Abstract
AIM: To assess the diagnostic value of serum interleukin-8 (IL-8) in the detection of colorectal cancer (CRC).
METHODS: An original study was conducted to explore the potential value of IL-8 in CRC diagnosis. Receiver operating characteristic (ROC) analysis was performed and the area under the curve (AUC) value was calculated. PUBMED and EMBASE were searched (to October, 2013), supplemented with manual screening for relevant publications. Meta-analysis methods were applied to pool sensitivity, specificity, positive and negative likelihood ratios, and diagnostic odds ratios and to construct a summary receiver-operating characteristic (sROC) curve. Heterogeneity across studies was checked by the I2 test and Deek’s funnel plot method was applied to test publication bias.
RESULTS: In our original study, serum IL-8 yielded an AUC of 0.742 [95%CI: 0.635-0.849]. The sensitivity and specificity were 85.42% and 54.05%, respectively, at a cut-off value of 5.39. In this meta-analysis, we included five studies with 668 CRC patients and 374 controls and one study in our own center with 48 CRC patients and 37 controls. The pooled sensitivity and specificity of IL-8 were 0.69 (95%CI: 0.42-0.87) and 0.85 (95%CI: 0.68-0.94) for CRC detection. Besides, the area under the sROC curve was 0.86 (95%CI: 0.82-0.88). Subgroup analysis suggested that there was no heterogeneity in the Asian subgroup but some in the European subgroup. In addition, no publication bias was found according to the Deek’s funnel plot asymmetry test.
CONCLUSION: Serum IL-8 is a promising biomarker for CRC detection and may become a clinically useful tool to identify high-risk patients.
Keywords: Interleukin-8, Colorectal cancer, Diagnostic value, Case-control study, Meta-analysis
Core tip: Serum cytokine concentrations may reflect inflammatory processes during the carcinogenesis of colorectal cancer (CRC). The authors conducted an original study to explore the diagnostic value of interleukin-8 (IL-8) in CRC. Besides, standard statistical methods for meta-analysis were applied in the present study. Six studies were finally included (including our study). The pooled results suggested that serum IL-8 is a promising biomarker for CRC detection and may represent a clinically useful tool to identify high-risk patients.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers worldwide and also an important source of cancer-related death[1,2]. Detection of early-stage CRC is critical for curative treatment interventions, which can significantly reduce the incidence and mortality[3-6]. The present clinical examinations, such as sigmoidoscopy, colonoscopy and fecal occult blood test etc., have been widely used for CRC screening[7,8]. However, sigmoidoscopy and colonoscopy are considered time consuming, invasive and cumbersome[9-11], and fecal occult blood test screening suffers for its low sensitivity for polyps, especially smaller ones[6,12]. Thus, non-invasive diagnostic biomarkers are critical for CRC early detection.
Cytokines are a category of soluble peptides that play an important role in inflammation and the initiation and promotion of carcinogenesis[11,13]. As a member of the CXC cytokine family, interleukin-8 (IL-8) is one of the most significantly upregulated chemokines in CRC, and contributes to tumor growth, invasion and metastasis in CRC[14-16]. IL-8 induces CRC cell proliferation and migration by a disintegrin and metalloprotease (ADAM)-dependent pathway, and heparin-binding epidermal growth factor (EGF)-like growth factor (HB-EGF) plays an important role as the major ligand for this pathway[17]. Therefore, several studies[10,11,18-20] have evaluated the application of IL-8 in the diagnosis of CRC; however, the results were contradictory. As a consequence, the aim of this meta-analysis was to systematically assess the diagnostic value of IL-8 for assisting in the diagnosis of CRC.
MATERIALS AND METHODS
Ethics statement
The study has obtained the approval from the Local Research Ethics Committee of Huzhou Central Hospital; data were from department of Anorectal branch, Huzhou Central hospital. Informed consent was obtained from all patients in the study.
Original study
We conducted an original study to explore the diagnostic value of IL-8 expression in CRC. Serum samples were collected in EDTA tubes from 37 healthy controls in Hangzhou First People’s Hospital and from 48 patients before surgery in Zhejiang Cancer Hospital, the Second Affiliated Hospital of Wenzhou Medical University and Huzhou Central hospital. All samples were immediately frozen in liquid nitrogen and kept at -80 °C until IL-8 extraction. No patients had received adjuvant treatment, including radiotherapy or chemotherapy before surgery and diagnosis. Serum IL-8 levels were measured using human IL-8/nucleosome assembly protein 1 (NAP-1) Platinum enzyme-linked immunosorbent assay kit (eBioscience, Inc. Vienna, Austria). The data of the original study were also included in the meta-analysis.
To determine the diagnostic performance of IL-8 level in CRC, ROC analysis was performed and the AUC value was calculated. The optimal cutoff threshold was determined at the point on the ROC curve at which (sensitivity + specificity - 100%) was maximal. Sensitivity and specificity were calculated using this cutoff value.
Meta-analysis
Literature search and selection criteria: PUBMED and EMBASE (to October, 2013) were searched without restrictions to identify eligible studies. The following terms were applied: “interleukin-8” OR “interleukin8” OR “interleukin 8” OR “IL-8” OR “IL8” OR “IL 8”; “colon” OR “rectal” OR “colorectal”; “cancer” OR “tumor” OR “tumour” OR “carcinoma” OR “neoplasm”. References of relevant articles and reviews were also scanned for potentially missing studies. Titles and abstracts were first scanned, and then full articles of potential eligible studies were reviewed. Meeting abstracts were excluded because of the limited data. The retrieved studies were carefully examined to exclude potential duplicates or overlapping data. This meta-analysis was designed, conducted and reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyse (PRISMA) and Meta-analysis Of Observational Studies in Epidemiology (MOOSE) statements[21,22].
Articles were included if they met all the following criteria: (1) the outcome of interest was CRC; (2) serum IL-8 levels were measured for the evaluation of CRC; (3) absolute numbers of true positive (TP), false positive (FP), true negative (TN) and false negative (FN) results were provided or could be derived; and (4) articles as full papers in English or Chinese were retrieved. Studies were excluded if they were performed in patients after induction of chemotherapy or surgery.
Data extraction and quality assessment: Two reviewers (Jin WJ and Xu JM) independently extracted the following information from each study, using a standardized protocol: authors, year of publication, country, study design (prospective or retrospective), patient characteristics (including sample size, gender and mean age) and number of TP, FP, TN, and FN was extracted. We finally identified five articles[10,11,18-20], and one study in our own center was extracted (current study). Discrepancies were resolved by a third investigator.
The quality of methodology for each study was assessed using the quality assessment of diagnostic accuracy studies (QUADAS)[23]. For each component, a score of 1 was applied if the answer was “yes”; otherwise, a score 0 was applied.
Data synthesis and statistical analysis: The analyses of sensitivity, specificity, likelihood ratios (LRs) and diagnostic odds ratio were performed and data were finally summarized in an sROC curve. The numbers of TP, FP, TN and FN were analyzed to calculate sensitivity and specificity. The formula for a positive LR is sensitivity/(1 - specificity), and the formula for a negative LR is (1 - sensitivity)/specificity. A clinical test that has a positive LR greater than 5.0 and a negative LR less than 0.2 could be defined useful[24,25]. Thus, the combined LRs provide the diagnostic odds ratio (positive LR/negative LR)[26,27].
Heterogeneity across studies was checked by the I2 test[28], which quantifies the proportion of total variation across studies caused by heterogeneity rather than chance. Deek’s funnel plot method was applied to test publication bias[29]. All P values presented were two-sided. The association was considered significant if the P ≤ 0.05. All analyses were conducted using Stata software (version 12.0; StatCorp, College Station, TX, United States) and Meta-Disc (version 1.4, Unit of clinical biostatics, the Ramoy Cajal Hospital, Madrid, Spain).
RESULTS
Study characteristics and data quality
After searching PUBMED and EMBASE, 1502 articles were identified. One thousand one hundred and fifty articles remained after removing 352 duplicated papers. Review of the titles and abstracts resulted in exclusion of 1073 articles. For the remaining 77 articles, 72 were excluded for the following reasons: insufficient data (n = 26), not on the right topic or targeted population (n = 42), not an original article (n = 2), language was neither English nor Chinese (n = 2). Finally, five studies[10,11,18-20] with 668 CRC patients and 374 controls and one original study in our own center with 48 CRC patients and 37 controls (current study) were included. The selection process is shown in Figure 1 and the characteristics of the included studies are shown in Table 1. Among the six studies, two were conducted in Asian countries and the remaining four were in European countries. All six studies were case-control studies. The participant age ranged from 31 to 99.1 years. All studies were of high quality.
Figure 1.
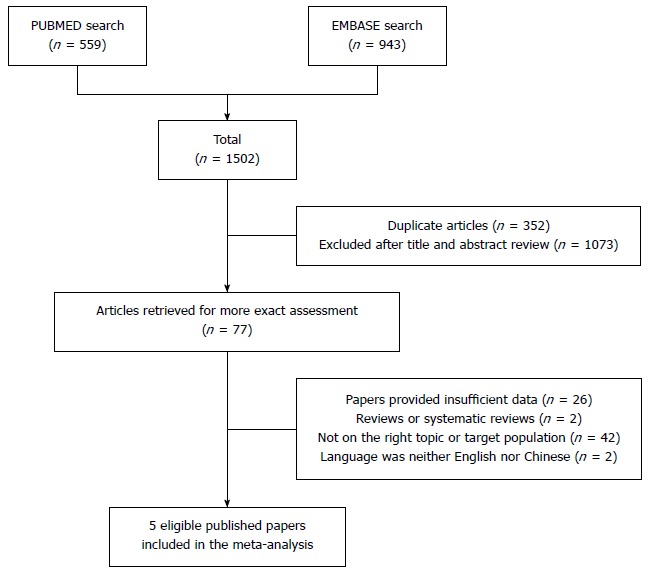
Flow diagram of the study selection process.
Table 1.
Characteristics of included studies
| Study | Country | Design | Male | Age (yr) | No. of participants | Sensitivity | Specificity | TP | FP | FN | TN | QUADAS |
| (%) | score | |||||||||||
| Pengjun et al[11], 2013 | China | Case-control | 52.4 | 31-74 | 149 CRC, 93 colorectal adenoma, 69 controls | 0.869 | 0.971 | 149 | 2 | 23 | 69 | 12 |
| (mean 58.0) | ||||||||||||
| Kantola et al[18], 2012 | Finland | Case-control | 51.0 | Mean 67.9 | 148 CRC, 86 controls | 0.809 | 0.821 | 115 | 18 | 27 | 84 | 12 |
| Bünger et al[10], 2012 | Germany | Case-control | 52.3 | 40.5-99.1 | 164 CRC, 34 colorectal adenomas, 119 controls | 0.220 | 0.900 | 81 | 6 | 287 | 52 | 12 |
| (mean 69.6) | ||||||||||||
| Bünger et al[19], 2011 | Germany | Case-control | 52.0 | 38-88 | 50 CRC, 50 controls | 0.280 | 0.940 | 50 | 3 | 129 | 50 | 12 |
| (mean 71.2) | ||||||||||||
| Kaminska et al[20], 2005 | Poland | Case-control | 49.0 | 32-82 | 157 CRC, 50 controls | 0.883 | 0.580 | 157 | 36 | 21 | 50 | 11 |
| Current study | China | Case-control | 52.1 | Mean 55.4 | 48 CRC, 37 controls | 0.854 | 0.541 | 41 | 17 | 7 | 20 | 12 |
TP: True positive; FP: False positive; TN: True negative; FN: False negative; QUADAS: Quality assessment of diagnostic accuracy studies; CRC: Colorectal cancer.
Original study
Forty-eight CRC patients and 37 normal controls were enrolled to assess the diagnostic value of IL-8 in CRC. The ROC analysis revealed that serum IL-8 might be a potential biomarker to discriminate patients with CRC from controls. The AUC value was 0.742 (95%CI: 0.635-0.849; Figure 2). The sensitivity and specificity were 85.42% and 54.05%, respectively, at a cut-off value of 5.39.
Figure 2.
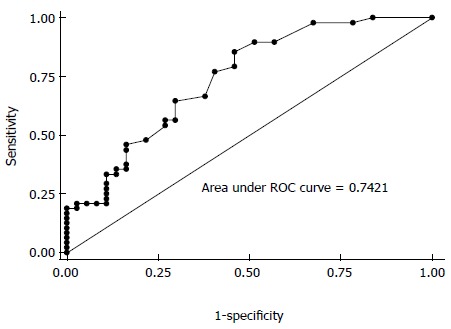
Area under the receiver operating characteristic curve value of interleukin-8 in colorectal cancer from the original study. ROC: Receiver operating characteristic.
Efficacy of IL-8 for the detection of CRC
The diagnostic value was evaluated by analyzing the five extracted studies and our original study. The pooled sensitivity was 0.69 (95%CI: 0.42-0.87), and the pooled specificity was 0.85 (95%CI: 0.68-0.94; Figure 3A). The area under the sROC curve was 0.86 (95%CI: 0.82-0.88; Figure 3B). Significant heterogeneity was found in both sensitivity (Q = 484.33; df = 5.00; P = 0.00; I2 = 98.97%) and specificity (Q = 69.59; df = 5.00; P = 0.00; I2 = 92.81%). The positive LR, negative LR and the diagnostic odds ratio value were 3.51 (95%CI: 1.93-6.35; Figure 4A), 0.33 (95%CI: 0.16-0.68; Figure 4B), and 12.03 (95%CI: 4.74-30.54; Figure 4C), respectively.
Figure 3.
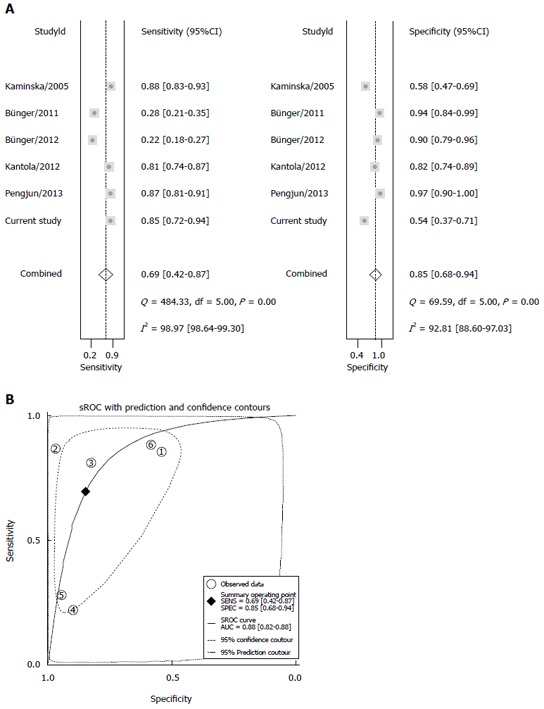
Diagnostic performance of interleukin-8 in the detection of colorectal cancer. A: Forest plots of the pooled sensitivity and specificity in CRC diagnosis; B: sROC curve analysis in CRC diagnosis. sROC: Summary receiver operating characteristic; CRC: Colorectal cancer.
Figure 4.
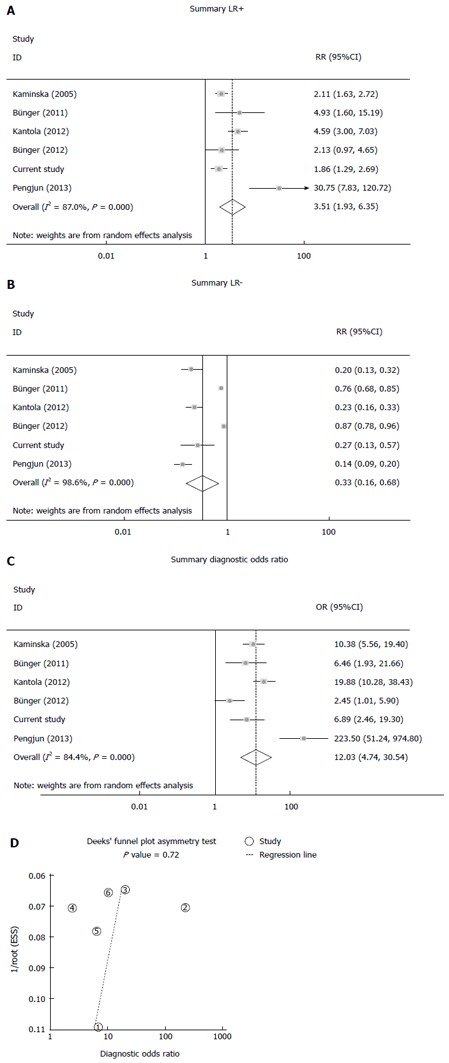
Positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio and Deek’s funnel plot. A: Positive LR; B: Negative LR; C: Diagnostic OR; D: Deek’s funnel plot asymmetry test. LR: Likelihood ratio; OR: Odds ratio.
Sensitivity and subgroup analyses
Sensitivity analysis was conducted to explore the potential impact of within-study heterogeneity. After removing the two studies of Bunger[10,19], the pooled sensitivity changed from 0.69 (95%CI: 0.42-0.87) to 0.86 (95%CI: 0.82-0.88), with a significant decrease in heterogeneity from Q = 484.33; df = 5.00; P = 0.00; I2 = 98.97% to Q = 3.56; df = 3.00; P = 0.31; I2 = 15.71%, as shown in Table 2.
Table 2.
Sensitivity and subgroup analyses
| Cases/controls | Sensitivity | SEN heterogeneity | Specificity | SPE heterogeneity | Positive LR | Negative LR | Diagnostic OR | |
| (χ2/γ/P/I2) | (χ2/γ/P/I2) | |||||||
| Sensitivity analysis | ||||||||
| All studies | 716/411 | 0.69 (0.42-0.87) | 484.33/5.00/0.00/98.97% | 0.85 (0.68-0.94) | 69.59/5.00/0.00/92.81% | 3.51 | 0.33 | 12.03 |
| Results without two from Bunger | 502/242 | 0.86 (0.82-0.88) | 3.56/3.00/0.31/15.71% | 0.79 (0.52-0.93) | 43.51/3.00/0.00/93.10% | 3.81 | 0.20 | 20.53 |
| Subgroup analysis | ||||||||
| Asia | 197/106 | 0.86 (0.81-0.91) | 0.05/1.00/0.83/0.00% | 0.82 (0.74-0.89) | 31.20/1.00/0.00/96.8% | 7.34 | 0.18 | 37.74 |
| Europe | 519/305 | 0.58 (0.25-0.85) | 322.69/3.00/0.00/99.07% | 0.85 (0.68-0.93) | 34.56/3.00/0.00/91.32% | 3.01 | 0.43 | 7.91 |
SEN: Sensitivity; SPE: Specificity; LR: Likelihood ratio; OR: Odds ratio.
Subgroup analysis was available in patients from different geographical regions. We found that the pooled sensitivity was 0.86 (95%CI: 0.81-0.91) in the Asian subgroup with no heterogeneity (P = 0.83, I2 = 0.00%). The pooled sensitivity was 0.58 (95%CI: 0.25-0.85) in the European subgroup with significant heterogeneity (P = 0.00, I2 = 99.07%), as shown in Table 2.
Publication bias
There was no significant publication bias according to the Deek’s funnel plot asymmetry test (Figure 4D).
DISCUSSION
The present meta-analysis first assessed the diagnostic value of IL-8 in the detection of CRC. Five studies with 668 CRC patients and 374 controls were included and an original study was also used to explore the potential value of IL-8 in CRC diagnosis. As the present meta-analysis showed, IL-8 has a pooled sensitivity of 0.69, a specificity of 0.85 and an AUC of 0.86, which suggested that IL-8 might be an non-invasive method for CRC diagnosis. The diagnostic odds ratio (DOR) combines the strengths of sensitivity and specificity as an independent indicator, and was reported to be a useful indicator for evaluation of a diagnostic method[30]. The DOR value of IL-8 was 12.03, indicating moderate diagnostic accuracy. However, the positive LR (3.51) and negative LR (0.33) suggested that IL-8 might be not adequate to rule in and rule out CRC patients.
Multiple mechanisms have been proposed to explain the diagnostic value of IL-8. It has been reported that IL-8 has a multifunctional role in CRC progression, and is involved in enhancing the survival of cancer cells, promoting tumor cell proliferation and regulating adhesion and invasion[31,32]; moreover, studies suggested that it induces cell migration in colon cancer cells, acting as an autocrine growth factor[33,34].
Cell lines derived from human colon carcinomas secrete IL-8 in vitro and this chemokine has also been detected immunohistochemically in human colon carcinoma specimens, in which it is tumor cell associated. Supplementing endogenously produced IL-8 by the recombinant chemokine led to stimulation of cell growth[33]. This may suggest that IL-8 acts as an autocrine growth factor for colon carcinoma cell lines.
IL-8 was reported to promote cell proliferation and migration through metalloproteinase-cleavage in human colon carcinoma cells[17]. The biological actions of IL-8 are mediated by binding to its receptors, IL-8RA (CXCR1) and IL-8RB (CXCR2), which are members of seven trans-membrane G-protein-coupled receptor (GPCR) family[35-39]. CXCR1 and CXCR2 both bind IL-8 with high affinity; however, CXCR2 also binds to other CXC chemokines[39,40]. These receptors also play an important role in tumor microenvironment and tumor progression[41,42]. Stimulation of the receptors can induce shedding of EGF ligands in CRC cells via activation of ADAM, with subsequent transactivation of the EGF receptor[17]. Subsequently, the carboxy-terminal fragments of HB-EGF are trafficked into the nucleus by special metalloproteinases, and exert some effects on regulation of cell proliferation[43]. Thus, IL-8 induces CRC cell proliferation and migration by an ADAM-dependent pathway, and HB-EGF plays an important role as the major ligand for this pathway[17]. Although these studies explained the association between IL-8 and CRC, further studies are required.
To determine the sources of heterogeneity is also an important goal of a meta-analysis. The different geographical regions and sample sizes may partially explain the heterogeneity observed in our study; however, there was still significant heterogeneity. Another important reason may be that there is wide range of cutoff values for IL-8 levels in the included studies.
The present meta-analysis has several strengths. First, this is the first meta-analysis to assess the diagnostic value of IL-8 to detect CRC. Second, most of the included studies were of high methodological quality. Third, an original study was also applied to explore the diagnostic value of IL-8 in CRC. In addition, no publication bias was observed.
This study also has some limitations. First, the number of studies involved in the meta-analysis was not large enough. Second, significant heterogeneity was found. Sensitivity and subgroup analyses were applied, while the results could not fully explain the observed heterogeneity.
In conclusion, the present meta-analysis confirmed the diagnostic value of IL-8 to detect CRC and it might represent a clinically useful tool to identify high-risk patients.
COMMENTS
Background
Detection of early-stage colorectal cancer (CRC) is critical for curative treatment interventions, which can significantly reduce the incidence and mortality. However, the current clinical screening is far from adequate. Thus, non-invasive diagnostic biomarkers are critical for early detection of CRC.
Research frontiers
Interleukin-8 (IL-8) is one of the most significantly upregulated chemokines in CRC, and contributes to tumor growth, invasion and metastasis in CRC. Several studies have evaluated the application of IL-8 in CRC diagnosis.
Innovations and breakthroughs
This is the first meta-analysis to assess the diagnostic value of IL-8 in detection of CRC. The authors also performed an original study to further support the conclusion.
Applications
IL-8 provided good specificity for CRC and it may represent a clinically useful tool to identify high-risk patients.
Peer review
This is a good meta-analysis in which the authors conducted an original study and a meta-analysis evaluating the diagnostic value of serum IL-8 levels in CRC. The results are interesting and suggest that IL-8 may represent a clinically useful tool to identify high-risk patients.
Footnotes
P- Reviewer: Bao BY S- Editor: Nan J L- Editor: Stewart G E- Editor: Liu XM
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Connell W. PRO: Endoscopic surveillance minimizes the risk of cancer. Am J Gastroenterol. 2004;99:1631–1633. doi: 10.1111/j.1572-0241.2004.40829.x. [DOI] [PubMed] [Google Scholar]
- 4.Müller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann Intern Med. 1995;123:904–910. doi: 10.7326/0003-4819-123-12-199512150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Bond JH. Colorectal cancer screening: the potential role of virtual colonoscopy. J Gastroenterol. 2002;37 Suppl 13:92–96. doi: 10.1007/BF02990108. [DOI] [PubMed] [Google Scholar]
- 6.Bond JH. Fecal occult blood test screening for colorectal cancer. Gastrointest Endosc Clin N Am. 2002;12:11–21. doi: 10.1016/s1052-5157(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 7.Parente F, Boemo C, Ardizzoia A, Costa M, Carzaniga P, Ilardo A, Moretti R, Cremaschini M, Parente EM, Pirola ME. Outcomes and cost evaluation of the first two rounds of a colorectal cancer screening program based on immunochemical fecal occult blood test in northern Italy. Endoscopy. 2013;45:27–34. doi: 10.1055/s-0032-1325800. [DOI] [PubMed] [Google Scholar]
- 8.Khalid-de Bakker C, Jonkers D, Smits K, Mesters I, Masclee A, Stockbrügger R. Participation in colorectal cancer screening trials after first-time invitation: a systematic review. Endoscopy. 2011;43:1059–1086. doi: 10.1055/s-0031-1291430. [DOI] [PubMed] [Google Scholar]
- 9.Ramos M, Llagostera M, Esteva M, Cabeza E, Cantero X, Segarra M, Martín-Rabadán M, Artigues G, Torrent M, Taltavull JM, et al. Knowledge and attitudes of primary healthcare patients regarding population-based screening for colorectal cancer. BMC Cancer. 2011;11:408. doi: 10.1186/1471-2407-11-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bünger S, Haug U, Kelly M, Posorski N, Klempt-Giessing K, Cartwright A, Fitzgerald SP, Toner V, McAleer D, Gemoll T, et al. A novel multiplex-protein array for serum diagnostics of colon cancer: a case-control study. BMC Cancer. 2012;12:393. doi: 10.1186/1471-2407-12-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pengjun Z, Xinyu W, Feng G, Xinxin D, Yulan L, Juan L, Xingwang J, Zhennan D, Yaping T. Multiplexed cytokine profiling of serum for detection of colorectal cancer. Future Oncol. 2013;9:1017–1027. doi: 10.2217/fon.13.71. [DOI] [PubMed] [Google Scholar]
- 12.Schulmann K, Reiser M, Schmiegel W. Colonic cancer and polyps. Best Pract Res Clin Gastroenterol. 2002;16:91–114. doi: 10.1053/bega.2002.0268. [DOI] [PubMed] [Google Scholar]
- 13.Doll D, Keller L, Maak M, Boulesteix AL, Siewert JR, Holzmann B, Janssen KP. Differential expression of the chemokines GRO-2, GRO-3, and interleukin-8 in colon cancer and their impact on metastatic disease and survival. Int J Colorectal Dis. 2010;25:573–581. doi: 10.1007/s00384-010-0901-1. [DOI] [PubMed] [Google Scholar]
- 14.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 15.Ning Y, Lenz HJ. Targeting IL-8 in colorectal cancer. Expert Opin Ther Targets. 2012;16:491–497. doi: 10.1517/14728222.2012.677440. [DOI] [PubMed] [Google Scholar]
- 16.Terada H, Urano T, Konno H. Association of interleukin-8 and plasminogen activator system in the progression of colorectal cancer. Eur Surg Res. 2005;37:166–172. doi: 10.1159/000085964. [DOI] [PubMed] [Google Scholar]
- 17.Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, Oshima T, Ogasawara N, Togawa S, Wada T, et al. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine. 2005;29:275–282. doi: 10.1016/j.cyto.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Kantola T, Klintrup K, Väyrynen JP, Vornanen J, Bloigu R, Karhu T, Herzig KH, Näpänkangas J, Mäkelä J, Karttunen TJ, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer. 2012;107:1729–1736. doi: 10.1038/bjc.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bünger S, Haug U, Kelly FM, Klempt-Giessing K, Cartwright A, Posorski N, Dibbelt L, Fitzgerald SP, Bruch HP, Roblick UJ, et al. Toward standardized high-throughput serum diagnostics: multiplex-protein array identifies IL-8 and VEGF as serum markers for colon cancer. J Biomol Screen. 2011;16:1018–1026. doi: 10.1177/1087057111414894. [DOI] [PubMed] [Google Scholar]
- 20.Kaminska J, Nowacki MP, Kowalska M, Rysinska A, Chwalinski M, Fuksiewicz M, Michalski W, Chechlinska M. Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: soluble tumor necrosis factor receptor type I--an independent prognostic factor. Tumour Biol. 2005;26:186–194. doi: 10.1159/000086951. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer JE, Bachmann LM, Jaeschke R. A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29:1043–1051. doi: 10.1007/s00134-003-1761-8. [DOI] [PubMed] [Google Scholar]
- 25.Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA. 1994;271:703–707. doi: 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- 26.Devillé WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, Bezemer PD. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deeks JJ. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ. 2001;323:157–162. doi: 10.1136/bmj.323.7305.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. doi: 10.1016/s0895-4356(03)00177-x. [DOI] [PubMed] [Google Scholar]
- 31.Rubie C, Frick VO, Pfeil S, Wagner M, Kollmar O, Kopp B, Graber S, Rau BM, Schilling MK. Correlation of IL-8 with induction, progression and metastatic potential of colorectal cancer. World J Gastroenterol. 2007;13:4996–5002. doi: 10.3748/wjg.v13.i37.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galffy G, Mohammed KA, Dowling PA, Nasreen N, Ward MJ, Antony VB. Interleukin 8: an autocrine growth factor for malignant mesothelioma. Cancer Res. 1999;59:367–371. [PubMed] [Google Scholar]
- 33.Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- 34.Wilson AJ, Byron K, Gibson PR. Interleukin-8 stimulates the migration of human colonic epithelial cells in vitro. Clin Sci (Lond) 1999;97:385–390. [PubMed] [Google Scholar]
- 35.Rollins BJ. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 36.Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, Miller LH. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261:1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- 37.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. [PubMed] [Google Scholar]
- 38.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- 39.Beckmann MP, Munger WE, Kozlosky C, VandenBos T, Price V, Lyman S, Gerard NP, Gerard C, Cerretti DP. Molecular characterization of the interleukin-8 receptor. Biochem Biophys Res Commun. 1991;179:784–789. doi: 10.1016/0006-291x(91)91885-g. [DOI] [PubMed] [Google Scholar]
- 40.Cerretti DP, Kozlosky CJ, Vanden Bos T, Nelson N, Gearing DP, Beckmann MP. Molecular characterization of receptors for human interleukin-8, GRO/melanoma growth-stimulatory activity and neutrophil activating peptide-2. Mol Immunol. 1993;30:359–367. doi: 10.1016/0161-5890(93)90065-j. [DOI] [PubMed] [Google Scholar]
- 41.Lee YS, Choi I, Ning Y, Kim NY, Khatchadourian V, Yang D, Chung HK, Choi D, LaBonte MJ, Ladner RD, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. Br J Cancer. 2012;106:1833–1841. doi: 10.1038/bjc.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 43.Nanba D, Mammoto A, Hashimoto K, Higashiyama S. Proteolytic release of the carboxy-terminal fragment of proHB-EGF causes nuclear export of PLZF. J Cell Biol. 2003;163:489–502. doi: 10.1083/jcb.200303017. [DOI] [PMC free article] [PubMed] [Google Scholar]


