Abstract
Acute pancreatitis (AP) is a frequent disease with degrees of increasing severity responsible for high morbidity. Despite continuous improvement in care, mortality remains significant. Because hypovolemia, together with microcirculatory dysfunction lead to poor outcome, fluid therapy remains a cornerstone of the supportive treatment. However, poor clinical evidence actually support the aggressive fluid therapy recommended in recent guidelines since available data are controversial. Fluid management remains unclear and leads to current heterogeneous practice. Different strategies may help to improve fluid resuscitation in AP. On one hand, integration of fluid therapy in a global hemodynamic resuscitation has been demonstrated to improve outcome in surgical or septic patients. Tailored fluid administration after early identification of patients with high-risk of poor outcome presenting inadequate tissue oxygenation is a major part of this strategy. On the other hand, new decision parameters have been developed recently to improve safety and efficiency of fluid therapy in critically ill patients. In this review, we propose a personalized strategy integrating these new concepts in the early fluid management of AP. This new approach paves the way to a wide range of clinical studies in the field of AP.
Keywords: Pancreatitis, Fluid, Passive leg raising, Preload, Central venous pressure
Core tip: Fluid therapy is a cornerstone of the early supportive treatment of acute pancreatitis. However, poor clinical evidence actually support the aggressive fluid therapy recommended in recent guidelines since available data are controversial. In this review, based on our experience of fluid management in the critically ill patients, we propose a tailored fluid administration relying on the individual benefit to risk balance, as a part of a global goal-directed hemodynamic strategy.
INTRODUCTION
The incidence of acute pancreatitis (AP), currently ranging from 13 to 45/100000 per year, increases steadily[1], making AP the first gastro-intestinal cause of hospitalization in the United States. Persistent organ failure occurring in the first few days is the main determinant of severity and defines severe AP[2]. Despite early management, in-hospital mortality of these patients, around 30%, remains high[3].
Due to numerous mechanisms, hypovolemia is a well-recognized risk factor of poor outcome in patients with AP[4]. During severe AP, an uncontrolled inflammatory response alters endothelial functions leading to vasodilation, capillary leakage and edema. Together with vomiting, ascite or ileus, this vascular dysfunction promotes hypovolemia and acute circulatory failure. Circulatory dysfunction leads to tissue hypoperfusion, ischemia and subsequently to self-sustaining disease with persistent pancreatic injury, extra-pancreatic tissue damage and organ failures[5].
Despite better knowledge of its pathophysiology[6,7], treatment of AP remains mostly supportive[8]. Rapid fluid perfusions, so called fluid loading or volume expansion are a cornerstone of AP management. Fluid loading allows rapid correction of hypovolemia, and efficient prevention of circulatory dysfunction[9]. Nevertheless, if appropriate fluid resuscitation prevents worsening of pancreas injury and development of organ failures, it may lead to poor outcome when excessive or insufficient[10-14]. Because of potential adverse effects, fluid resuscitation should therefore be cautiously administered in accordance with relevant evidence.
OPTIMIZING FLUID RESUSCITATION IN ACUTE PANCREATITIS: WHAT IS RECOMMENDED? WHAT IS CURRENTLY DONE?
When taking care of patients suffering from AP, it is strongly recommended to immediately assess hemodynamic status and begin resuscitative measures[15]. Early and aggressive fluid resuscitation is usually recommended and seems to reduce morbidity and mortality[1,15-19].
Early resuscitation refers mostly to fluid loading within the first 24 h of management[2,9,20]. Aggressive resuscitation is a liberal strategy of fluid administration to reach predetermined endpoints. In the latest guidelines, aggressive fluid therapy is defined as the administration of 250-500 mL per hour to all patients, except for those suffering from cardiovascular, renal and other comorbid conditions. Moreover, in case of suspicion of severe volume depletion, additional fluids are recommended. Proposed endpoints for guiding fluid therapy are mostly based on clinical parameters [arterial blood pressure, heart rate (HR) and urinary output (UO)], blood urea nitrogen (BUN)[3,15], hematocrit changes at 12-24 h after admission, and optionally central venous pressure (CVP)[4,9,21]. Finally, based on these endpoints, reassessment of fluid requirement is advised every 6 h within the first 24 to 48 h.
Nevertheless, there is poor consistent evidence to support such fluid strategy[5]. Recommendations are based on moderate levels of evidence, since studies are mostly observational with conflicting results[6,9]. As a result, current practice shows great heterogeneity, with various attitudes regarding fluid administration and chosen endpoints. In a recent New Zealand survey, physicians declared using aggressive fluid therapy in AP with organ failure. More than 70% of physicians estimated giving more than 4 L of fluids in patients with severe AP during the first 24 h after hospital admission. In theory, fluid administration as recommended might lead to an amount of about 6-12 L of fluids during the first 24 h[7,9,15]. However, aggressive fluid therapy as routinely performed corresponds to an average of 4.5 L of fluid over the first 24 h[8,9], against 3.5 L for non-aggressive therapy. In the same survey, fluid loading was mostly guided by UO, HR, blood pressure, hematocrit, BUN and lactate, even if the latter is not mentioned in the recommendations.
This explains the current controversy in the literature about necessary fluid volume, adequate timing and endpoints to achieve[5,8,9]. Moreover, some studies rather support restrictive strategies and report a positive impact on mortality[5,10,16]. Indeed, aggressive fluid loading may be detrimental, not only for patients suffering from AP[2,11,12,22] but more generally when any significant fluid therapy is needed[3,5-7,10,13-15,23-25]. The failure to clearly demonstrate the superiority of one fluid strategy over another may come from the great variability of individual response to volume expansion and the specific hemodynamic status of each patient at a given time. Consequently, aggressive therapy may be appropriate for some patients and deleterious for others.
New methods allowing better hemodynamic and fluid management have been developed over the last 15 years. These strategies aim to restore specific hemodynamic parameters with an individualized management named “early goal-directed therapy”, in which fluid expansion takes a major part. The first step of this method is to clearly determine the specific population to which it should be applied. The second step is to assess tissue perfusion and oxygenation goals to be achieved. The last step is to choose the appropriate therapy in order to reach these predetermined goals. Fluid management then becomes part of a global hemodynamic strategy that has proved to be valuable in high-risk surgical patients and severe sepsis[16,26]. Understanding how hemodynamic criteria can be used to guide fluid therapy in these patients would help improving care and research in the field of AP[1,17,27].
GLOBAL HEMODYNAMIC RESUSCITATION: THE EARLY GOAL-DIRECTED THERAPY
Early goal-directed therapy (EGDT) is an aggressive, time-sensitive and individualized approach of global hemodynamic management. It is started within the very first hours after admission, before the occurrence of persistent organ failure that it aims to prevent[13,27].
This strategy arises from the finding that early aggressive therapy in acute diseases such as stroke, trauma or acute myocardial infarction improves mortality and outcomes[27]. EGDT has been conceived for optimizing treatment when tissue oxygenation is impaired by hemodynamic failure. It is a multifaceted strategy aiming to adjust oxygen delivery to oxygen consumption[13,14]. The concept of a global hemodynamic strategy guided by oxygen transport variables was first proposed in 1983 for high-risk surgical patients[28]. EGDT as a time-sensitive method has been initially applied to patients suffering from severe sepsis and septic shock[27], then in all patients with elevated lactate level, regardless of etiology[13]. It also has been proposed for perioperative management of patients undergoing major surgery, like cardiovascular or gastro-intestinal surgeries[29-33]. In these populations, EGDT is a now widely performed strategy that reduces morbidity, mortality and healthcare resource consumption[26,27,34]. Although no human trial evaluated such strategy in AP, most patients suffering from AP share similar pathophysiology, risk factors and severity with patients in whom this approach has been studied. Thus, even though clinical studies are needed to allow transposition to AP, EGDT may be suitable for this severe disease in the course of which many rapid hemodynamic changes can happen[35,36].
Immediate identification on admission of patients requiring EGDT based on the evaluation of the patient severity and potential outcome constitutes the very first step of the strategy. In severe sepsis and septic shock, EGDT is performed when patients present persistent hypotension with systolic blood pressure < 90 mmHg after a volume expansion of 20-30 mL/kg over a 30-min period or hyperlactatemia > 4 mmol/L[14]. In their study, Jansen et al[13] performed EGDT for every patient with lactatemia > 3 mmol/L on admission to the ICU. When included, patients were stratified into four groups: sepsis, neurologic, cardiac arrest and other nonsepsis, which accounted for 38% of the inclusions. Even though the authors did not mention whether some AP were included, these patients frequently meet these inclusion criteria.
Twenty percent of patients will develop moderately severe to severe AP[37], characterized by the presence of either local or systemic complication, or organ failures. The resolution of organ failures in the first two days defines moderately severe AP. This group has prolonged hospitalizations and requires ICU care in 50% of cases, but maintains a mortality rate similar to the mild AP group[38]. Persistent organ failure is the main determinant of severity in AP and defines severe AP. Eighty percent of patients with severe AP will stay in the ICU. As patients with severe AP are at high risk of poor outcome, patients with high risk of severe AP would be considered at risk of poor outcome too. Despite the lack of reliable markers for early prediction of AP severity, several indices have been proposed[15]. Thus, along with refractory hypotension and elevated lactatemia, established risk factors for severe AP might be good candidates for early detection of patients at risk of poor outcome (Table 1). Nevertheless, further studies are needed to determine the most suitable parameters for early identification of at risk-patients in whom EGDT would be needed in this setting.
Table 1.
Diagnostic criteria for acute pancreatitis with high risk of poor outcome
| Criteria for high risk of poor outcome | Hospitalization setting | Organ or system dysfunction |
| Severe AP: Persistent organ or system dysfunction (> 48 h) | Intensive care | Cardio-vascular: SAP < 90 mmHg despite 20-30 mL/kg fluid loading Respiratory: PaO2 < 60 mmHg |
| Risk factors for severe AP: Organ or system dysfunction (< 48 h) Lactate > 3 mmol/L Persistent SIRS1 (> 24 h) Pancreatic necrosis Pleural effusion or pulmonary infiltrates BUN > 20 mg/dL or rising BUN Hematocrit > 40% or rising hematocrit Age > 55 yr or comorbid disease or obesity | Intermediate or intensive care | Renal: Creatinine ≥ 2 mg/dL or UO < 0.5 mL/kg of body weight/h for 1 h, despite 20-30 mL/kg fluid loading Hematological: Platelet count < 80000/mm3 or decrease > 50% of initial platelet count Metabolic: pH ≤ 7.30 or base deficit ≥ 5.0 mmol/L in association with lactate > 3 mmol/L Gastro-intestinal: Gastro-intestinal bleeding (> 500 mL/24 h) Neurological: Altered mental status |
SIRS is defined by the presence of ≥ 3 of the following criteria: Pulse > 90 beats/min, Respirations > 20/min or PaCO2 < 32 mmHg, Temperature > 38 °C or < 36 °C, WBC count > 12000 or < 4000 cells /mm3 or > 10% immature neutrophils. AP: Acute pancreatitis; BUN: Blood urea nitrogen; PaCO2: Partial pressure of carbon dioxide in arterial blood; PaO2: Partial pressure of oxygen in arterial blood; SAP: Systolic arterial pressure; SIRS: Systemic inflammatory response syndrome; UO: Urinary output; WBC: White blood cell.
For those pre-selected patients, optimization of parameters reflecting tissue perfusion and oxygenation remains the major goal to achieve during severe sepsis and high-risk surgery. Thus, essential determinants or estimates of oxygen delivery are assessed step by step and corrected if needed.
In order to monitor and optimize microcirculatory function, HR and mean arterial pressure (MAP) are mainly used. As tachycardia remains a clinical sign of circulatory failure therapeutic strategy aims to lower HR under 100 beats/min. MAP, reflecting effective organ perfusion pressure, has to be maintained above 65 mmHg[27].
Microcirculatory function, finally ensuring tissue perfusion, can be estimated by lactate level and UO[27,39,40]. Lactate level increases when aerobic cellular respiration is impaired and switched towards anaerobic metabolism. UO, in roughly reflecting glomerular perfusion, provides valuable information on general tissue perfusion. Both are good clues to evaluate tissue perfusion even if not entirely specific. For instance, lactate levels can possibly increase in rare metabolic diseases or when liver failure occurs. UO can be altered during organic renal failure, independently of hemodynamic disorders[41]. Similarly, mottling score, reflecting skin hypoperfusion can also be helpful to estimate global tissue perfusion[42,43]. EGDT aims to normalize lactate level and Jansen and al. targeted a 20%-decrease every two hours[13]. Therapeutic intervention also aims to maintain UO over 0.5 mL/kg per hour and make mottling disappear.
The balance between oxygen delivery (DO2) and systemic oxygen consumption (VO2) is approached by measurement of central venous oxygen saturation (ScvO2). Its measurement can be easily performed on a blood sample taken from a central venous catheter inserted in the superior vena cava territory. SvO2 depends on global oxygen transport and tissue oxygen extraction and consumption as can be seen in the modified Fick equation: SvO2 ≈ SaO2 - [VO2 /(CO × Hb × 1.34)] where SaO2 represents arterial oxygen saturation, CO cardiac output and Hb hemoglobin[44]. Each parameter described previously should be optimized to reach an ScVO2 level > 70%, associated with a normal lactate level. Importantly, when ScVO2 is superior to 70% but lactate level remains high, the presence of microcirculatory dysfunction with oxygen extraction impairment leading to persistent tissue hypoxia despite adequate oxygen transport should be suspected.
To carry out this step-by-step strategy, patients should be closely monitored. Together with standard monitoring of vital signs, specific devices including central venous catheters and urinary catheters have to be implemented when patients meet severity criteria. EGDT is then implemented during the first 6-8 h of the patient’s management. Previously described endpoints should be closely and regularly checked to assess treatment efficiency. For instance, Rivers et al[14] checked endpoints every 30 min. Jansen et al[13] measured blood lactate level together with other chosen endpoints every two hours.
Global hemodynamic goals are achieved by numerous treatments (e.g., fluids, red blood cell transfusion, oxygen, ventilation, analgesics, sedatives, antipyretics, vasoconstrictors, vasodilators and cardiac treatments) depending on the presence of hypovolemia, anemia, low SaO2, vasoplegia and cardiac dysfunction. In this global approach, fluid therapy plays an early and major role (Figure 1). A rigorous management of fluid loading is essential to succeed in reaching endpoints and requires simple but adequate guiding tools.
Figure 1.
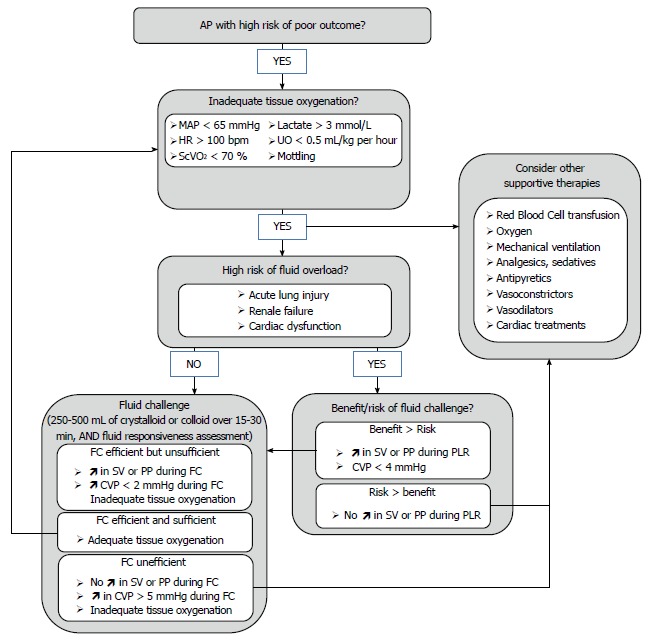
Suggested algorithm for fluid management in acute pancreatitis. AP: Acute pancreatitis; MAP: Mean arterial pressure; HR: Heart rate; ScvO2: Central venous oxygen saturation; UO: Urinary output; SV: Stroke volume; PP: Arterial pulse pressure; PLR: Passive leg raising; CVP: Central venous pressure; FC: Fluid challenge.
MANAGEMENT OF FLUID RESUSCITATION: A CORNERSTONE OF THE EARLY GOAL-DIRECTED THERAPY
The clinician’s major concerns are to assess for each fluid prescription whether fluid infusion would improve the patient’s hemodynamics and organ perfusion, with minimal risk of adverse effect. Three situations can be encountered. The first one is a patient with undisputed need for volume expansion, presenting obvious hypovolemia with a clearly identified etiology. For instance patients with severe AP or sepsis at the very beginning of the treatment are very likely hypovolemic and usually receive 20-30 mL/kg of fluids within the first 60-90 min. In this case, the benefit to risk balance is obvious. The second situation is obvious fluid overload such as a patient with congestive heart failure and acute pulmonary edema for whom volume expansion would clearly be deleterious. The last situation concerns patients with hemodynamic impairment for whom volume expansion represents a major therapeutic option, but with uncertain benefit to risk balance. This remains the most frequently encountered case for which specific tools have been created. Indeed, when only based on clinical parameters (e.g., mottling, HR, blood pressure or UO), barely one half of the critically ill patients will respond positively to fluid loading[45]. Because of the potential adverse effects of inappropriate fluid perfusions[10-14,46], tools intended to assess and predict the effects of fluid loading may be helpful to guide fluid therapy and improve patients outcome.
When applied in practice, EGDT leads to differences in patients’ fluid management. Rivers and al. found that a greater amount of fluid was given to the EGDT group compared with the standard group in the first 6 h (4981 mL vs 3499 mL; P < 0.001), even though the total amount of fluid over the first 72 h was similar (13443 mL vs 13358 mL; P = 0.73). As a result, a 30% decrease in hematocrit associated with a larger amount of transfusion of red blood cells was observed in the EGDT group compared with standard care in the first 6 h[27]. As the beneficial effect of this strategy is based on the adaptation of hemodynamic management on tissue oxygenation, there is still a lack of evidence concerning the best tools to use for guiding fluid resuscitation.
Assessing fluid responsiveness: fluid challenge
Fluid challenge (FC) intends to assess a patient’s fluid responsiveness during a volume expansion test. First described by Weil and Henning[47], FC is a titrated administration of 50-200 mL of fluid over a 10 min interval, with a concomitant close monitoring of patient’s cardiovascular response. Fluid responsiveness is defined by a fluid-induced increase in stroke volume (SV), or in CO as the product of SV by HR. A positive response is considered when fluid loading leads to an increase in SV ≥ 10%-15%[45]. Indeed, if optimization of systemic hemodynamics and tissue perfusion remains the ultimate goal of fluid therapy, increase in SV is considered as a prerequisite to achieve it[48]. FC is the reference standard method to distinguish responders from non-responders to fluid loading[34]. Current international guidelines recommend 250-1000 mL of crystalloids or 250-500 mL of colloids over 15-30 min, repeated after reassessment until endpoints are achieved[26,34,49].
When performed in anesthesiology, where invasive monitoring techniques such as trans-esophageal Doppler, esophageal echocardiography or thermodilution enable continuous assessment of CO, fluid infusion is continued as long as CO increases[50,51]. However, continuous CO measurement is often not available for non-surgical patients. In that case, noninvasive measurement of SV before and after FC with transthoracic echocardiography is a relevant parameter to estimate fluid responsiveness[52].
If SV monitoring cannot be performed, blood pressure derived indexes may help to predict fluid responsiveness. Indeed, fluid-induced changes in arterial pulse pressure (PP) are correlated to some extent to changes in SV[53,54]. Monnet et al[54] found that a fluid-induced increase in invasive PP over 17% attested of fluid responsiveness with a sensitivity of 65% and a specificity of 85%. Lakhal et al[53] showed that an increase beyond 23% for invasive PP, or 35% for noninvasive PP reliably predicted fluid responsiveness. On the opposite, fluid responsiveness was unlikely under 5% of PP change. Nonetheless, the large range of inconclusive results (i.e., 5%-17% of changes in PP) represents a major limit of this method.
In parallel, dynamic analysis of CVP can be monitored as an indicator of safety limits[13,47,55]. CVP is commonly used as an estimation of cardiac preload at the bedside. Preload is defined as the load in cardiac chambers present before isovolumetric ventricular contraction has started. It represents the stress exerted on ventricular walls in end diastole. Venous return is a major determinant of preload and is mostly dependent on volemia. Thus, hypovolemia decreases preload whereas volume expansion increases it. Described by Frank and Starling, there is up to a certain limit a positive relationship between end-diastolic ventricular load and systolic SV, called preload-dependence[45]. In that case, fluid administration leads to a large increase in SV while CVP remains stable or presents only a minimal increase. Preload-dependence is thus associated with a positive response to volume expansion. However, beyond a certain individual threshold, an increase in preload does not increase SV anymore, which corresponds to a preload-independence state. For those patients, fluid administration leads to poor SV improvement but consistent increase in CVP with high risk of fluid overload (Figure 2). Subsequently, volume expansion-induced changes in CVP have been proposed as a safety limit of FC[47,55]. As long as changes in CVP remain below 2 mmHg FC is continued until hemodynamic endpoints are fulfilled. For an increase in CVP ranging from 2-5 mmHg, fluid infusion should be stopped for a while then restarted. Over a 5 mmHg increase, FC should be stopped. The time interval to assess filling pressures and fluid responsiveness was every 10 min in the initial description. However, with the availability of continuous vital signs monitoring, the intervals may be extended to 30 min.
Figure 2.
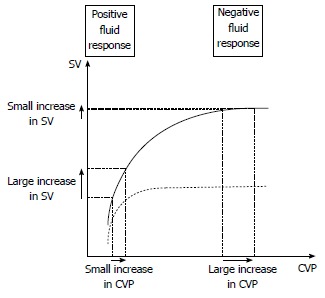
Schematic representation of central venous pressure/stroke volume of normal (solid line) and failing heart (dotted line). When the heart is fluid responsive, a fluid challenge induces a large increase in stroke volume (SV) and a small increase in central venous pressure (CVP). When the heart is fluid unresponsive, a fluid challenge induces a small increase in SV and a large increase in CVP. In contrast, there is no reliable threshold of CVP that can be used in current practice to predict a positive or negative response to fluid loading. This threshold depends mostly on the cardiac function at the time of fluid infusion.
FC allows a prompt correction of fluid deficit, with a shorter duration of hypovolemia and organ hypoperfusion, compared with a protracted fluid infusion strategy over 12 h or more[55]. FC only requires a central venous catheter to control safety limits, together with conventional monitoring of vital signs and CO if available (Figure 1). Nevertheless, this strategy, although approved by experts and routinely used in intensive care has never been confirmed by a prospective controlled trial[55]. In addition, despite close monitoring, the effect of fluid infusion is retrospectively assessed, and the repetition of FC might lead to fluid overload. Such risk remains a major concern for patients with AP, as they present an increased risk of acute lung injury[56]. Therefore, fluid responsiveness should ideally be estimated before fluid is administered to avoid ineffective or deleterious fluid administration for patients with unclear benefit to risk balance, such as those who develop pulmonary, cardiac or renal dysfunction[11,12,57]. New parameters aiming to predict fluid responsiveness have been developed to this end.
Predicting fluid-responsiveness: preload and preload-dependence
The ultimate goal of tools aiming to predict fluid-responsiveness is to find where individual ventricular hemodynamic status is located on the Franck-Starling curve (Figure 2). In other terms, indexes predicting fluid responsiveness are assessing cardiac preload-dependence[58].
Based on aforementioned physiological concepts, one could postulate that low preload values are more likely to be associated with preload-dependence and conversely for high preload values. However, several studies show that this assertion is not true. When CVP or pulmonary artery occlusion pressure (PAOP) are used as estimates of cardiac preload, they usually fail to predict fluid responsiveness[45,59]. This can easily be understood because Franck-Starling curve is specific to each patient[45]. Thus, there is no way to know whether a single absolute CVP or PAOP level corresponds to a preload-dependence or -independence zone[60] (Figure 2). Even for extreme values of CVP or PAOP, there is no reliable threshold that can be used in current practice to predict a positive or negative response to volume expansion[45,59]. However, preload evaluation, and particularly CVP measurements are still recommended in hemodynamic management algorithms for several reasons[34]. First, it is easy to assess, only requiring a central venous catheter. Second, as detailed above dynamic analysis of CVP is still valuable in evaluating FC response. Eventually, CVP values standing below 4 mmHg, even if not predictive of fluid responsiveness, ensure safe fluid loading with little risk of overfilling[34,61] (Figure 1).
Consequently, indexes predicting fluid-responsiveness focus on preload-dependence rather than preload assessment[58]. Passive leg raising (PLR) maneuver is an easy maneuver that mimics volume expansion by shifting venous blood from the lower limbs and the splanchnic vessels toward the intrathoracic vessels[62]. Thus, PLR leads to a rapid and reversible increase in cardiac preload and subsequently in SV in case of preload dependence. To be efficient, PLR maneuver has to be performed as follows[63]: the patient’s baseline position is lying down on a bed, half-sitting in semirecumbent position, with a 45° angle between trunk and lower limbs, which are horizontal. Then, a 45° bascule of the bed should be done, so that the trunk becomes horizontal and the lower limbs rise up. Impact of the maneuver appears within the first minute, while the hemodynamic measurements are recorded. PLR mimics an approximate 300-450 mL FC[63,64]. A close correlation is observed between changes in SV measured with TTE or esophageal Doppler, after PLR and after a 500 mL of fluid loading in critically ill patients with sepsis or acute pancreatitis[64-67]. When considering a recent meta-analysis enrolling 9 clinical studies that evaluated the accuracy of PLR to predict fluid responsiveness, a PLR-induced change in SV superior to 8%-15% predicted fluid responsiveness with a sensitivity of 89% and specificity of 91%[68]. When considering PP as a surrogate of SV, a PLR-induced change in PP > 9%-12% predicts fluid responsiveness with sensitivity of 60% and a specificity of 86%[68] (Figure 1). The main limit of the PLR technique is the presence of intra-abdominal hypertension. Indeed, in ventilated critically ill patients, Mahjoub et al[69] showed that PLR failed to predict fluid responsiveness when intra-abdominal pressure exceeded 16 mmHg due to false negatives. As demonstrated by Kitano et al[70], Takata et al[71] when intra-abdominal pressure exceeds right atrial pressure, the inferior vena cava collapses and impairs venous return. The PLR-induced change in cardiac preload is decreased making the PLR maneuver inefficient[69-71]. As intra-abdominal hypertension is a common complication of AP, intra-abdominal pressure should be measured before using PLR.
Other indexes based on heart-lung interactions have also been developed. However, they are only validated for mechanically ventilated patients under strict conditions of sedation, ventilation and cardiac rhythm[72]. Because the proportion of patients requiring mechanical ventilation during AP remains very low, with specific multidisciplinary management in intensive care[56], these parameters are not discussed in this review. In spontaneously breathing patients, respiratory variations in inferior vena cava diameter or PP are still in development[73-75]. As existing data were not confirmed in large population studies, and as most of them didn’t include patients with AP, the use of such parameters in spontaneously breathing patients with AP seems hazardous and yet to be validated.
The impact of fluid therapy based on preload-dependence parameters has been evaluated in studies involving surgical patients[76-78]. When compared with liberal or preload-based fluid administration, the use of preload-dependence parameters drives to a decrease in lactate level, perioperative complications and time to discharge. Interestingly, this strategy leads either to a greater[77,78] or to a lesser[76] amount of fluid compared with the control group. These results suggest that the efficiency of such strategy comes from volume expansion adjustment to patient’s needs rather than from the total amount of fluid administered. Patients involved in these studies were mechanically ventilated and no similar trial exists in spontaneously breathing patients. Moreover, there is a great lack of data in patients with AP. Nevertheless, a recent study performed on anesthetized pigs with experimental AP compared a fluid therapy based on preload-dependence indexes to a CVP-based strategy. The fluid therapy guided by preload-dependence parameters increased survival (29.4% vs 11.8%; P < 0.05) by preventing microcirculation dysfunction, pancreatic damages and pulmonary edema. These results are concordant with human findings described just before, and confirm the inability of CVP to guide fluid therapy[79]. These encouraging data might open the way to further research in humans with AP.
CONCLUSION
Adopting an individualized early goal-directed strategy seems very promising to optimize fluid resuscitation in patients with AP. However, since AP has specific pathophysiology, evolution, complications and outcome, further studies are required to provide a suitable algorithm. The first step will be to define parameters allowing early identification of patients needing EGDT, notably those at risk to develop severe or necrotizing AP. Among parameters previously described in the literature, elevated lactate level and refractory hypotension could be good candidates. The second step is to clearly define ultimate goals of hemodynamic resuscitation reflecting tissue perfusion and oxygenation. If those are not achieved, EGDT should immediately be implemented and carried on until adequate systemic perfusion is restored. Close reassessment of initial endpoints has to be performed every 30 min to readjust treatment without delay. Because volume expansion plays a major role in this strategy, fluids should be administered early. Inadequate fluid replacement can occur when guided on clinical parameters alone, static preload assessment with CVP or worse, blindly. A safe and practical way to perform fluid loading remains FC, with simultaneous assessment of fluid-responsiveness and control for risk of overload. However, for patients with high risk of fluid overload, predicting fluid-responsiveness before volume expansion may reduce the number of FC and improve patient outcomes. PLR is an accurate validated maneuver to predict fluid-responsiveness. It can widely be used provided the absence of intra-abdominal hypertension. These considerations open the way to a wide range of clinical studies aiming to adapt and validate such strategies in the specific population of patients with AP.
Footnotes
P- Reviewer: Capolongo G, Pellicano R S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 3.Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Brown A, Baillargeon JD, Hughes MD, Banks PA. Can fluid resuscitation prevent pancreatic necrosis in severe acute pancreatitis? Pancreatology. 2002;2:104–107. doi: 10.1159/000055899. [DOI] [PubMed] [Google Scholar]
- 5.Trikudanathan G, Navaneethan U, Vege SS. Current controversies in fluid resuscitation in acute pancreatitis: a systematic review. Pancreas. 2012;41:827–834. doi: 10.1097/MPA.0b013e31824c1598. [DOI] [PubMed] [Google Scholar]
- 6.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 7.Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518–530. doi: 10.1002/bjs.5316. [DOI] [PubMed] [Google Scholar]
- 8.McKay CJ, Imrie CW. The continuing challenge of early mortality in acute pancreatitis. Br J Surg. 2004;91:1243–1244. doi: 10.1002/bjs.4750. [DOI] [PubMed] [Google Scholar]
- 9.Haydock MD, Mittal A, Wilms HR, Phillips A, Petrov MS, Windsor JA. Fluid therapy in acute pancreatitis: anybody’s guess. Ann Surg. 2013;257:182–188. doi: 10.1097/SLA.0b013e31827773ff. [DOI] [PubMed] [Google Scholar]
- 10.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 11.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 13.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 14.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 15.Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–1416. doi: 10.1038/ajg.2013.218. [DOI] [PubMed] [Google Scholar]
- 16.Warndorf MG, Kurtzman JT, Bartel MJ, Cox M, Mackenzie T, Robinson S, Burchard PR, Gordon SR, Gardner TB. Early fluid resuscitation reduces morbidity among patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:705–709. doi: 10.1016/j.cgh.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wall I, Badalov N, Baradarian R, Iswara K, Li JJ, Tenner S. Decreased mortality in acute pancreatitis related to early aggressive hydration. Pancreas. 2011;40:547–550. doi: 10.1097/MPA.0b013e318215368d. [DOI] [PubMed] [Google Scholar]
- 18.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, Thiny MT, Stizenberg K, Morgan DR, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187.e1-e3. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baillargeon JD, Orav J, Ramagopal V, Tenner SM, Banks PA. Hemoconcentration as an early risk factor for necrotizing pancreatitis. Am J Gastroenterol. 1998;93:2130–2134. doi: 10.1111/j.1572-0241.1998.00608.x. [DOI] [PubMed] [Google Scholar]
- 20.Solanki NS, Barreto SG. Fluid therapy in acute pancreatitis. A systematic review of literature. JOP. 2011;12:205–208. [PubMed] [Google Scholar]
- 21.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–2400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 22.Mao EQ, Fei J, Peng YB, Huang J, Tang YQ, Zhang SD. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin Med J (Engl) 2010;123:1639–1644. [PubMed] [Google Scholar]
- 23.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 24.Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6:107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 25.Silva JM, de Oliveira AM, Nogueira FA, Vianna PM, Pereira Filho MC, Dias LF, Maia VP, Neucamp Cde S, Amendola CP, Carmona MJ, et al. The effect of excess fluid balance on the mortality rate of surgical patients: a multicenter prospective study. Crit Care. 2013;17:R288. doi: 10.1186/cc13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivers EP, Coba V, Whitmill M. Early goal-directed therapy in severe sepsis and septic shock: a contemporary review of the literature. Curr Opin Anaesthesiol. 2008;21:128–140. doi: 10.1097/ACO.0b013e3282f4db7a. [DOI] [PubMed] [Google Scholar]
- 28.Shoemaker WC, Appel P, Bland R. Use of physiologic monitoring to predict outcome and to assist in clinical decisions in critically ill postoperative patients. Am J Surg. 1983;146:43–50. doi: 10.1016/0002-9610(83)90257-x. [DOI] [PubMed] [Google Scholar]
- 29.Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103:637–646. doi: 10.1093/bja/aep279. [DOI] [PubMed] [Google Scholar]
- 30.Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445] Crit Care. 2005;9:R687–R693. doi: 10.1186/cc3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arulkumaran N, Corredor C, Hamilton MA, Ball J, Grounds RM, Rhodes A, Cecconi M. Cardiac complications associated with goal-directed therapy in high-risk surgical patients: a meta-analysis. Br J Anaesth. 2014;112:648–659. doi: 10.1093/bja/aet466. [DOI] [PubMed] [Google Scholar]
- 32.Aya HD, Cecconi M, Hamilton M, Rhodes A. Goal-directed therapy in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth. 2013;110:510–517. doi: 10.1093/bja/aet020. [DOI] [PubMed] [Google Scholar]
- 33.Yadav D, Garg PK. Spectrum of perforation peritonitis in delhi: 77 cases experience. Indian J Surg. 2013;75:133–137. doi: 10.1007/s12262-012-0609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonelli M, Levy M, Andrews PJ, Chastre J, Hudson LD, Manthous C, Meduri GU, Moreno RP, Putensen C, Stewart T, et al. Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007;33:575–590. doi: 10.1007/s00134-007-0531-4. [DOI] [PubMed] [Google Scholar]
- 35.Buter A, Imrie CW, Carter CR, Evans S, McKay CJ. Dynamic nature of early organ dysfunction determines outcome in acute pancreatitis. Br J Surg. 2002;89:298–302. doi: 10.1046/j.0007-1323.2001.02025.x. [DOI] [PubMed] [Google Scholar]
- 36.de-Madaria E, Martínez J, Pérez-Mateo M. The dynamic nature of fluid resuscitation in acute pancreatitis. Clin Gastroenterol Hepatol. 2012;10:95–6; author reply 96. doi: 10.1016/j.cgh.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Tonsi AF, Bacchion M, Crippa S, Malleo G, Bassi C. Acute pancreatitis at the beginning of the 21st century: the state of the art. World J Gastroenterol. 2009;15:2945–2959. doi: 10.3748/wjg.15.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vege SS, Gardner TB, Chari ST, Munukuti P, Pearson RK, Clain JE, Petersen BT, Baron TH, Farnell MB, Sarr MG. Low mortality and high morbidity in severe acute pancreatitis without organ failure: a case for revising the Atlanta classification to include “moderately severe acute pancreatitis”. Am J Gastroenterol. 2009;104:710–715. doi: 10.1038/ajg.2008.77. [DOI] [PubMed] [Google Scholar]
- 39.Mégarbane B. Severe lactic acidosis except for shock states. Réanimation. 2013;22:435–445. [Google Scholar]
- 40.Hernandez G, Boerma EC, Dubin A, Bruhn A, Koopmans M, Edul VK, Ruiz C, Castro R, Pozo MO, Pedreros C, et al. Severe abnormalities in microvascular perfused vessel density are associated to organ dysfunctions and mortality and can be predicted by hyperlactatemia and norepinephrine requirements in septic shock patients. J Crit Care. 2013;28:538.e9–538.14. doi: 10.1016/j.jcrc.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88:1127–1140. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ait-Oufella H, Bourcier S, Alves M, Galbois A, Baudel JL, Margetis D, Bige N, Offenstadt G, Maury E, Guidet B. Alteration of skin perfusion in mottling area during septic shock. Ann Intensive Care. 2013;3:31. doi: 10.1186/2110-5820-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ait-Oufella H, Lemoinne S, Boelle PY, Galbois A, Baudel JL, Lemant J, Joffre J, Margetis D, Guidet B, Maury E, et al. Mottling score predicts survival in septic shock. Intensive Care Med. 2011;37:801–807. doi: 10.1007/s00134-011-2163-y. [DOI] [PubMed] [Google Scholar]
- 44.Teboul JL, Hamzaoui O, Monnet X. SvO2 to monitor resuscitation of septic patients: let’s just understand the basic physiology. Crit Care. 2011;15:1005. doi: 10.1186/cc10491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121:2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 46.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 47.Weil MH, Henning RJ. New concepts in the diagnosis and fluid treatment of circulatory shock. Thirteenth annual Becton, Dickinson and Company Oscar Schwidetsky Memorial Lecture. Anesth Analg. 1979;58:124–132. [PubMed] [Google Scholar]
- 48.Teboul JL, Asfar P, Bernardin G, Cariou A, Chemla D. Indicateurs du remplissage vasculaire au cours de l’insuffisance circulatoire. Réanimation. 2004;13:255–263. doi: 10.1016/j.annfar.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Pottecher T, Calvat S, Dupont H, Durand-Gasselin J, Gerbeaux P. Haemodynamic management of severe sepsis: recommendations of the French Intensive Care Societies (SFAR/SRLF) Consensus Conference, 13 October 2005, Paris, France. Crit Care. 2006;10:311. doi: 10.1186/cc4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vallet B, Blanloeil Y, Cholley B, Orliaguet G, Pierre S, Tavernier B. Guidelines for perioperative haemodynamic optimization. Ann Fr Anesth Reanim. 2013;32:e151–e158. doi: 10.1016/j.annfar.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Phan TD, Ismail H, Heriot AG, Ho KM. Improving perioperative outcomes: fluid optimization with the esophageal Doppler monitor, a metaanalysis and review. J Am Coll Surg. 2008;207:935–941. doi: 10.1016/j.jamcollsurg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Lewis JF, Kuo LC, Nelson JG, Limacher MC, Quinones MA. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation. 1984;70:425–431. doi: 10.1161/01.cir.70.3.425. [DOI] [PubMed] [Google Scholar]
- 53.Lakhal K, Ehrmann S, Perrotin D, Wolff M, Boulain T. Fluid challenge: tracking changes in cardiac output with blood pressure monitoring (invasive or non-invasive) Intensive Care Med. 2013;39:1953–1962. doi: 10.1007/s00134-013-3086-6. [DOI] [PubMed] [Google Scholar]
- 54.Monnet X, Letierce A, Hamzaoui O, Chemla D, Anguel N, Osman D, Richard C, Teboul JL. Arterial pressure allows monitoring the changes in cardiac output induced by volume expansion but not by norepinephrine. Crit Care Med. 2011;39:1394–1399. doi: 10.1097/CCM.0b013e31820edcf0. [DOI] [PubMed] [Google Scholar]
- 55.Vincent JL, Weil MH. Fluid challenge revisited. Crit Care Med. 2006;34:1333–1337. doi: 10.1097/01.CCM.0000214677.76535.A5. [DOI] [PubMed] [Google Scholar]
- 56.Mole DJ, Olabi B, Robinson V, Garden OJ, Parks RW. Incidence of individual organ dysfunction in fatal acute pancreatitis: analysis of 1024 death records. HPB (Oxford) 2009;11:166–170. doi: 10.1111/j.1477-2574.2009.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Charpentier J, Luyt CE, Fulla Y, Vinsonneau C, Cariou A, Grabar S, Dhainaut JF, Mira JP, Chiche JD. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32:660–665. doi: 10.1097/01.ccm.0000114827.93410.d8. [DOI] [PubMed] [Google Scholar]
- 58.Cherpanath TG, Geerts BF, Lagrand WK, Schultz MJ, Groeneveld AB. Basic concepts of fluid responsiveness. Neth Heart J. 2013;21:530–536. doi: 10.1007/s12471-013-0487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar A, Anel R, Bunnell E, Habet K, Zanotti S, Marshall S, Neumann A, Ali A, Cheang M, Kavinsky C, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32:691–699. doi: 10.1097/01.ccm.0000114996.68110.c9. [DOI] [PubMed] [Google Scholar]
- 60.Tavernier B, Makhotine O, Lebuffe G, Dupont J, Scherpereel P. Systolic pressure variation as a guide to fluid therapy in patients with sepsis-induced hypotension. Anesthesiology. 1998;89:1313–1321. doi: 10.1097/00000542-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 62.Rutlen DL, Wackers FJ, Zaret BL. Radionuclide assessment of peripheral intravascular capacity: a technique to measure intravascular volume changes in the capacitance circulation in man. Circulation. 1981;64:146–152. doi: 10.1161/01.cir.64.1.146. [DOI] [PubMed] [Google Scholar]
- 63.Jabot J, Teboul JL, Richard C, Monnet X. Passive leg raising for predicting fluid responsiveness: importance of the postural change. Intensive Care Med. 2009;35:85–90. doi: 10.1007/s00134-008-1293-3. [DOI] [PubMed] [Google Scholar]
- 64.Préau S, Saulnier F, Dewavrin F, Durocher A, Chagnon JL. Passive leg raising is predictive of fluid responsiveness in spontaneously breathing patients with severe sepsis or acute pancreatitis. Crit Care Med. 2010;38:819–825. doi: 10.1097/CCM.0b013e3181c8fe7a. [DOI] [PubMed] [Google Scholar]
- 65.Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, Teboul JL. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34:1402–1407. doi: 10.1097/01.CCM.0000215453.11735.06. [DOI] [PubMed] [Google Scholar]
- 66.Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33:1125–1132. doi: 10.1007/s00134-007-0646-7. [DOI] [PubMed] [Google Scholar]
- 67.Maizel J, Airapetian N, Lorne E, Tribouilloy C, Massy Z, Slama M. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med. 2007;33:1133–1138. doi: 10.1007/s00134-007-0642-y. [DOI] [PubMed] [Google Scholar]
- 68.Cavallaro F, Sandroni C, Marano C, La Torre G, Mannocci A, De Waure C, Bello G, Maviglia R, Antonelli M. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010;36:1475–1483. doi: 10.1007/s00134-010-1929-y. [DOI] [PubMed] [Google Scholar]
- 69.Mahjoub Y, Touzeau J, Airapetian N, Lorne E, Hijazi M, Zogheib E, Tinturier F, Slama M, Dupont H. The passive leg-raising maneuver cannot accurately predict fluid responsiveness in patients with intra-abdominal hypertension. Crit Care Med. 2010;38:1824–1829. doi: 10.1097/CCM.0b013e3181eb3c21. [DOI] [PubMed] [Google Scholar]
- 70.Kitano Y, Takata M, Sasaki N, Zhang Q, Yamamoto S, Miyasaka K. Influence of increased abdominal pressure on steady-state cardiac performance. J Appl Physiol (1985) 1999;86:1651–1656. doi: 10.1152/jappl.1999.86.5.1651. [DOI] [PubMed] [Google Scholar]
- 71.Takata M, Robotham JL. Effects of inspiratory diaphragmatic descent on inferior vena caval venous return. J Appl Physiol (1985) 1992;72:597–607. doi: 10.1152/jappl.1992.72.2.597. [DOI] [PubMed] [Google Scholar]
- 72.Marik PE, Lemson J. Fluid responsiveness: an evolution of our understanding. Br J Anaesth. 2014;112:617–620. doi: 10.1093/bja/aet590. [DOI] [PubMed] [Google Scholar]
- 73.Muller L, Bobbia X, Toumi M, Louart G, Molinari N, Ragonnet B, Quintard H, Leone M, Zoric L, Lefrant JY. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16:R188. doi: 10.1186/cc11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Préau S, Dewavrin F, Soland V, Bortolotti P, Colling D, Chagnon JL, Durocher A, Saulnier F. Hemodynamic changes during a deep inspiration maneuver predict fluid responsiveness in spontaneously breathing patients. Cardiol Res Pract. 2012;2012:191807. doi: 10.1155/2012/191807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monge García MI, Gil Cano A, Díaz Monrové JC. Arterial pressure changes during the Valsalva maneuver to predict fluid responsiveness in spontaneously breathing patients. Intensive Care Med. 2009;35:77–84. doi: 10.1007/s00134-008-1295-1. [DOI] [PubMed] [Google Scholar]
- 76.Forget P, Lois F, de Kock M. Goal-directed fluid management based on the pulse oximeter-derived pleth variability index reduces lactate levels and improves fluid management. Anesth Analg. 2010;111:910–914. doi: 10.1213/ANE.0b013e3181eb624f. [DOI] [PubMed] [Google Scholar]
- 77.Lopes MR, Oliveira MA, Pereira VO, Lemos IP, Auler JO, Michard F. Goal-directed fluid management based on pulse pressure variation monitoring during high-risk surgery: a pilot randomized controlled trial. Crit Care. 2007;11:R100. doi: 10.1186/cc6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, Pradl R, Stepan M. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14:R118. doi: 10.1186/cc9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trepte CJ, Bachmann KA, Stork JH, Friedheim TJ, Hinsch A, Goepfert MS, Mann O, Izbicki JR, Goetz AE, Reuter DA. The impact of early goal-directed fluid management on survival in an experimental model of severe acute pancreatitis. Intensive Care Med. 2013;39:717–726. doi: 10.1007/s00134-012-2775-x. [DOI] [PubMed] [Google Scholar]


