Abstract
AIM: To investigate the efficacy of lubiprostone compared to Senna on bowel symptoms and constipation in post-operative orthopedic patients treated with opioids.
METHODS: In this double blind, randomized, active comparator trial, adults who required opioids for analgesia following orthopedic procedures and who were admitted in inpatient rehabilitation were randomized following baseline assessments to lubiprostone (Amitza®), orally twice a day or Senna (generic) two capsules administered daily for six days. Subjects were assessed using the patient assessment of constipation (PAC)-symptoms (PAC-SYM) and the PAC-quality of life (PAC-QOL) scales measured at baseline and Day 7; Subjects were assessed daily for secondary measures included the Bristol stool scale bowel consistency, specific bowel symptom score (Nausea, cramping, straining, completeness, abdominal pain, time per lavatory attempt, assistance needed), adverse events and rescue medications required. Function was measured using the functional independence measure (FIM) at admission and discharge; length of stay (LOS) and missed treatments due to gastrointestinal symptoms were also assessed.
RESULTS: 64 adults were enrolled; 56 participants (28 in each group) had baseline and follow up measures and were included in the intention to treat (ITT) analyses. 43 participants completed the study, 21 in the active lubiprostone and 22 in the active Senna group. The mean age of the participants was 71.5 years (SD = 11.4 years, range: 28-96 years). In the ITT analyses, participants showed significant improvement in bowel symptoms as measured by the PAC-SYM (mean ± SD, -0.28 ± 0.60, range: -1-2.33) and PAC-QOL (mean ± SD, 0.33 ± 0.81, range: -1.5-2.0) over time, but there were no significant differences between the lubiprostone and Senna groups in mean change in the PAC-SYM (-0.20 ± 0.60 vs -0.36 ± 0.61, P = 0.61 respectively) or the PAC-QOL (0.29 ± 0.76 vs 0.37 ± 0.87, P = 0.61 respectively). The mean change in each bowel symptom also did not significantly differ between treatment groups on ITT analyses, except for completeness of bowel movement, with the Senna group showing greater negative mean change in bowel movement completeness (-0.56 ± 1.01 vs -2.00 ± 1.41, P = 0.03) and for reduction of abdominal pain, favoring Senna (-0.14 ± 0.73 vs -0.73 ± 1.08, P = 0.04). Fifteen (75%) participants in the lubiprostone and in the Senna group requested rescue treatments. Participants made significant functional improvement from admission to discharge over a median LOS of 12 d, with a mean FIM change of 29.13 ± 13.58 and no significant between group differences (27.0 ± 9.2 vs 31.5 ± 16.6, P = 0.27).
CONCLUSION: Both lubiprostone and Senna improved constipation-related symptoms and QOL in opioid-induced constipation, with no significant between-group differences.
Keywords: Constipation, Opioids, Lubiprostone, Senna, Orthopedics, Rehabilitation
Core tip: Constipation is frequent in postoperative orthopedic patients treated with opioids. Opioid side effects are associated with poorer pain control, and thus may limit the ability to mobilize patients immediately following orthopedic surgery. There is very limited information comparing the efficacy and safety of pharmacologic interventions for opioid-induced constipation. In this study we found that two medications, lubiprostone and Senna, were associated with improvement in bowel-related symptoms in opioid-induced constipation in post-operative orthopedic patients, with no differences found between the two medications. Rescue bowel medications were frequently required by participants in both groups, indicating that multiple laxative medications may be required for constipation symptom control in this setting.
INTRODUCTION
The use of opioid analgesics is often required to provide adequate pain relief following major orthopedic surgical procedures, however the use of this class of medications for pain control frequently results in symptoms of constipation, which may worsen patient distress[1]. Constipation symptoms have also been demonstrated to impact health-related quality of life[2-4]. In a meta-analysis of eleven trials using opioid analgesics in non-cancer patients, constipation was among the most common side effects described, and these symptoms were reported by 41% of patients[5].
Lubiprostone (Amitiza®) is a locally acting type-2 chloride channel activator that increases intestinal fluid and electrolyte secretion, and thus increases intestinal motility[6]. It has been shown to be efficacious for chronic constipation and constipation predominant irritable bowel syndrome. Lubiprostone was also recently approved for opioid-induced constipation in adults with chronic non-cancer pain[7,8]. Lubiprostone’s action to improve intestinal secretion may counter opioid activity on the μ-opioid receptors in the gastrointestinal tract. With the binding of opioid agonists to these μ-receptors, there is release of excitatory and inhibitory neurotransmitters, resulting in reduced intestinal mobility and mucosal secretions[9,10]. Research suggests lubiprostone may reverse morphine induced anti-secretory effects through its direct action on mucosal chloride channels[11,12]. In mice, lubiprostone has been found to increase circular small intestinal smooth muscles contraction[13].
Among patients requiring opioids for pain control, those managed postoperatively following elective orthopedic surgery often require both short and long acting opioids. While opioids are generally an effective method for pain control, they are associated with significant constipation specifically within the orthopedic population[14]. Opioid-related adverse effects have been noted in up to 54% of patients receiving opioids after orthopedic surgery, and those patients with constipation have a significantly longer postoperative length of stay in acute care[15]. Selected postoperative patients may require hospitalization in acute rehabilitation following orthopedic procedures due to significant functional loss and co-morbid conditions. During the period of rehabilitation, patients often continue opioid medications for pain control as functional mobility training is accelerated. Such individuals are already at heightened risk for chronic constipation, as constipation has been found to be associated with reduced functional status; constipation is also present with increased frequency in the frail elderly, a common group seen in acute rehabilitation[16,17].
The purpose of this study is to assess the efficacy of lubiprostone compared to standard care for the treatment of constipation in orthopedic patients receiving opioids for pain control during inpatient rehabilitation care. At the time of study initiation, Senna, a contact stimulant laxative that increases propulsive peristaltic activity of the colon through local effects on the mucosa, was the medication used for initial treatment of constipation at our institution and thus this drug was thus chosen as the comparator. The study was undertaken to compare the efficacy of lubiprostone compared to Senna on opioid-associated constipation symptoms and to assess the impact of these constipation interventions on rehabilitation outcomes in adults hospitalized following orthopedic surgery.
MATERIALS AND METHODS
Design
In this double blind, active comparator trial, participants were randomized to lubiprostone or Senna for six days, and bowel symptoms followed daily, with primary outcome assessments performed at baseline and following completion of the study drug intervention. This study was approved by the local Institutional Review Board, and potential participants were approached for participation after referral from their inpatient attending physicians. All participants provided written informed consent prior to study enrollment and randomization.
The study was funded by a grant from Takeda Pharmaceuticals North America, Inc. The study was registered at clinicaltrials.gov website, study number NCT00662363.
Study population
Participants were recruited from the inpatient population of an academic freestanding rehabilitation hospital following admission for impairments related to an orthopedic surgery. Qualifying participants were adults reporting constipation, 18 years old or greater who were able to provide informed consent. At baseline, opioids must have been received within the prior 24 h of hospitalization for pain control. The use of opioids for immediate post-op analgesia following orthopedic surgical procedures could be intravenous, intramuscular, transdermal or oral. For study enrollment, it was also required that the continued use of opioids was expected to be required for pain control treatment during rehabilitation. Opioid medications taken during rehabilitation were always administered by the transdermal or oral route.
Inclusion criteria: Additional inclusion criteria required for study participation were the following: anticipated duration of rehabilitation hospitalization of at least 7 d; Woman of childbearing potential were required to have a negative serum pregnancy test; at least one associated symptom of constipation needed to be present at the time of baseline assessment: lumpy or hard stools, feeling of incomplete evacuation of bowels, abdominal cramping or pain straining with movement of bowels or painful bowel movement effort and/or need for manual assistance to have a bowel movement.
Exclusion criteria: The following exclusion criteria were used for this study: known allergy or sensitivity to the study medications; current pregnancy, diarrhea on the day of enrollment; diagnosis of Clostridium difficile infection during the current hospitalization; pre-existing medical or neurologic condition or surgical procedures (other than their recent orthopedic procedure) which are known to commonly lead to bowel dysfunction such as, but not limited to Crohn’s disease, ulcerative colitis, multiple sclerosis, cerebral palsy, spinal cord injury, malabsorption syndrome, irritable bowel syndrome, abdominopelvic neoplasm (gastric, colon cancer), or severe liver disease. Subjects with a history of colon cancer and/or resection who developed persistent gastrointestinal symptoms following the resection were excluded while subjects with colonic or ileocolonic resection greater than 2 years from study enrollment and with no gastrointestinal problems following that procedure were included.
Predetermined criteria for removal: Subjects were interviewed daily while on the study treatments, including daily assessment of bowel movements and abdominal symptoms. Subjects who required rescue medications for three consecutive days or with severe abdominal symptoms as assessed by the investigators were also to be withdrawn.
Procedures
This study included an initial visit (Day 0) for screening and baseline data collection, daily follow-up visits (Days 1-6) while on study medications, and an exit visit (Day 7). Potential subjects were identified from a list of admitted patients with the rehabilitation diagnosis of orthopedic impairment. Subjects admitted to rehabilitation following orthopedic procedures were approached for participation after confirmation and approval from their primary admitting physician and identification regarding whether they were on opioid medications for pain control. At the baseline visit, subjects were screened for inclusion and exclusion criteria and pregnancy testing performed, if applicable. Demographic, medical and functional information was obtained and baseline assessments performed. Enrolled participants were taken off any scheduled bowel medications they were currently receiving to treat their constipation on the day of randomization. Subjects were randomized to one of two treatment groups (active Senna or lubiprostone with corresponding placebo) for Days 1-6 of the study. Subjects were interviewed daily for symptoms, and final assessments performed at Day 7.
Participants were randomized by pharmacy staff at Day 1 in a 1:1 ratio in blocks of 4 to either Senna (generic) or lubiprostone (Amitiza®, Takeda Pharmaceuticals). The drugs were administered as follows: lubiprostone 24 μg orally twice a day given with meals and placebo Senna capsules, two orally at noon, or two Senna tablets daily at noon and placebo lubiprostone capsules twice a day. Thus participants received either active lubiprostone with placebo Senna tabs or active Senna with placebo lubiprostone tabs. Lubiprostone and identically appearing lubiprostone placebo tablets were provided by Takeda. Senna tablets were over-encapsulated by the institution’s pharmacy staff and identically appearing Senna placebo tablets were also provided by the institution’s pharmacy. The study medication treatments were started in the morning after the baseline visit and administered for a total of six days. The physician investigators, the research assistants administering study questionnaires, nurses, and subjects were blinded to the treatment regimen.
Pill counts were performed daily and the subject’s medication administration record reviewed to assess compliance.
Outcome measures
Baseline assessments included bowel symptoms and a quality of life measure, the patient assessment of constipation (PAC), described below, as well as the following bowel symptoms: nausea, cramping, straining, completeness, bowel pain, time per lavatory attempt, assistance needed, number of unsuccessful attempts for bowel movement, number, and size of bowel movement. The consistency of bowel movements was rated on the Bristol stool scale.
Subjects were assessed daily while on the study medication and asked to rate bowel symptoms over the past 24 h. Daily opioid use (over 24 h) was recorded, as well as missed therapies due to bowel symptoms, adverse events, and rescue laxatives taken due to persistent bowel symptoms. On Day 7, subjects completed the bowel symptoms assessments and in addition they were reassessed using the PAC.
Subjects were also monitored daily for adverse events. Subjects requesting withdrawal from the study medication interventions were asked to continue completion of the study assessment measures and these findings were used in the intention to treat analysis. Subjects discharged before Day 7 completed the follow up measures on the day of discharge.
The predetermined main outcome measure was between group comparisons on the patient constipation questionnaire. Secondary endpoints were between group comparisons of spontaneous bowel movements, bowel symptoms, adverse events, need for rescue medications, missed therapies, length of stay and admission and discharge functional status.
Gastrointestinal
PAC: The PAC has previously been found to be a valid and reliable way to measure constipation symptoms and clinical course[18]. The PAC has both a symptom (SYM) component, composed of 12 items and a quality of life (QOL) component consisting of five items. The PAC-SYM questionnaire has shown good concurrent and clinical validity for opioid-induced constipation in a number of pain populations and has demonstrated responsiveness to treatment. There are three symptom domains within the PAC-SYM: Abdominal symptoms (4 items), rectal symptoms (3 items) and stool symptoms (5 items). This questionnaire was completed by the subjects at baseline and Day 7 of the trial. The PAC-SYM is a symptom scale where higher numbers indicate more symptoms. Change from baseline to 7 d was calculated and larger negative differences indicated greater improvement in constipation symptoms. The PAC-QOL is a quality of life scale where higher numbers indicate better quality of life. Change from baseline to 7 d was calculated and a larger positive difference indicated greater improvement.
Constipation symptoms[19]: The following symptoms were rated (as over the past 24 h) on a 5 point scale from 0 (never) to 4 (always) at baseline (Day 0), daily during study medication administration (Days 1-6) and Day 7: nausea, cramping, straining, completeness, and abdominal pain. The number of bowel movements in the past 24 h was also recorded.
Time per lavatory attempt: This was rated by number of minutes per attempted bowel movement in the past 24 h on a scale from 0 (less than 5 min) to 4 (more than 30 min). Ratings were performed at baseline and Days 1-7.
Assistance needed: The amount of assistance required for a bowel movement in the past 24 h was rated on a three point scale: 0 (without assistance), 1 (with laxatives), or 2 (use of finger or enema to have a bowel movement). The level of assistance was rated at baseline and Days 1-7.
Number of unsuccessful attempts for bowel movement: This was rated over the past 24 h on a five point scale: 0 (never), 1 (1-3 times) 2 (3-6 times), 3 (6-9 times) and 4 (more than 9 times). Ratings were performed at baseline and Days 1-7.
Bristol stool scale[20]: Bowel movements were assess daily and rated using this scale. This scale rates the stool form by 7 types, where Type 1 describes separate hard lumpy stool to Type 7 (watery stool with no solid pieces). This was rated at baseline and Days 1-7.
Rehabilitation measures
Functional independence measure[21]: The functional independence measure (FIM) is a standard rehabilitation scale that measures 18 items in two domains, motor and cognition. Each domain is rated on a seven-point scale where 1 indicates complete dependence for the activity and 7 complete independence without physical or equipment assistance. This was measured at admission and discharge from rehabilitation. The bowel sub-score of the FIM was separately evaluated. FIM efficiency was calculated by taking the FIM change over the rehabilitation stay divided by the length of the rehabilitation admission.
Length of stay: This was measured in days and calculated from admission to discharge from rehabilitation.
Missed therapies: Any physical therapy, occupational therapy, or speech therapy visits missed during days one through six of the study due to medical symptoms was recorded; sessions missed due to bowel symptoms were separately described.
Statistical analysis
Based on the power analysis, a sample size of n = 29 was determined per group. Using this sample size, it was calculated that the study would have over 80% power to detect a difference of 0.5 units in the change in constipation symptoms score between the lubiprostone and the Senna treatment groups, with a significance level of 0.05. This was based on a two-sample t-test and assumed that the standard deviation of the change for the constipation symptoms score was 0.66 units[18]. Given expected screening failures and study dropout, we planned on screening approximately 70 patients and enrolling 64.
Continuous measures (those reported as means and standard deviations) and their differences were compared between groups using an independent two-sample t-test. Categorical measures (those reported as frequencies and percentages) were compared between groups using the χ2 test or Fisher’s exact test if frequencies were low. If no significant differences were found, then the groups were combined and change from baseline to 7 d was assessed using paired t-tests for continuous data or McNemar’s test for categorical data. Analyses were done for the intent-to-treat population as well as for the participants completing study medication and assessments.
RESULTS
Patients were recruited for the study. Figure 1 depicts participant randomization and study progression. There were eight individuals screened who did not meet inclusion and/or exclusion criteria. Reasons for exclusion were: history of Crohn’s disease (1 person), history of irritable bowel disease (1), diarrhea on baseline visit day (1), or no associated bowel symptom (5). Subjects were enrolled and randomized. Sixty subjects received at least one dose of the study drugs. Fifty-six subjects had baseline and follow up measures (28 in each treatment group) and were used for intention to treat analyses (ITT). Forty-three subjects completed the study as defined by receiving at least 80 percent of the study drug, baseline assessments and final assessments. This group is designated on the tables as “Completers”. Twenty-one of these 43 subjects received lubiprostone and 22 received Senna.
Figure 1.
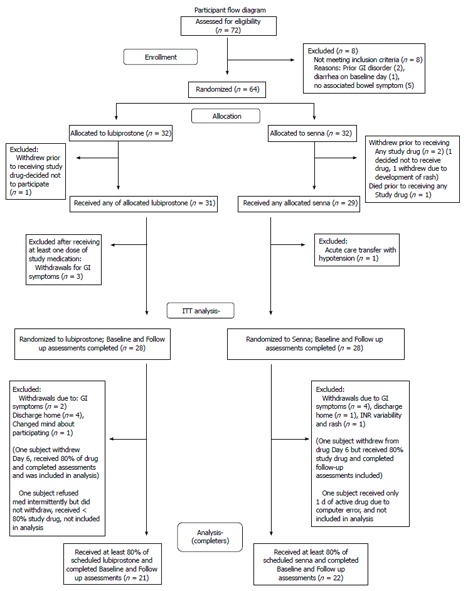
Participant flow diagram. ITT: Intention to treat; GI: Gastrointestinal.
Table 1 contains demographic information. The mean age of the participants in the ITT groups was 70.6 years for those receiving lubiprostone and 72.5 years for those receiving Senna. There were no significant differences in the baseline demographics between the two treatment groups (Table 1). Thirty of the subjects were on at least one scheduled laxative at screening (range: 1-3/d) (which was discontinued at Day 1), and also additional as needed (prn) laxatives. The remainder of the subjects were taking laxatives as needed for constipation symptoms. The subject groups also did not differ in their baseline total daily opioid dose, as compared by calculating the equi-analgesic dose for the narcotic taken, with doses reported as morphine equivalents (mean ± SD, Day dose: 70.8 ± 95.5 mg vs 53.4 ± 41.5 mg, P = 0.44 for lubiprostone and Senna groups). Subjects groups also did not differ between the total opioid doses taken over Days 1-7 (total dosage: 501.3 ± 628.8 mg vs 408.0 ± 329.5 mg, P = 0.54 for lubiprostone and Senna).
Table 1.
Participant characteristics
| Characteristics |
Intent to treat |
Completers |
||||
| Lubiprostone | Senna | P value | Lubiprostone | Senna | P value | |
| n = 28 | n = 28 | n = 21 | n = 22 | |||
| Age, yr | 70.6 ± 12.1 | 72.5 ± 10.8 | 0.54 | 68.9 ± 12.6 | 69.6 ± 9.6 | 0.85 |
| Weight, kg | 88.5 ± 23.4 | 84.0 ± 16.7 | 0.42 | 93.4 ± 21.7 | 85.4 ± 17.0 | 0.20 |
| Days postoperative, d | 5.81 ± 2.72 | 7.71 ± 6.95 | 0.19 | 6.10 ± 2.90 | 6.50 ± 4.21 | 0.72 |
| Sex, female | 20 (74) | 15 (54) | 0.16 | 15 (71) | 13 (59) | 0.53 |
| Race/ethnicity African American Hispanic Unknown White | 5 (18) 1 (4) 2 (7) 20 (71) | 7 (25) 0 (0) 3 (11) 18 (64) | 0.76 | 6 (27) 0 (0) 0 (0) 16 (73) | 4 (19) 1 (5) 1 (5) 15 (71) | 0.72 |
| Primary surgery Diagnosis Total knee or hip replacement Spinal surgery Hip/femur fracture Other | 14 (50) 8 (14) 5 (9) 1 (2) | 21 (75) 5 (18) 1 (4) 1 (4) | 0.18 | 13 (62) 5 (24) 2 (10) 1 (5) | 11 (50) 8 (32) 3 (18) 0 (0) | 0.64 |
Data are expressed as absolute n (%) or mean ± SD.
All gastrointestinal study parameters indicated comparability between randomized groups at baseline. The results for the primary and secondary efficacy for gastrointestinal measures are reported in Table 2. There were no significant differences in study parameters between randomized groups for the ITT population. In particular, there were no significant differences between the lubiprostone and Senna groups in mean change from baseline to Day 7 assessments in the PAC-SYM (-0.20 ± 0.60 vs -0.36 ± 0.61, P = 0.61) or PAC-QOL (0.25 ± 0.78 vs 0.50 ± 0.80, P = 0.61). Group data were combined to assess the significance of changes from baseline to 7 d. Within group analyses showed significant differences between Day 0 and 7 ratings on the PAC SYM and QOL. For only the Completer groups comparison, the ratings of bowel symptoms (completeness of bowel movement) was significantly different between the lubiprostone and Senna groups in mean change from baseline to Day 7 assessments, with the Senna group showing greater negative change in bowel movement completeness (-0.56 ± 1.01 vs -2.00 ± 1.41, P = 0.03). There was also a significant difference in the reduction of abdominal pain, favoring Senna (P = 0.04). No significant difference were found for PAC-SYM subscales comparing the two treatment groups for any of these measures, with a mean overall change score of -0.38 (SD = 0.66) for the abdominal symptom score, -0.14 (SD = 0.71) for rectal symptom score, and -0.29 (SD = 0.96) for the stool symptom score. For within group analyses, participants demonstrated significant improvement in bowel symptoms as measured by the PAC-SYM (mean ± SD, baseline: 0.86 ± 0.71, Day: 0.58 ± 0.51, P = 0.002) and PAC-QOL (baseline: 1.37 ± 0.61, Day 7: 1.75 ± 0.67, P = 0.007) over time.
Table 2.
Gastrointestinal outcomes: Patient assessment in constipation
| Outcome measure |
Intent to treat |
Completers |
||||
| Lubiprostone | Senna | P value | Lubiprostone | Senna | P value | |
| n = 28 | n = 28 | n = 21 | n = 22 | |||
| PAC-SYM | ||||||
| Baseline | 0.72 ± 0.56 | 0.93 ± 0.77 | 0.10 | 0.76 ± 0.60 | 0.95 ± 0.81 | 0.25 |
| Day 7 | 0.51 ± 0.41 | 0.63 ± 0.59 | 0.06 | 0.57 ± 0.44 | 0.59 ± 0.58 | 0.35 |
| Change | -0.22 ± 0.57 | -0.29 ± 0.58 | 0.89 | -0.20 ± 0.60 | -0.36 ± 0.61 | 0.61 |
| PAC-QOL | ||||||
| Baseline | 1.50 ± 0.64 | 1.41 ± 0.73 | 0.63 | 1.42 ± 0.55 | 1.32 ± 0.66 | 0.63 |
| Day 7 | 1.66 ± 0.59 | 1.83 ± 0.79 | 0.35 | 1.67 ± 0.56 | 1.82 ± 0.77 | 0.35 |
| Change | 0.99 ± 0.78 | 0.42 ± 0.84 | 0.30 | 0.25 ± 0.78 | 0.50 ± 0.80 | 0.61 |
Data are expressed as absolute numbers (percentage) or mean ± SD. PAC-SYM: Patient assessment of constipation (PAC)-symptoms; PAC-QOL: PAC-quality of life.
Rehabilitation outcome measures are reported in Table 3. The overall mean length of stay was 14.7 d with SD = 7.72 (median = 12; range: 2-37 d) and length of stay did not differ by group. No differences were found in functional outcomes between the two treatment groups; admission FIM score was 69.1 (SD = 13.0) and discharge 98.4 (SD = 14.2), with a FIM efficiency of 2.38 (SD = 1.26). Subjects showed improvement on their FIM bowel score across the study time frame; at admission, the bowel FIM item rating was 4.12 (SD = 1.50, range: 1-6) and at discharge 5.33 (SD = 1.25, range: 2-7) with no between group differences.
Table 3.
Gastrointestinal symptoms
| Outcome measure |
Intent to treat |
Completers |
||||
| Lubiprostone | Senna | P value | Lubiprostone | Senna | P value | |
| n = 28 | n = 28 | n = 21 | n = 22 | |||
| Nausea Baseline Day 7 Change | 0.61 ± 0.79 0.39 ± 0.79 0.21 ± 0.92 | 0.64 ± 0.99 0.46 ± 0.96 0.18 ± 0.86 | 0.88 0.76 0.88 | 0.57 ± 0.81 0.38 ± 0.80 0.19 ± 1.03 | 0.64 ± 1.05 0.50 ± 1.01 0.14 ± 0.83 | 0.82 0.67 0.85 |
| Cramping Baseline Day 7 Change | 0.25 ± 0.70 0.14 ± 0.36 0.11 ± 0.57 | 0.54 ± 0.84 0.11 ± 0.31 0.43 ± 0.92 | 0.17 0.69 0.12 | 0.29 ± 0.78 0.14 ± 0.36 0.14 ± 0.57 | 0.63 ± 0.90 0.04 ± 0.21 0.59 ± 0.96 | 0.18 0.28 0.07 |
| Painful bowel movement evacuation effort (straining) Baseline Day 7 Change | 1.40 ± 1.50 0.47 ± 0.70 0.64 ± 0.93 | 0.87 ± 1.46 0.75 ± 1.37 0.42 ± 1.38 | 0.98 0.55 0.62 | 1.53 ± 1.55 0.60 ± 0.74 0.58 ± 0.90 | 1.18 ± 1.60 0.87 ± 1.51 0.78 ± 1.30 | 0.58 0.72 0.69 |
| Completeness of bowel movement Baseline Day 7 Change | 1.56 ± 1.42 0.67 ± 0.97 0.82 ± 1.17 | 2.00 ± 1.41 0.55 ± 1.23 1.55 ± 2.11 | 0.23 1.00 0.33 | 1.53 ± 1.51 0.86 ± 1.03 0.56 ± 1.01 | 2.10 ± 1.29 0.46 ± 1.06 2.00 ± 1.41 | 0.12 0.24 0.03 |
| Abdominal pain Baseline Day 7 Change | 0.29 ± 0.71 0.07 ± 0.26 0.21 ± 0.69 | 0.59 ± 1.01 0.04 ± 0.19 0.56 ± 1.05 | 0.20 0.56 0.16 | 0.19 ± 0.68 0.05 ± 0.22 0.14 ± 0.73 | 0.73 ± 1.08 0.00 ± 0.00 0.73 ± 1.08 | 0.06 0.34 0.04 |
| Time per lavatory attempt Baseline < 5 min 5-10 min > 10 min NA Day 7 < 5 min 5-10 min > 10 min NA | 2 (7) 12 (43) 8 (29) 6 (21) 8 (30) 8 (30) 4 (15) 7 (26) | 4 (14) 7 (25) 10 (36) 7 (25) 9 (32) 6 (21) 6 (21) 7 (25) | 0.40 0.87 | 0 (5) 11 (52) 7 (33) 3 (14) 7 (32) 3 (14) 5 (23) 7 (32) | 3 (14) 5 (23) 8 (36) 6 (27) 7 (33) 7 (33) 4 (19) 3 (14) | 0.08 0.66 |
Data are expressed as absolute numbers (percentage) or mean ± SD. NA: Not significant.
Adverse events were reported for subjects (n = 60) receiving at least one dose of the two study medications and these events are detailed in Table 4. Gastrointestinal disorders were the most common events reported. There were three serious adverse events. Two events occurred in subjects who received at least one dose of the study medication. One death occurred Day 1, however this subject had not received any active study drug before the time of the event. One serious adverse event occurred in one subject receiving Senna when the subject required transfer to acute care due to hypotension on Day 5. She was found to have an elevated troponin, with hypotension thought to be related to stress cardiomyopathy and dehydration. The final event was a subject on lubiprostone who dislocated her knee and required transfer to acute care. None of these three events were assessed related to study treatments (Table 5).
Table 4.
Bowel movements and Bristol stool scale
| Outcome measure | Intent to treat | Completers | ||||
|
Lubiprostone |
Senna |
P value |
Lubiprostone |
Senna |
P value |
|
| n = 28 | n = 28 | n = 21 | n = 22 | |||
| Number of unsuccessful attempts for bowel movement | ||||||
| Baseline | 0.17 | 0.29 | ||||
| 0 | 10 (36) | 11 (39) | 5 (24) | 7 (32) | ||
| 1 | 4 (14) | 8 (29) | 3 (14) | 7 (32) | ||
| 2-3 | 10 (36) | 5 (18) | 9 (43) | 4 (18) | ||
| 4+ | 4 (14) | 4 (14) | 4 (19) | 4 (18) | ||
| Day 7 | 0.31 | 0.60 | ||||
| 0 | 15 (75) | 17 (61) | 10 (48) | 14 (64) | ||
| 1 | 6 (21) | 7 (25) | 6 (29) | 5 (23) | ||
| 2-3 | 7 (25) | 4 (14) | 5 (24) | 3 (14) | ||
| 4+ | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Change (improved) | 9 (32) | 12 (43) | 0.42 | 12 (57) | 13 (59) | 0.92 |
| Number of bowel movement | ||||||
| Baseline | 0.96 ± 1.17 | 0.61 ± 0.83 | 0.19 | 0.95 ± 0.92 | 0.64 ± 0.90 | 0.26 |
| Day 7 | 0.93 ± 0.90 | 0.93 ± 0.86 | 1.00 | 0.95 ± 0.92 | 0.91 ± 0.87 | 0.87 |
| Change | -0.04 ± 1.50 | 0.32 ± 0.90 | 0.29 | 0.00 ± 1.22 | 0.27 ± 0.98 | 0.42 |
| Consistency of bowel movement (Bristol stool scale) | ||||||
| Baseline | 0.18 | 0.20 | ||||
| Type 1 | 4 (14) | 0 (0) | 4 (19) | 0 (0) | ||
| Type 2 | 1 (4) | 2 (17) | 1 (5) | 2 (9) | ||
| Type 3 | 3 (11) | 4 (14) | 2 (5) | 3 (14) | ||
| Type 4 | 1 (4) | 3 (11) | 1 (5) | 1 (5) | ||
| Type 5 | 2 (7) | 0 (0) | 2 (10) | 0 (0) | ||
| Type 6 | 4 (14) | 2 (7) | 2 (10) | 2 (9) | ||
| Type 7 | 1 (4) | 0 (0) | 1 (5) | 0 (0) | ||
| NA | 12 (43) | 17 (59) | 8 (38) | 14 (64) | ||
| Day 7 | 0.32 | 0.22 | ||||
| Type 1 | 4 (14) | 0 (0) | 4 (19) | 0 (0) | ||
| Type 2 | 1 (4) | 4 (14) | 1 (5) | 4 (18) | ||
| Type 3 | 2 (7) | 2 (7) | 1 (5) | 1 (5) | ||
| Type 4 | 6 (21) | 10 (36) | 5 (24) | 8 (36) | ||
| Type 5 | 1 (4) | 0 (0) | 0 (0) | 0 (0) | ||
| Type 6 | 2 (7) | 2 (7) | 2 (10) | 1 (5) | ||
| Type 7 | 1 (4) | 1 (4) | 0 (0) | 1 (5) | ||
| NA | 11 (39) | 9 (32) | 8 (38) | 7 (32) | ||
Data are expressed as absolute numbers (percentage) or mean ± SD. NA: Not significant.
Table 5.
Adverse event
| System/adverse event |
Any adverse event |
|
| Lubiprostone | Senna | |
| n = 31 | n = 29 | |
| Number receiving at least one dose | ||
| Number of participants with any event | 14 (45.2) | 12 (41) |
| Gastrointestinal disorders | ||
| Nausea | 3 (9.7) | 5 (17.2) |
| Diarrhea | 5 (16.1) | 2 (6.9) |
| Abdominal pain | 8 (25.8) | 2 (6.9) |
| Abdominal cramping | 6 (19.4) | 6 (20.7) |
| Constipation | 0 | 1 (3.4) |
| Nervous system disorders | ||
| Headache | 0 | 1 (3.4) |
| Skin disorders rash | 0 | 1 (3.4) |
| Musculoskeletal disorders | ||
| Patellar dislocation | 1 (3.2) | 0 |
| Laboratory evaluations | 0 | |
| Hematologic | 0 | 1 (3.4) |
| Cardiovascular disorders | ||
| Cardiomyopathy | 1 (3.4) | |
| Total events | 23 | 20 |
All adverse events in participants receiving at least one dose of study medication. Data are expressed as absolute numbers (percentage) or mean ± SD.
Information on subject withdrawals is detailed in Figure 1. Twenty-one subjects withdrew before completing the six days of study medication. Three subjects withdrew from the study following randomization but before receiving any study medications. One subject in the lubiprostone group and one in the Senna group changed their mind about participating. A third subject in the Senna group withdrew to a rash. One subject died before receiving active study drug (see above). Reasons for withdrawal, with some participants reporting more than 1 reason, included: abdominal pain (4 participants), diarrhea (2), nausea (2), loose stools (3), cramping (5), early discharge (5), knee dislocation (1), unstable blood test (international normalized ratio in a subject on warfarin) (1), rash (1), and headache (1).
Two subjects who did not withdraw from the study received less than 80 percent of the active drug. For the initial subject enrolled in the trial, the computer electronic order task did not work properly for nurse administration and the subject received one day of study drug. An additional subject refused doses due to gastrointestinal symptoms, but did not withdraw, and received less than 80 percent of the study interventions over the six days of medication administration. These two subjects were included in the ITT analysis, but not the analysis of subjects completing trial interventions. Other protocol deviations included: missed one day of data collection (1 subject), one dose of study medications missed (2), one dose of incorrect medication administered (1 subject), scheduled constipation medications were not initially changed (1), rescue meds taken for three days, but the subject was not withdrawn (1).
Rescue medications for symptoms of constipation were requested by 15 (75%) of the lubiprostone and 18 (78.2%) of the Senna participants, which was not significantly different (P = 0.93). Rescue medications used by subjects in this study included milk of magnesia, polyethylene glvcol. magnesium citrate, lactulose and suppositories. Subjects required a mean of 2.25 doses/enrolled subjects in the lubiprostone group and 2.26 doses in the Senna group (P = 0.87).
Rehabilitation outcome measures are reported in Table 3. The overall mean length of stay was 14.7 d with SD = 7.72 (median = 12; range: 2-37 d) and length of stay did not differ by group. No differences were found in functional outcomes between the two treatment groups; admission FIM score was 69.1 (SD = 13.0) and discharge 98.4 (SD = 14.2), with a FIM efficiency of 2.38 (SD = 1.26). Subjects showed improvement on their FIM bowel score across the study time frame; at admission, the bowel FIM item rating was 4.12 (SD = 1.50, range: 1-6) and at discharge 5.33 (SD = 1.25, range: 2-7) with no between group differences.
DISCUSSION
In this study, we found that both Senna and lubiprostone were associated with improvement in bowel related symptoms over the treatment period, however we did not find significant differences in the primary efficacy measures between these two medications. Rescue bowel medication use was frequently required by the participants in both groups however, and thus may have limited our ability to distinguish differences between these regimens. Nevertheless, this latter finding indicates that multiple laxative medications may be required for constipation symptom control with opioids in this setting.
Bowel related symptoms in patients with opioid constipation are frequent. While involved in physical and occupational therapy, both control of pain with activity as well control of bowel symptoms is required in order to participate and benefit from such interventions. Despite the need for frequent rescue medication for bowel symptoms by the participants in this study, it is of interest that we did not find bowel symptoms precluded therapy attendance to any great degree, though any impact on other activities outside of therapy was not examined.
In a survey of patients taking oxycodone for nonmalignant pain, constipation was reported by 53.1%; overall opioid-related side effects in this study was found to impact adherence to prescribed medication dosages and has been found to be associated with poorer pain control[22].
Initiation of laxatives to prevent constipation symptoms has been recommended when opioids are prescribed for pain control, however satisfactory results with laxatives for control of this symptom have been reported to be less than 50%[23,24]. Common interventions to control constipation symptoms include bowel stimulants and osmotic laxatives, but such medications may be ineffective or result in unpredictable bowel function. Mu-opioid receptor antagonists appear to be safe and effective for laxation in palliative care patients, however at this time only subcutaneous methylnaltrexone is available in the US[25]. In subjects in rehabilitation, naltrexone resulted in earlier laxation compared to placebo[26]. A multi-institutional study assessing the impact of prophylactic medications on the incidence of opioid-induced gastrointestinal dysfunction in hospitalized cancer patients found that premedication significantly lowered the rate of constipation, however, constipation was still present in 34% of these patients despite the use of laxatives[25,27]. Identification of medications that provide better control of bowel symptoms is needed, as are comparison trials to identify the most effective regimens to reduce the resultant morbidity. Additionally, although effectiveness did not differ, costs do differ for the two medications evaluated in the present study in that Senna is available as a generic medication and thus less costly; a full cost benefit analysis was not the performed as a component of this study, however.
Whether the physical activity that these subjects performed as part of their rehabilitation treatment could have influenced the improvement in constipation symptoms found in this study is not known. There has been little evaluation of the effects of physical activity as an intervention for bowel dysfunction. There were not between group differences in baseline functional status. This finding and the absence of significant differences in change in function between groups, as well as absent differences in missed therapies suggests that activity levels were similar and was unlikely to have affected measured outcomes between groups.
Limitations to this study include its smaller sample size. In addition, we describe results for a specific patient population, which may limit generalizability of the result of this study to other subjects with opioid induced constipation.
Subjects utilizing either lubiprostone or Senna demonstrated improvement in symptoms of constipation and quality of life as measured by the PAC-SYM and PAC-QOL. Most participants in each of the treatment groups required additional medications to control symptoms. Thus the analyses reflect both the treatment group medications as well as the additional medication interventions required. This may have impacted our ability to detect differences between the treatment groups, but does confirm that more than one medication may be required for control of constipation symptoms in this patient population.
COMMENTS
Background
Constipation is a frequent adverse effect of opioid analgesics in post-operative orthopedic patients. This symptom is associated with longer postoperative lengths of stay and may worsen patient distress.
Research frontiers
Lubiprostone, a locally acting type-2 chloride channel activator enhances intestinal motility through increases in intestinal fluid and electrolyte secretion. Research suggests that lubiprostone may reverse the morphine-induced anti-secretory effects of opioids through its direct action on mucosal chloride channels. It is approved in the United States for opioid-induced constipation in non-cancer patients, but efficacy has not been demonstrated in the post-operative setting, nor have trials been done to compare its efficacy with other medications used for control of constipation in this setting. This randomized, double-blind, active comparator trial assessed the effects of lubiprostone compared to Senna on opioid-induced constipation in postoperative patients hospitalized in rehabilitation following orthopedic surgery.
Innovations and breakthroughs
Both Senna and lubiprostone were associated with improvement in constipation and other bowel-related symptoms over the treatment period, with no significant differences in efficacy between the two treatment arms. However, rescue bowel medications were frequently utilized by both groups, suggesting that multiple laxative medications may be required for control of constipation symptoms in this setting.
Applications
Both medications evaluated in this trial improved constipation and thus may be utilized, however as no differences were noted in efficacy between the two treatment groups, other factors such as medication cost may be a consideration to guide the choice of intervention. In addition, patients may require the prescription of more than one medication for control of constipation symptoms.
Terminology
Mu-opioid receptors are a class of opioid receptors that are found within the intestinal tract in addition to the central nervous system. Mu-agonists cause constipation due to their effects on intestinal peristaltic activity.
Peer review
This study addresses an important clinical issue. It investigated the efficacy of lubiprostone-based new therapy for opioid use-associated constipation in post-operative orthopedic patients. primarily in a geriatric population. It appears that the new drug did not show better efficacy than the conventional Senna in symptoms of constipation and quality of life. Most participants in each of the treatment groups required additional medications to control symptoms. The study also demonstrated that more than one medication may be required for control of constipation symptoms for the study population. These results would have potential benefits to the physicians who treat patients in this setting.
Footnotes
Supported by A grant from Takeda Pharmaceuticals North America, Inc
P- Reviewer: Basilisco G, Chen YK, Cheng SX, Furnari M, Wexner SD S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Holmes S. Use of a modified symptom distress scale in assessment of the cancer patient. Int J Nurs Stud. 1989;26:69–79. doi: 10.1016/0020-7489(89)90047-3. [DOI] [PubMed] [Google Scholar]
- 2.Irvine EJ, Ferrazzi S, Pare P, Thompson WG, Rance L. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002;97:1986–1993. doi: 10.1111/j.1572-0241.2002.05843.x. [DOI] [PubMed] [Google Scholar]
- 3.Dennison C, Prasad M, Lloyd A, Bhattacharyya SK, Dhawan R, Coyne K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics. 2005;23:461–476. doi: 10.2165/00019053-200523050-00006. [DOI] [PubMed] [Google Scholar]
- 4.Lapane KL, Quilliam BJ, Benson C, Chow W, Kim MS. Gastrointestinal events after opioid treatment in nonmalignant pain: correlates of occurrence and impact on health-related quality of life. J Opioid Manag. 2013;9:205–216. doi: 10.5055/jom.2013.0161. [DOI] [PubMed] [Google Scholar]
- 5.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112:372–380. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173–C1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 7.Barish CF, Drossman D, Johanson JF, Ueno R. Efficacy and safety of lubiprostone in patients with chronic constipation. Dig Dis Sci. 2010;55:1090–1097. doi: 10.1007/s10620-009-1068-x. [DOI] [PubMed] [Google Scholar]
- 8.Schey R, Rao SS. Lubiprostone for the treatment of adults with constipation and irritable bowel syndrome. Dig Dis Sci. 2011;56:1619–1625. doi: 10.1007/s10620-011-1702-2. [DOI] [PubMed] [Google Scholar]
- 9.Holzer P. Treatment of opioid-induced gut dysfunction. Expert Opin Investig Drugs. 2007;16:181–194. doi: 10.1517/13543784.16.2.181. [DOI] [PubMed] [Google Scholar]
- 10.Mehendale SR, Yuan CS. Opioid-induced gastrointestinal dysfunction. Dig Dis. 2006;24:105–112. doi: 10.1159/000090314. [DOI] [PubMed] [Google Scholar]
- 11.Sun X, Wang X, Wang GD, Xia Y, Liu S, Qu M, Needleman BJ, Mikami DJ, Melvin WS, Bohn LM, et al. Lubiprostone reverses the inhibitory action of morphine on mucosal secretion in human small intestine. Dig Dis Sci. 2011;56:330–338. doi: 10.1007/s10620-010-1515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiller LR. New and emerging treatment options for chronic constipation. Rev Gastroenterol Disord. 2004;4 Suppl 2:S43–S51. [PubMed] [Google Scholar]
- 13.Chan WW, Mashimo H. Lubiprostone Increases Small Intestinal Smooth Muscle Contractions Through a Prostaglandin E Receptor 1 (EP1)-mediated Pathway. J Neurogastroenterol Motil. 2013;19:312–318. doi: 10.5056/jnm.2013.19.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kivitz A, Cohen S, Dowd JE, Edwards W, Thakker S, Wellborne FR, Renz CL, Segurado OG. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther. 2006;28:1619–1629. doi: 10.1016/j.clinthera.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Pizzi LT, Toner R, Foley K, Thomson E, Chow W, Kim M, Couto J, Royo M, Viscusi E. Relationship between potential opioid-related adverse effects and hospital length of stay in patients receiving opioids after orthopedic surgery. Pharmacotherapy. 2012;32:502–514. doi: 10.1002/j.1875-9114.2012.01101.x. [DOI] [PubMed] [Google Scholar]
- 16.Johanson JF, Sonnenberg A, Koch TR. Clinical epidemiology of chronic constipation. J Clin Gastroenterol. 1989;11:525–536. doi: 10.1097/00004836-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Sonnenberg A, Koch TR. Epidemiology of constipation in the United States. Dis Colon Rectum. 1989;32:1–8. doi: 10.1007/BF02554713. [DOI] [PubMed] [Google Scholar]
- 18.Frank L, Kleinman L, Farup C, Taylor L, Miner P. Psychometric validation of a constipation symptom assessment questionnaire. Scand J Gastroenterol. 1999;34:870–877. doi: 10.1080/003655299750025327. [DOI] [PubMed] [Google Scholar]
- 19.Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39:681–685. doi: 10.1007/BF02056950. [DOI] [PubMed] [Google Scholar]
- 20.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton B, Grange RC, Sherwin FF, Zielezny M, Tashman J. A uniform data system for medical rehabilitation. In: Fuhrer MJ, editor Rehabilitation outcomes: analysis and measurement, editors. Baltimore: Brooks; 1987. pp. 137–147. [Google Scholar]
- 22.Anastassopoulos KP, Chow W, Ackerman SJ, Tapia C, Benson C, Kim MS. Oxycodone-related side effects: impact on degree of bother, adherence, pain relief, satisfaction, and quality of life. J Opioid Manag. 2011;7:203–215. doi: 10.5055/jom.2010.0063. [DOI] [PubMed] [Google Scholar]
- 23.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182:11S–18S. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 24.Swetz KM, Kamal AH. In the clinic. Palliative care. Ann Intern Med. 2012;156:ITC2–IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, ITC2-IT1, quiz ITC2-IT1. doi: 10.7326/0003-4819-156-3-201202070-01002. [DOI] [PubMed] [Google Scholar]
- 25.Candy B, Jones L, Goodman ML, Drake R, Tookman A. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev. 2011;(1):CD003448. doi: 10.1002/14651858.CD003448.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Anissian L, Schwartz HW, Vincent K, Vincent HK, Carpenito J, Stambler N, Ramakrishna T. Subcutaneous methylnaltrexone for treatment of acute opioid-induced constipation: phase 2 study in rehabilitation after orthopedic surgery. J Hosp Med. 2012;7:67–72. doi: 10.1002/jhm.943. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara M, Ikesue H, Matsunaga H, Suemaru K, Kitaichi K, Suetsugu K, Oishi R, Sendo T, Araki H, Itoh Y. A multi-institutional study analyzing effect of prophylactic medication for prevention of opioid-induced gastrointestinal dysfunction. Clin J Pain. 2012;28:373–381. doi: 10.1097/AJP.0b013e318237d626. [DOI] [PubMed] [Google Scholar]


