lett. 2005;579(3):705–11. doi: 10.1016/j.febslet.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 185.Hougee S, Sanders A, Faber J, Graus YM, van den Berg WB, Garssen J, Smit HF, Hoijer MA. Decreased pro-inflammatory cytokine production by LPS-stimulated PBMC upon in vitro incubation with the flavonoids apigenin, luteolin or chrysin, due to selective elimination of monocytes/macrophages. Biochem Pharmacol. 2005;69(2):241–8. doi: 10.1016/j.bcp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 186.Lin CM, Shyu KG, Wang BW, Chang H, Chen YH, Chiu JH. Chrysin suppresses IL-6-induced angiogenesis via down-regulation of JAK1/STAT3 and VEGF: an in vitro and in ovo approach. J Agric Food Chem. 2010;58(11):7082–7. doi: 10.1021/jf100421w. [DOI] [PubMed] [Google Scholar]
- 187.Wang YH, Ge B, Yang XL, Zhai J, Yang LN, Wang XX, Liu X, Shi JC, Wu YJ. Proanthocyanidins from grape seeds modulates the nuclear factor-kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int Immunopharmacol. 2011 doi: 10.1016/j.intimp.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 188.Li WG, Zhang XY, Wu YJ, Tian X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol Sin. 2001;22(12):1117–20. [PubMed] [Google Scholar]
- 189.Park MK, Park JS, Cho ML, Oh HJ, Heo YJ, Woo YJ, Heo YM, Park MJ, Park HS, Park SH, Kim HY, Min JK. Grape seed proanthocyanidin extract (GSPE) differentially regulates Foxp3(+) regulatory and IL-17(+) pathogenic T cell in autoimmune arthritis. Immunol Lett. 2011;135(1–2):50–8. doi: 10.1016/j.imlet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 190.Gonthier MP, Cheynier V, Donovan JL, Manach C, Morand C, Mila I, Lapierre C, Remesy C, Scalbert A. Microbial aromatic acid metabolites formed in the gut account for a major fraction of the polyphenols excreted in urine of rats fed red wine polyphenols. J Nutr. 2003;133(2):461–7. doi: 10.1093/jn/133.2.461. [DOI] [PubMed] [Google Scholar]
- 191.Li XL, Cai YQ, Qin H, Wu YJ. Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Can J Physiol Pharmacol. 2008;86(12):841–9. doi: 10.1139/Y08-089. [DOI] [PubMed] [Google Scholar]
- 192.Wang YH, Yang XL, Wang L, Cui MX, Cai YQ, Li XL, Wu YJ. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Can J Physiol Pharmacol. 2010;88(9):888–98. doi: 10.1139/y10-071. [DOI] [PubMed] [Google Scholar]
- 193.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6(6):461–7. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- 194.Cho JY, Chi SG, Chun HS. Oral administration of docosahexaenoic acid attenuates colitis induced by dextran sulfate sodium in mice. Mol Nutr Food Res. 2011;55(2):239–46. doi: 10.1002/mnfr.201000070. [DOI] [PubMed] [Google Scholar]
- 195.Kono H, Fujii H, Ogiku M, Tsuchiya M, Ishii K, Hara M. Enteral diets enriched with medium-chain triglycerides and N-3 fatty acids prevent chemically induced experimental colitis in rats. Transl Res. 2010;156(5):282–91. doi: 10.1016/j.trsl.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 196.Hassan A, Ibrahim A, Mbodji K, Coeffier M, Ziegler F, Bounoure F, Chardigny JM, Skiba M, Savoye G, Dechelotte P, Marion-Letellier R. An alpha-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-kappaB in rats with TNBS-induced colitis. J Nutr. 2010;140(10):1714–21. doi: 10.3945/jn.109.119768. [DOI] [PubMed] [Google Scholar]
- 197.Purnak T, Beyazit Y. Nitric oxide inhibition by dietary n-3 polyunsaturated fatty acids associated with decreased incidence of ulcerative colitis. Eur J Gastroenterol Hepatol. 2010;22(8):1023. doi: 10.1097/MEG.0b013e32833be8d1. [DOI] [PubMed] [Google Scholar]
- 198.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27(16):2712–25. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hering J, Garrean S, Dekoj TR, Razzak A, Saied A, Trevino J, Babcock TA, Espat NJ. Inhibition of proliferation by omega-3 fatty acids in chemoresistant pancreatic cancer cells. Ann Surg Oncol. 2007;14(12):3620–8. doi: 10.1245/s10434-007-9556-8. [DOI] [PubMed] [Google Scholar]
- 200.Turner D, Shah PS, Steinhart AH, Zlotkin S, Griffiths AM. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): a systematic review and meta-analyses. Inflamm Bowel Dis. 2011;17(1):336–45. doi: 10.1002/ibd.21374. [DOI] [PubMed] [Google Scholar]
- 201.Jia Q, Ivanov I, Zlatev ZZ, Alaniz RC, Weeks BR, Callaway ES, Goldsby JS, Davidson LA, Fan YY, Zhou L, Lupton JR, McMurray DN, Chapkin RS. Dietary fish oil and curcumin combine to modulate colonic cytokinetics and gene expression in dextran sodium sulphate-treated mice. Br J Nutr. 2011:1–11. doi: 10.1017/S0007114511000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- Crohn’s and Colitis Foundation of America. [Accessed August 16, 2004];Inflammatory Bowel Disease Research Enhancement Act. http://www.ccfa.org/advocacy/IBDResearchEnhancementAct.
- Centers for Disease Control and Prevention. [Accessed August 16, 2004];Inflammatory Bowel Disease (IBD) http://www.cdc.gov/ibd/