Highlights
-
•
A peptide was discovered that disrupts HSP20–phosphodiesterase 4D (PDE4D) complex formation.
-
•
HSP20–PDE4D complex disruption reversed hypertrophic-induced changes in electrical signalling in human cardiac myocytes.
-
•
HSP20–PDE4D complex disruption attenuated the physiological response to pressure/volume overload.
-
•
This physiological response normally results in an increase in cardiac myocyte size.
-
•
Cardiac fibrosis was reduced in mice following treatment with the HSP20–PDE4D disruptor peptide.
Abbreviations: APD, action potential duration; FS, fractional shortening; HSP20, heat shock protein 20; hiPSC-CMs, human induced pluripotent stem cell-derived cardiac myocytes; ISO, isoprenaline; LVEDD, left ventricle end diastolic dimension; LVESD, left ventricle end systolic dimension; LV, left ventricle; MTAB, minimally invasive transverse aortic banding; PBS, phosphate buffered saline; PDE4D, phosphodiesterase 4D; PKA, protein kinase-A
Keywords: cAMP, PDE4D, HSP20, Cardiac remodeling, Cardiac hypertrophy, Peptide disruption
Abstract
Phosphorylated heat shock protein 20 (HSP20) is cardioprotective. Using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and a mouse model of pressure overload mediated hypertrophy, we show that peptide disruption of the HSP20–phosphodiesterase 4D (PDE4D) complex results in attenuation of action potential prolongation and protection against adverse cardiac remodelling. The later was evidenced by improved contractility, decreased heart weight to body weight ratio, and reduced interstitial and perivascular fibrosis. This study demonstrates that disruption of the specific HSP20–PDE4D interaction leads to attenuation of pathological cardiac remodelling.
1. Introduction
The small heat shock protein, HSP20 is transiently up-regulated during stress/injury to the heart and underpins a plethora of cardio-protective properties including protection against apoptosis, inflammatory signalling, β-agonist-induced remodelling, and regulation of Ca2+ cycling [2]. HSP20 confers its protective properties via a number of diverse signalling systems including, prevention of caspase activity, enhancement of Akt/PKB signaling, inhibition of NF-κB signalling, reduction in cardiac myocyte apoptosis and necrosis, stabilisation of the cytoskeleton and increased cardiac myocyte shortening rates due to faster calcium transients [3]. Crucially, phosphorylation of HSP20 at Ser16 by PKA is critical for its protective actions [4], as illustrated by a variety of overexpression studies either using genetic engineering [5] or phospho-HSP20 analogues [6]. Cellular PKA activity is tightly regulated both spatially and temporally by the compartmentalisation of cAMP [7,8]. In cardiac myocytes, cAMP is a pivotal second messenger influencing inotropic and chronotropic activities, as well as hypertrophy and apoptosis [9]. Targeted intracellular hydrolysis by cAMP-specific phosphodiesterases (PDEs) enables the creation of subcellular compartments with high levels of cAMP relative to the surrounding environment [10]. Recent research has shown that HSP20 sequesters the PDE4D sub-family, with targeted disruption of this complex resulting in hyper-phosphorylation of HSP20 and protection of neonatal cardiomyocytes from β-adrenergic-induced hypertrophy [11]. Disruption of the HSP20–PDE4 complex was achieved following mapping of the protein–protein interface by peptide array, which allowed selection of a peptide (called peptide 906) of PDE4 sequence that was capable of interfering with HSP20 binding [11]. Phosphorylation of HSP20 by PKA is also facilitated by virtue of association to the PKA anchor protein, AKAP Lbc [12]. Here, using in vitro and in vivo techniques, we show that peptide 906-mediated disruption of the HSP20–PDE4D complex normalises ISO-induced elongation of action potential duration and reduces the development of the hypertrophic response in aortic-banded mice by attenuating cardiac remodelling, improving left ventricular function and decreasing cardiac fibrosis.
2. Methods
2.1. Action potential measurements in human induced pluripotent stem cell-derived cardiomyocytes
Human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs; obtained from Cellular Dynamics Inc.) were maintained in culture for 10 days. Cells were incubated for 2 h in the presence of vehicle (PBS), HSP20–PDE4D disruptor peptide (bs906; 10 μM) or scrambled control peptide (10 μM) followed by 24 h in the presence or absence of isoproterenol (ISO, 10 μM). Peptide bs906 and scrambled control peptide correspond to the sequence of “peptide 906” and “peptide control” respectively, previously used in cellular studies [11]. Of note, both bs906 and scrambled control peptide contained N-terminal stearoyl making them cell permeable. To obtain the action potential recordings the cells were washed in serum free medium and exposed transiently to 3 μM di-4-ANEPPS and left for 60–90min before electrical recordings were made. The multi-well plate was placed on a stage incubator of an inverted microscope and the spontaneous electrical activity was recorded from the di-4-ANEPPS fluorescence signal (using CellOPTIQ platform) from areas of hiPSC-CMs in individual wells visualised using a 40× (NA0.6) objective. Fluorescence signals were digitised at 10 KHz and the digital records subsequently analysed off-line. Each point corresponds to the mean ± S.E.M of 15 values obtained by measuring different fields per well in 3 plates. Statistical analysis was carried out using one-way ANOVA tests followed by post hoc Tukey’s tests using Origin 7.5. Significance was set at p ⩽ 0.05.
2.2. MTAB surgery and transthoracic echocardiography
Healthy adult male C57BL/6J mice (Harlan, UK) weighing between 25 and 30 g were used for these experiments. Procedures conformed to the UK Animals (Scientific Procedures) Act 1986 and were approved by institutional ethical review committees. MTAB and sham surgical protocols were performed as previously described [13]. Immediately following surgery, animals were injected subcutaneously with either HSP20–PDE4D disruptor peptide [11] (bs906) or a scrambled control peptide (10 mg/kg) twice weekly and were left for 4 weeks to allow cardiac remodeling to occur. Echocardiographic assessment of left ventricular function was performed 4 weeks after MTAB or sham surgery, as described previously [13]. LV end systolic dimension (LVESD) and LV end diastolic dimension (LVEDD) were assessed from M-mode traces and % fractional shortening calculated. Fractional shortening (FS) is expressed as [(LVEDD − LVESD)/LVEDD] × 100. An average of three measurements of each variable was used.
2.3. Post-mortem measurements
After 4 weeks, animals were euthanised with an intravenous injection of pentobarbital sodium (Euthatal, 200 mg/kg). Terminal anaesthesia was confirmed by testing loss of the pedal reflex. The heart, liver and lungs were rapidly excised and washed thoroughly in ice-cold Ca2+-free Krebs solution (120 mM NaCl, 5.4 mM KCl, 0.52 mM NaH2PO4, 20 mM HEPES, 11.1 mM glucose, 3.5 mM MgCl2, 20 mM Taurine, 10 mM Creatine; pH 7.4). Tissue was either homogenised or processed as described in the following sections.
2.4. Picro-sirius red staining
LV tissue was processed, embedded in paraffin and sectioned. Slides were stained for 1 h in picrosirius red solution containing 0.1% (w/v) direct red 80 dye in a saturated aqueous solution of 1.3% picric acid as previously described [14]. Five random sections per heart (5 μm sections with a distance of 200 μm between sections), and 5 areas of interest per section were photographed at 10× magnification using non-polarised light with a Leica DM LB2 microscope and a Leica DFC 320 camera (Leica Microsystems, Germany). Quantification of picrosirius red staining used ImageProPlus software (version 5.0; MediaCybernetics), with stained area expressed as a percentage of the total area of interest. Values were averaged to give one representative value per heart.
2.5. Statistics
Results were expressed as the mean ± S.E.M. of n observations, where n refers to the number of animals or samples. Statistical comparisons were performed using the student’s unpaired t test and one-way ANOVA, unless otherwise stated. p < 0.05 was considered statistically significant.
3. Results
3.1. Attenuation of isoprenaline-induced action potential elongation by bs906
The hypertrophied heart displays remodelling of its metabolic, biological, and electrophysiological properties, all of which are risk factors for ventricular arrhythmias and cardiac failure [15]. Recently, HSP20 has been shown to regulate Ca2+ cycling and SR Ca2+ load [16], with transgenic mice overexpressing HSP20 displaying enhanced basal cardiac function and protection against β-adrenergic-induced LV dysfunction [2,17]. Tight regulation of SR Ca2+ transients is critical for normal/synchronised cardiac cycles and action potential duration (APDs). While rodent neonatal and adult cardiomyocytes are appropriate for mechanistic studies of HSP20–PDE4 disruption, the use of a human-derived cell line may be more appropriate to evaluate the clinical relevance of drug effects [18]. To this end, we pre-treated human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs) in a similar manner to that used previously for mouse neonatal cardiac myocytes [11], that is for 2 h with either vehicle (PBS), bs906 (10 μM) or scrambled control peptide (10 μM), followed by 24 h in the presence or absence of ISO (10 μM) to determine if disruption of the HSP20–PDE4D interaction could reverse hypertrophy and associated electrical remodelling (Fig. 1). ISO-induced increases in APD at 50% and 90% repolarisation (i.e. a hypertrophic phenotype) were reversed when cells were pre-treated with bs906, but not with scrambled peptide (50%: 463.9 ± 22.4 ms cf. 542.8 ± 27.0 ms, 90%: 581.3 ± 20.9 ms cf. 665.2 ± 19.6 ms, ISO + bs906 and ISO + scrambled peptide, respectively, p < 0.05; Fig. 1A and B respectively,). Importantly, there was no effect on APD 50 or 90 when cells were pre-treated with bs906 or scrambled peptide in the absence of ISO (50%: 446.1 ± 23.5 ms cf. 441.2 ± 59.5 ms, 90%: 531.8 ± 22.2 ms cf. 543.5 ± 62.9 ms, bs906 and scrambled peptide, respectively, p > 0.05), suggesting that bs906 acts only to normalise hypertrophic induced changes in electrical signalling in hiPSC-CMs, possibly via prolonged inhibition of protein phosphatase 1 (PP1) by HSP20[16].
Fig. 1.
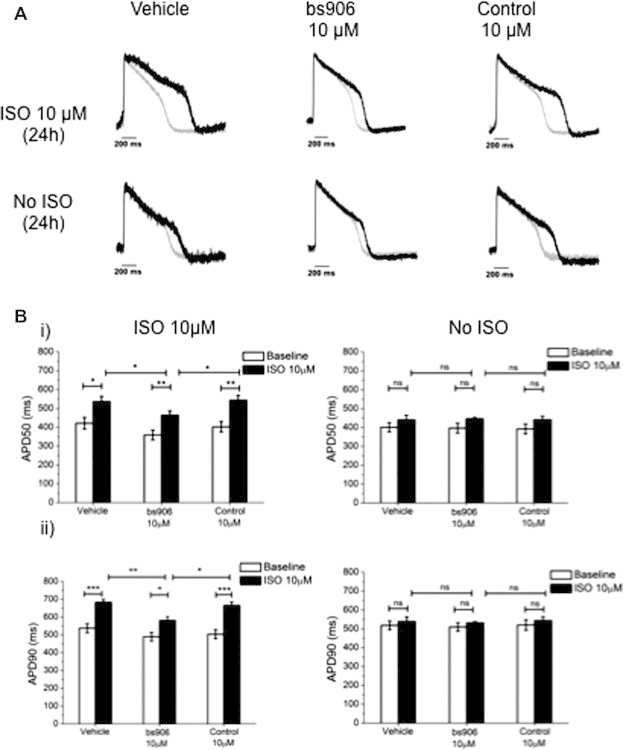
HSP20-PDE4D peptide disruptor modulates APD in hiPSC-CMs. hiPSC-CMs (Cellular Dynamics International, Madison, Wisconsin) were pre-treated for 2 h with vehicle (PBS), bs906 (10 μM) or control peptide (10 μM), followed by 24 h co-incubation with ISO (10 μM). Cells were loaded with 3 μM di-4-ANEPPS and electrical activity of spontaneously beating cardiomyocytes was registered using CellOPTIQ platform (Clyde Biosciences Ltd.). (A) Example AP traces (grey trace baseline, black trace 24 h 10 μM ISO/Vehicle). (B) (i) Average values of APD at 50% (APD50) and (ii) 90% of repolarisation (APD90) from baseline (white columns) or after treatment (black columns). Data represents mean ± S.E.M, measured from 15 areas per treatment from n = 3 separate cultures, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, one-way ANOVA post hoc Tukey’s test.
3.2. Preserved cardiac function in bs906-treated hypertrophic mice
Another key feature of the hypertrophied and remodelling heart is impaired LV function. To investigate whether HSP20–PDE4D complex disruption could protect against LV dysfunction and remodelling, we used a minimally invasive transverse aortic banding (MTAB) model of pressure overload hypertrophy. Following MTAB, mice were injected with either 10 mg/kg bs906 or scrambled peptide over a 4-week period, followed by echocardiography to determine the extent of LV remodelling. As shown in Fig. 2A(i)), twice weekly injections of bs906, but not control, prevented MTAB-induced decreases in left ventricular (LV) contractility (%fractional shortening: 47.1 ± 1.7% cf. 29.6 ± 2.0%, p < 0.0001, MTAB + bs906 and MTAB + control, respectively, and 47.1 ± 1.7% cf. 46.1 ± 1.1%, p > 0.05, MTAB + bs906 and sham + bs906, respectively). Interestingly, a one-weekly dose of bs906 proved sufficient in preventing LV dysfunction in MTAB mice (Fig. 2A(ii)), 38.5 ± 2.5% cf. 29.2 ± 2.2%, p < 0.5, MTAB + bs906 and MTAB + control, respectively, and 38.5 ± 2.5% cf. 40.4 ± 1.3%, p > 0.05 MTAB + bs906 and sham + bs906, respectively).
Fig. 2.
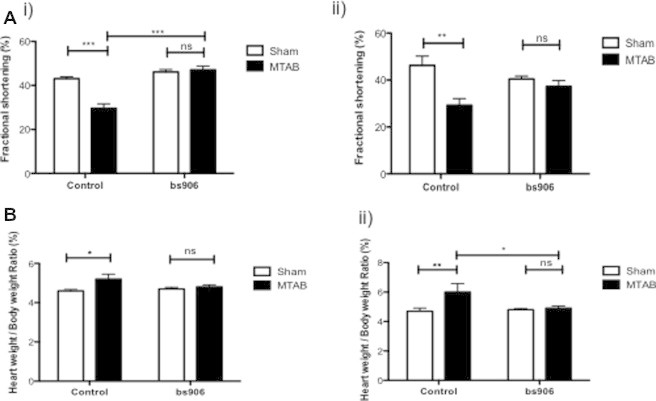
Disruption of HSP20–PDE4D complex reverses MTAB induced cardiac dysfunction. Histograms of % fractional shortening (A) and post-mortem heart weight to body weight ratios (B) Mice undergoing sham or MTAB surgery were treated with either 10 mg/kg bs906 or control twice (i) or once weekly (ii) for 4 weeks. Data represent mean ± S.E.M., n = 3–8, ∗p < 0.05 ∗∗p < 0.01, ∗∗∗p < 0.001, ns = p > 0.05, one-way ANOVA post hoc Tukey’s test.
3.3. Attenuation of MTAB-induced cardiac remodelling
Aberrant chronic and prolonged β-adrenergic stimulation is a key feature of the diseased and remodelling heart. Cardiac hypertrophy is a physiological response to pressure/volume overload that results in an increase in cardiac myocyte size. We have previously shown that bs906 (but not scrambled control) could attenuate cardiac myocyte hypertrophy in culture [11]; however, our current work has now validated this approach in an animal model (Fig. 2B). Pressure-overload mediated cardiac remodelling was attenuated with twice-weekly doses of bs906 (Fig 2B(i); 5.2 ± 0.3 cf. 4.6 ± 0.07, n ⩾ 6, p < 0.001, MTAB + scrambled and sham + scrambled, respectively and 4.8 ± 0.1 cf. 4.7 ± 0.09, n = 8, p > 0.05, MTAB + bs906 and sham + bs906, respectively). In fact, one weekly dose of bs906 was adequate in reducing MTAB induced remodelling (Fig. 2B(ii); 4.9 ± 0.2 cf. 6.0 ± 0.6, n ⩾ 3, p < 0.05, MTAB + bs906 and MTAB + scrambled, respectively, and 4.9 ± 0.2 cf. 4.8 ± 0.1, n ⩾ 3, p > 0.05, MTAB + bs906 and sham + bs906, respectively).
3.4. Reduced MTAB-induced interstitial fibrosis by bs906
Cardiac fibrosis is another important feature of the remodelling heart that contributes to the pathogenesis of diastolic dysfunction [13]. Cardiac fibrosis is the result of aberrant fibroblast proliferation, remodelling of the extracellular matrix and increased collagen synthesis and deposition [19]. Analysis of picrosirius red stained LV sections showed increased collagen deposition in MTAB hearts treated with scrambled peptide twice weekly (Fig. 3A and B; % stained area, 5.5 ± 0.9% cf. 1.7 ± 0.4%, n ⩾ 6, p < 0.001, MTAB + scrambled and sham + scrambled, respectively), in correspondence with previously published data [13]. By contrast, MTAB mice treated twice weekly with bs906 showed a significant reduction in fibrosis (Fig. 3A and B; 2.4 ± 0.2% cf. 5.5 ± 0.9%, n ⩾ 6, p < 0.001, MTAB + bs906 and MTAB + scrambled, respectively, and 2.4 ± 0.2% cf. 1.5 ± 0.2%, n ⩾ 7, p > 0.5, MTAB + bs906 and sham + bs906, respectively). This observation is gratifyingly similar to studies by other labs that have shown that excessive collagen deposition can be reversed following treatment with a phosphopeptide-analogue of HSP20 [20] or cardiac overexpression of HSP20 [17].
Fig. 3.
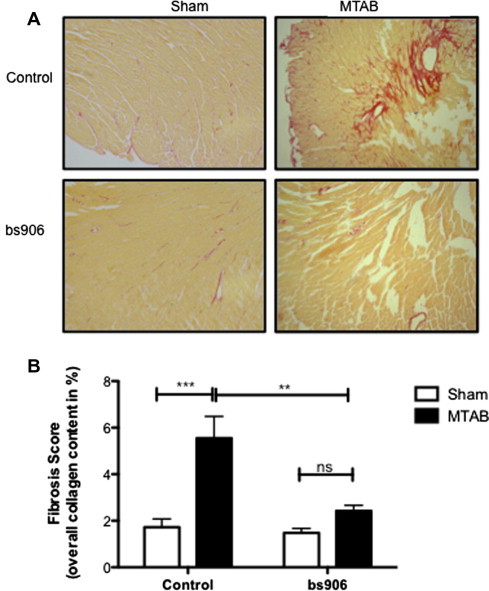
Cardiac fibrosis is modulated with disruption of HSP20–PDE4D complex. Picrosirius red staining of sham and MTAB hearts following treatment with 10 mg/kg bs906 or control peptide twice weekly (A). Histogram values (B) represent stained area expressed as a percentage of the total area of interest. Data represents mean ± S.E.M., n ⩾ 6, ∗∗p < 0.01, ∗∗∗p < 0.001, ns = p > 0.05, one-way ANOVA post hoc Tukey’s test.
4. Discussion
In summary, we have shown that targeted disruption of the HSP20–PDE4D interaction attenuates myocardial remodelling following cardiac insult in a minimally invasive transverse aortic banding model of pressure overload hypertrophy. This strategy [10], previously validated in cellular systems [11], has advantages over simple cell permeable phospho-peptide mimics of HSP20 [6,21] as these contain a labile phosphate group which can easily be targeted by phosphatases and are difficult to mass produce. High-throughput screening of small molecule libraries could also be applied to unlock the cardio-protective potential of HSP20. This approach has been used to discover a small molecule modulator of HSP20 that promotes relaxation of human airway smooth muscle cells and intact tissue ex vivo [1]. Our novel approach in targeting the interaction of HSP20 with an inhibitory protein (PDE4D) may represent a new therapeutic strategy for the treatment of a myriad of cardiovascular disorders, including cardiac hypertrophy.
Acknowledgments
This work was supported by Heart Research UK (Grant No: RG2610). G.S.B. is supported by MRC grant (MR/J007412/1). We are extremely grateful to Mr Graeme MacKenzie for technical assistance. MPH-V is recipient of a postdoctoral fellowship from Fundacion Alfonso Martin Escudero, Spain. GSB and SC conceived and designed the project, TPM, MPV-H, JEF and CE acquired and analyzed the data, TPM, GSB and SC wrote the paper.
References
- 1.An S.S., Askovich P.S., Zarembinski T.I., Ahn K., Peltier J.M., von Rechenberg M., Sahasrabudhe S., Fredberg J.J. A novel small molecule target in human airway smooth muscle for potential treatment of obstructive lung diseases: a staged high-throughput biophysical screening. Respir. Res. 2011;12:8. doi: 10.1186/1465-9921-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan G.C., Chu G., Kranias E.G. Hsp20 and its cardioprotection. Trends Cardiovasc. Med. 2005;15:138–141. doi: 10.1016/j.tcm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Edwards H.V., Cameron R.T., Baillie G.S. The emerging role of HSP20 as a multifunctional protective agent. Cell Signal. 2011;23:1447–1454. doi: 10.1016/j.cellsig.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Edwards H.V., Scott J.D., Baillie G.S. PKA phosphorylation of the small heat-shock protein Hsp20 enhances its cardioprotective effects. Biochem. Soc. Trans. 2012;40:210–214. doi: 10.1042/BST20110673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Zingarelli B., O’Connor M., Zhang P., Adeyemo A., Kranias E.G., Wang Y., Fan G.C. Overexpression of Hsp20 prevents endotoxin-induced myocardial dysfunction and apoptosis via inhibition of NF-kappaB activation. J. Mol. Cell. Cardiol. 2009;47:382–390. doi: 10.1016/j.yjmcc.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLemore E.C., Tessier D.J., Flynn C.R., Furnish E.J., Komalavilas P., Thresher J.S., Joshi L., Stone W.M., Fowl R.J., Brophy C.M. Transducible recombinant small heat shock-related protein, HSP20, inhibits vasospasm and platelet aggregation. Surgery. 2004;136:573–578. doi: 10.1016/j.surg.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Baillie G.S. Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J. 2009;276:1790–1799. doi: 10.1111/j.1742-4658.2009.06926.x. [DOI] [PubMed] [Google Scholar]
- 8.McCormick K., Baillie G.S. Compartmentalisation of second messenger signalling pathways. Curr. Opin. Genet. Dev. 2014;27C:20–25. doi: 10.1016/j.gde.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Tomita H., Nazmy M., Kajimoto K., Yehia G., Molina C.A., Sadoshima J. Inducible cAMP early repressor (ICER) is a negative-feedback regulator of cardiac hypertrophy and an important mediator of cardiac myocyte apoptosis in response to beta-adrenergic receptor stimulation. Circ. Res. 2003;93:12–22. doi: 10.1161/01.RES.0000079794.57578.F1. [DOI] [PubMed] [Google Scholar]
- 10.Lee L.C., Maurice D.H., Baillie G.S. Targeting protein–protein interactions within the cyclic AMP signaling system as a therapeutic strategy for cardiovascular disease. Future Med. Chem. 2013;5:451–464. doi: 10.4155/fmc.12.216. [DOI] [PubMed] [Google Scholar]
- 11.Sin Y.Y., Edwards H.V., Li X., Day J.P., Christian F., Dunlop A.J., Adams D.R., Zaccolo M., Houslay M.D., Baillie G.S. Disruption of the cyclic AMP phosphodiesterase-4 (PDE4)-HSP20 complex attenuates the beta-agonist induced hypertrophic response in cardiac myocytes. J. Mol. Cell. Cardiol. 2011;50:872–883. doi: 10.1016/j.yjmcc.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Edwards H.V., Scott J.D., Baillie G.S. The A-kinase-anchoring protein AKAP-Lbc facilitates cardioprotective PKA phosphorylation of Hsp20 on Ser(16) Biochem. J. 2012;446:437–443. doi: 10.1042/BJ20120570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin T.P., Robinson E., Harvey A.P., MacDonald M., Grieve D.J., Paul A., Currie S. Surgical optimization and characterization of a minimally invasive aortic banding procedure to induce cardiac hypertrophy in mice. Exp. Physiol. 2012;97:822–832. doi: 10.1113/expphysiol.2012.065573. [DOI] [PubMed] [Google Scholar]
- 14.Junqueira L.C., Bignolas G., Brentani R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 15.Szentandrassy N., Farkas V., Barandi L., Hegyi B., Ruzsnavszky F., Horvath B., Banyasz T., Magyar J., Marton I., Nanasi P.P. Role of action potential configuration and the contribution of C(2)(+)a and K(+) currents to isoprenaline-induced changes in canine ventricular cells. Br. J. Pharmacol. 2012;167:599–611. doi: 10.1111/j.1476-5381.2012.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian J., Vafiadaki E., Florea S.M., Singh V.P., Song W., Lam C.K., Wang Y., Yuan Q., Pritchard T.J., Cai W., Haghighi K., Rodriguez P., Wang H.S., Sanoudou D., Fan G.C., Kranias E.G. Small heat shock protein 20 interacts with protein phosphatase-1 and enhances sarcoplasmic reticulum calcium cycling. Circ. Res. 2011;108:1429–1438. doi: 10.1161/CIRCRESAHA.110.237644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan G.C., Yuan Q., Song G., Wang Y., Chen G., Qian J., Zhou X., Lee Y.J., Ashraf M., Kranias E.G. Small heat-shock protein Hsp20 attenuates beta-agonist-mediated cardiac remodeling through apoptosis signal-regulating kinase 1. Circ. Res. 2006;99:1233–1242. doi: 10.1161/01.RES.0000251074.19348.af. [DOI] [PubMed] [Google Scholar]
- 18.Schweikart K., Guo L., Shuler Z., Abrams R., Chiao E.T., Kolaja K.L., Davis M. The effects of jaspamide on human cardiomyocyte function and cardiac ion channel activity. Toxicol. In Vitro. 2013;27:745–751. doi: 10.1016/j.tiv.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreo A., Ambrosio G., De Chiara B., Pu M., Tran T., Mauri F., Raman S.V. Influence of myocardial fibrosis on left ventricular diastolic function: noninvasive assessment by cardiac magnetic resonance and echo. Circ. Cardiovasc. Imaging. 2009;2:437–443. doi: 10.1161/CIRCIMAGING.108.838367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes L.B., Furnish E.J., Komalavilas P., Flynn C.R., Ashby P., Hansen A., Ly D.P., Yang G.P., Longaker M.T., Panitch A., Brophy C.M. Cell permeant peptide analogues of the small heat shock protein, HSP20, reduce TGF-beta1-induced CTGF expression in keloid fibroblasts. J. Invest. Dermatol. 2009;129:590–598. doi: 10.1038/jid.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreiza C.M., Brophy C.M., Komalavilas P., Furnish E.J., Joshi L., Pallero M.A., Murphy-Ullrich J.E., von Rechenberg M., Ho Y.S., Richardson B., Xu N., Zhen Y., Peltier J.M., Panitch A. Transducible heat shock protein 20 (HSP20) phosphopeptide alters cytoskeletal dynamics. FASEB J. 2005;19:261–263. doi: 10.1096/fj.04-2911fje. [DOI] [PubMed] [Google Scholar]


