Abstract
Repeated anastomotic recurrence (AR) of colonic cancer is uncommon. We report a case of a double-isolated AR after sigmoidectomy. In 2003, a 60-year-old woman underwent stapled sigmoid resection for a moderately differentiated adenocarcinoma. Further rectal bleeding occurred after six months, and colonoscopy detected an AR. Thus, an additional stapled colorectal anastomosis was performed. Ten months later, a colonoscopy detected a circumferential AR that prompted the completion of a second colorectal resection, with a double-stapled colorectal anastomosis. Twenty-four hours after surgery, a massive pulmonary embolism occurred, and the patient died within a few hours. At present, only six cases of repeated isolated AR have been described. Repeated segmental colorectal resections are generally associated with a favourable prognosis, with a median survival rate of 45 mo (range, 13-132 mo). Repeated isolated ARs are rare, and segmental colorectal resections are generally associated with long-term disease-free survival.
Keywords: Repeated Anastomotic Recurrence, Stapled Anastomosis, Viable Exfoliated Cells
Core tip: We report a case of a double isolated anastomotic recurrence (AR) after sigmoidectomy. At present, only six cases of repeated isolated AR have been described. Repeated isolated ARs are rare, and segmental colorectal resections are generally associated with long-term disease-free survival.
INTRODUCTION
Colorectal cancer is the second most common neoplasia in Western countries. Sometimes, within 24 mo after curative surgery, a locoregional extraluminal recurrence may occur; the resultant 5-year mortality rate is 80%-90%[1]. Emergency surgery, poor colorectal surgery skills, poor tumour differentiation and advanced disease stages are the main risk factors of death[2,3].
Many series have focused on local recurrences following the surgical treatment of rectal cancer, whereas only a few published studies have investigated locoregional recurrences among patients undergoing colonic cancer surgery[4].
However, isolated (without disseminated disease) anastomotic recurrence (AR) inside the bowel lumen is uncommon, although it is generally associated with better long-term survival compared to locoregional recurrence. Historically, the risk is greater for rectal cancer than colonic cancer, with a reported AR incidence of 2%-4%[5].
Moreover, repeated AR following colorectal resections for colonic cancer is unusual, and all published data are case reports.
AR usually results from the luminal implantation of viable exfoliated tumoral cells during surgical manipulation or from proliferative instability at the anastomotic site promoting metachronous carcinogenesis[6,7].
Herein, we describe a rare case in which an isolated double AR following curative mechanical sigmoid resection was observed.
Using the keywords “colorectal anastomotic recurrence”, “local recurrence”, “locoregional recurrence”, “repeated recurrence”, “local relapse”, and “viable exfoliated cells”, a PubMed database search (PubMed, National Library of Medicine, Bethesda, MD, United States) was performed. Few cases of subsequent colorectal AR following colonic resections have been previously described, and each case was diagnosed by rectal bleeding or follow-up colonoscopy. In most cases, a favourable prognosis following repeated segmental colorectal resections was observed.
CASE REPORT
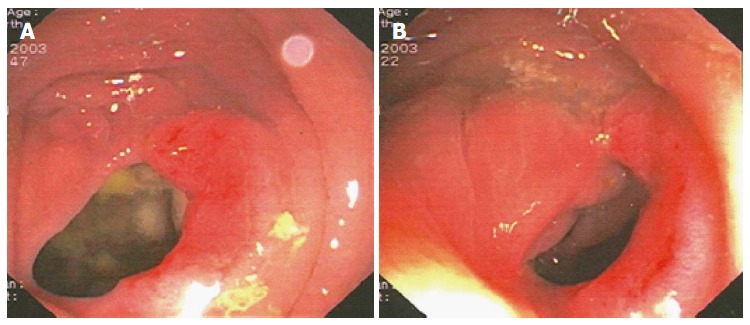
In June 2003, we observed a 60-year-old woman complaining of constipation and rectal bleeding. She was not affected by any coagulation disorder or major comorbidities. Colonoscopy revealed a circumferential friable neoplasm, with large necrotic areas in the sigmoid colon, 35 cm from the anal verge. The neoplasm strictured the lumen, preventing further passage of the endoscope (13 mm) (Figure 1).
Figure 1.
June 2003-Lumen stricture caused by circumferential sigmoid colon cancer 35 cm from the anal verge.
The patient underwent a preoperative work-up consisting of routine blood tests, including tumour markers, and a whole body computed tomography (CT) scan, which detected neither local infiltration nor liver and pulmonary metastases. A segmental sigmoid colon resection was performed, followed by a stapled colorectal side-to-end anastomosis (Premium Plus CEEA™ Stapler 31, Covidien®, Dublin, Ireland). Pathological examination showed a moderately differentiated (G2) adenocarcinoma, without any vascular or lymphatic invasion; the tumour was stage 1, according to the TNM classification (pT2N0M0), and B1, according to the Astler and Coller classification. No metastases were detected in the twenty excised lymph nodes, and the resection margins were 5 cm proximal and 3 cm distal.
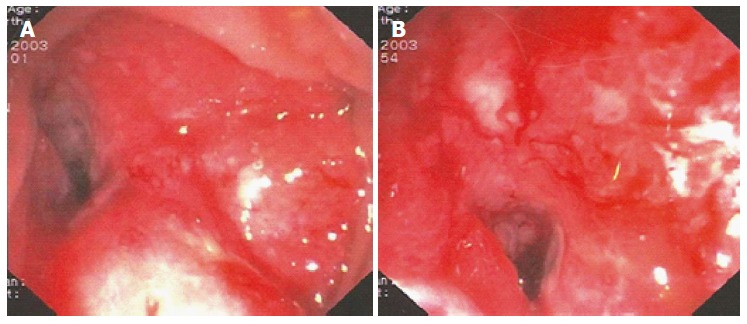
Based on the Italian Association of Medical Oncology guidelines[8], the patient did not undergo chemotherapy, and she was enrolled in a follow-up program. After six months, new rectal bleeding occurred, and colonoscopy detected an area of hypertrophic and oedematous mucosa approximately 18 cm from the anal verge that occupied half of the circumference of the colorectal anastomosis (Figure 2).
Figure 2.
December 2003 - First colorectal AR, six months after primary surgery.
Several biopsies showed infiltration of the lamina propria related to an adenocarcinoma recurrence. Contrast-enhanced whole-body CT scan did not rule out any distant or local metastases.
In January 2004, a segmental colorectal resection was performed (Premium Plus CEEA™ Stapler 31, Covidien®, Dublin, Ireland), followed by a stapled colorectal side-to-end anastomosis. Pathological examination confirmed the pre-operative diagnosis of G2 adenocarcinoma with tumour-free aortocaval lymph nodes (pT2N0M0), and a normal mucosal pattern 8 cm proximal and 6 cm distal from the tumour was reported. A 10-cycle chemotherapy regimen with 5-fluorouracile 425 mg/m2 and folic acid 20 mg/m2 was performed weekly. The colonoscopy that was performed 10 mo later detected a further circumferential recurrence at the previous anastomotic site (Figure 3).
Figure 3.
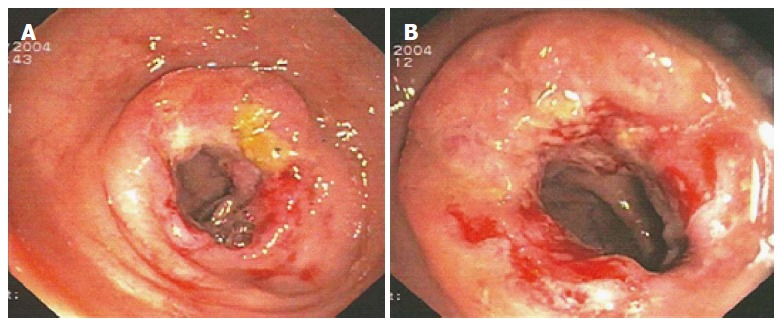
November 2004 - Second AR, seventeen months after primary surgery.
Contrast-enhanced CT scan did not reveal any distant metastases. The serum CEA level was normal. In November 2004, a colorectal resection (with a double-stapled colorectal anastomosis approximately 5 cm from the anal verge) and an abdominopelvic lymphadenectomy were performed. Pathological examination showed a G2 adenocarcinoma AR infiltrating the muscular layer (pT3N0M0-Astler and Coller stage B2); the circumferential margin of resection and 10 excised lymph nodes were tumour free. Twenty-four hours after surgery, a massive pulmonary embolism occurred, and the patient died few hours later, despite thromboprophylaxis therapy with low-weight heparin (nadroparin 2.850 UI) that had been continuously administered since the night before surgery.
DISCUSSION
The literature review found only a few previous reports of repeated isolated AR, all of which occurred fewer than two years after curative resection for colonic cancer. The first case was described by Roncoroni et al[9] in a 65- year-old female patient (double AR). AR (pT2N0M0) was observed one year after a stapled left hemicolectomy, and the second occurrence (pT2N0M0) came nine months after the colorectal resection. The patient underwent stapled colorectal and, subsequently, a double-stapled coloanal anastomosis and adjuvant radiotherapy. She was disease free 54 mo after the first operation. The second case was described by Yajima et al[10] in a 44 -year-old woman. The first recurrence occurred after 12 mo, and the second occurred after 34 mo following a mechanical sigmoidectomy for G2 adenocarcinoma of the sigmoid colon. A double-stapled colorectal anterior resection for the repeated AR was performed, followed by adjuvant chemotherapy. Perioperative rectal remnant irrigation was performed with 2000 mL of normal saline. No evidence of further recurrence was found 48 mo after the patient’s initial operation. Futami et al[11] reported another case in a 64-year-old female patient (double recurrence). The first AR was observed only 3 mo after a stapled sigmoidectomy for a pN0 adenocarcinoma, with no lymphatic or vascular invasion. Preoperative colonic retrograde irrigation and perioperative lavage of a rectal remnant by a polyvinylpyrrolidone iodine (PVP-I) solution were performed, along with the stapled colorectal resection. An abdominoperineal resection for a second (pN0) AR was carried out after twelve months. The survival rate was not reported. Funahashi et al[12] described an exceptional case of repeated (three times) AR in a 51-year-old man at 6, 15 and 24 mo after the first sigmoidectomy. This patient received an anterior rectal resection, associated with colonic irrigation by 5% PVP-I; a low anterior resection, associated with an en-bloc resection of small intestine and bladder; and finally, an abdominoperineal resection with lateral lymphadenectomy. Approximately 8 years after surgery, the patient was well, demonstrating favourable oncological outcomes for AR.
In the reported cases, the AR diagnosis was confirmed by colonoscopy or rectal bleeding. Most serum CEA values were normal, and repeated segmental colorectal resections were associated with a favourable prognosis, with a median survival rate of 45 mo (range, 13-132 mo). Only 2 cases required the performance of an abdominoperineal resection (Table 1). Repeated AR that occurred despite colorectal irrigation represented 3 of the 6 reported cases.
Table 1.
Subsequent isolated anastomotic recurrences following colorectal resections for colonic cancer: the characteristics of reported cases
| Ref. | Year | Age (yr) | AR1 (number) | First AR1 interval time (mo) | CEA level (ng/mL) | Survival (mo) | Colorectal irrigation |
| Roncoroni et al[9] | Dis Colon Rectum 2004 | 65 Female | 2 | 12 | Normal | 54 | - |
| Futami et al[11] | J Nippon Med Sch 2007 | 64 Male | 2 | 3 | Normal | 13 | Yes |
| Funahashi et al[12] | WJSO 2007 | 51 Male | 3 | 6 | Normal | 42 | Yes |
| Yajima et al[10] | Surg Today 2007 | 44 Female | 2 | 12 | Normal | 48 | Yes |
| Costi et al[19] | WJS 2011 | 65 Female | 2 | 21 | Normal | 132 | - |
| Conzo et al | - | 60 Female | 2 | 6 | Normal | 17 | - |
AR: Anastomotic recurrences.
Regardless of notable therapeutic progress, local recurrence after curative colorectal resection occurs in up to 32% of patients[11]. More frequently, locoregional extraluminal recurrence occurs within 24 mo after surgery, often without disseminated disease and generally resulting in a poor prognosis. This incidence is higher for distal localisation (sigmoid colon and rectum), confirming the higher malignancy of left colon cancer. Inadequate resection margins and occult lymphatic involvement are the most frequent causes.
However, isolated colonic recurrences at the anastomotic site following colorectal resections for colonic cancer are uncommon, with a 4%-12% rate reported[5]. A higher risk is observed in the treatment of rectal cancer. AR is generally associated with a better long term survival[13,14], which has also been confirmed in the treatment of rectal cancer by Matsuda et al[15] who found a higher survival rate in AR compared to pelvic recurrence among 21 patients undergoing rectal cancer surgery.
In most cases, incomplete resection or implantation of exfoliated cells was involved[2,16].
Biological, surgical and genetic factors should be considered in determining colorectal local recurrence. Aggressive histological type, poor differentiation (G3) and advanced tumour stage are the main biological risk factors[17]. Radial margins, inadvertent rectal perforation, distal and proximal rectal margins, and en-bloc resections of adherent tumours are considered to be the main surgical risk factors[18]. Costi et al[19] also demonstrated a high genetic instability in two patients who were affected by repeated AR.
In our case, the pathological examination of all specimens showed tumour-free margins, as well as the absence of lymph-vascular invasion and no lymph node involvement or occult metastases. Hence, this condition was considered to be a double isolated primary AR rather than a perianastomotic locoregional case. The implantation of viable exfoliated cells appears to be the main cause of recurrence. Exfoliation and subsequent cancer cell implantation remain a controversial subject of active research. Exfoliated cells may proliferate along the anastomotic site, often after stapled anastomosis, precipitating a local relapse. The isolation of viable desquamated cancer cells from colonic lavage solution or from stapling devices has been well demonstrated by different studies. Although these cells are capable of implanting on the raw surface of suture materials, the AR rate after curative colorectal resections, which are often performed without perioperative colorectal irrigation, remains low. Therefore, different unknown AR pathogenetic factors should be investigated. Moreover, it is well known that anal fissure or fistula, haemorrhoidectomy sites, and other types of mucosal injury can promote the implantation and growth of viable cancer cells[20]. To decrease implantation risk, Jenner et al[21] proposed intraluminal irrigation with the cytocidal agent PVP-I. This procedure decreased suture line recurrence rates from 10%-16% to 2%-3%[11]. However, after curative resections, we do not routinely perform colorectal remnant irrigation; in the literature, the indication data are debatable. In addition, in the subsequent AR-presenting cases, perioperative colorectal lavage by PVP-I or by normal saline, as performed in 3 out of 6 cases, was still unable to prevent AR.
Keighley et al[22] described another mechanism for local recurrence based on the biological instability at the anastomotic site, which may increase susceptibility to cancer. Animal studies have shown an increased yield of bowel tumours at the site of anastomosis after resection, regardless of whether the insult occurred before or after the administration of common chemical carcinogens.
It has been suggested that the proliferative instability along the anastomotic line caused by suture materials can allow the engraftment of exfoliated malignant cells[23-25]. Titanium and descending braided sutures can provide a substrate for exfoliated malignant cells, thereby resulting in recurrences along the suture line. However, despite reports of AR caused by cancer cell implantation using the double-stapling technique[26-29], no statistically significant difference has been reported in the local recurrence rate between stapled and hand-sewn anastomoses[26,29,30]. Experimental studies recommend the use of absorbable monofilament manual sutures because of their lower carcinogenic potential. Nevertheless, there is no statistical evidence supporting this hypothesis[31].
In our case, we cannot exclude the possibility that the isolated AR was caused by metachronous carcinogenesis, as demonstrated in Roncoroni’s case by the appearance of polyps at anastomosis site[9].
Because of the rarity of isolated repeated AR, there is no standardised surgical strategy. Whenever possible, AR should be addressed by the re-excision of the anastomosis, with 5-cm clear margins. Segmental colectomy and total colectomy are both acceptable options[32].
The blood supply to the remaining bowel should always be assessed when planning these subsequent resections. Segmental colorectal resections are generally associated with long-term, disease-free survival.
In conclusion, after curative stapled surgical resection for colonic cancer, isolated repeated AR is rare, and a favourable prognosis has mostly been reported; however, disease management may be associated with significant morbidity or mortality rates. This outcome may be because of either the implantation of viable cells or the proliferative instability of the stapled suture line; furthermore, genetic factors may also be involved. Colorectal irrigation by PVP-I or by normal saline did not prevent repeated AR in the reported cases. An early diagnosis by intensive endoscopic follow-up can allow for curative colorectal resections. Based on our limited experience in managing operable AR, segmental resection followed by intensive careful endoscopic monitoring could result in long-term disease-free survival.
COMMENTS
Case characteristics
A 60-year-old woman with a history of double isolated anastomotic recurrence after sigmoidectomy.
Clinical diagnosis
Constipation and rectal bleeding.
Differential diagnosis
Local recurrence, metachronous carcinogenesis.
Laboratory diagnosis
WBC 7.3 k/uL; HBG 10.5gm/dL; CEA 1.3 ng/mL; metabolic panel and liver function test were within normal limits.
Imaging diagnosis
Colonoscopy revealed a circumferential friable neoplasm of the sigmoid colon. Whole body computer tomography scan did not detect either local infiltration or liver and pulmonary metastases.
Pathological diagnosis
Pathological examination showed a moderately differentiated (G2) adenocarcinoma, without vascular or lymphatic invasion, stage 1 according to TNM classification (pT2N0M0) and B1 according to Astler and Coller classification after the first operation.
Treatment
A segmental sigmoid colon resection, followed by a stapled colorectal side-to-end anastomosis, was performed, followed by segmental colectomies for the treatment of anastomotic recurrences (ARs).
Related reports
Because of the rarity of isolated repeated AR, there is no standardized surgical strategy; when is possible an AR should be addressed by reexcision of the anastomosis with 5-cm clear margins.
Term explanation
The Authors believe that there are no uncommon terms in the case report.
Experiences and lessons
After curative stapled surgical resection for colonic cancer, isolated repeated AR is rare; it may be due either to the implantation of viable cells or to the proliferative instability of the stapled suture line, and yet genetic factors may be involved; Segmental resection, followed by intensive endoscopic careful monitoring, could result in a long-term disease-free survival.
Peer review
Because of the rarity of isolated repeated AR, there is no standardized surgical strategy; AR may be due either to the implantation of viable cells or to the proliferative instability of the stapled suture line, and yet genetic factors may be involved; although limited experience in the management of operable AR, segmental resection, followed by intensive endoscopic careful monitoring, could result in a long-term disease-free survival.
Footnotes
P- Reviewer: Consten ECJ, Wong TM S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Thomson WH, Foy CJ, Longman RJ. The nature of local recurrence after colorectal cancer resection. Colorectal Dis. 2008;10:69–74. doi: 10.1111/j.1463-1318.2007.01223.x. [DOI] [PubMed] [Google Scholar]
- 2.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996–999. doi: 10.1016/s0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 3.Gricouroff G. Pathogenesis of recurrences on the suture line following surgical resection for carcinoma of the colon. Cancer. 1967;20:673–676. doi: 10.1002/1097-0142(1967)20:5<673::aid-cncr2820200517>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Elferink MA, Visser O, Wiggers T, Otter R, Tollenaar RA, Langendijk JA, Siesling S. Prognostic factors for locoregional recurrences in colon cancer. Ann Surg Oncol. 2012;19:2203–2211. doi: 10.1245/s10434-011-2183-4. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Kahi CJ, Levin B, Smith RA, Bond JH, Brooks D, Burt RW, Byers T, Fletcher RH, Hyman N, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130:1865–1871. doi: 10.1053/j.gastro.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 6.van den Tol PM, van Rossen EE, van Eijck CH, Bonthuis F, Marquet RL, Jeekel H. Reduction of peritoneal trauma by using nonsurgical gauze leads to less implantation metastasis of spilled tumor cells. Ann Surg. 1998;227:242–248. doi: 10.1097/00000658-199802000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gertsch P, Baer HU, Kraft R, Maddern GJ, Altermatt HJ. Malignant cells are collected on circular staplers. Dis Colon Rectum. 1992;35:238–241. doi: 10.1007/BF02051014. [DOI] [PubMed] [Google Scholar]
- 8.Umpleby HC, Williamson RC. Anastomotic recurrence in large bowel cancer. Br J Surg. 1987;74:873–878. doi: 10.1002/bjs.1800741003. [DOI] [PubMed] [Google Scholar]
- 9.Roncoroni L, Costi R, Marchesi F, Bottarelli L, Sarli L, Violi V, Bordi C. Second anastomotic recurrence after radical left hemicolectomy: report of a case. Dis Colon Rectum. 2004;47:1547–1549. doi: 10.1007/s10350-004-0617-9. [DOI] [PubMed] [Google Scholar]
- 10.Yajima K, Matsuo H, Kobayashi T, Ajioka Y, Hatakeyama K. Curative resection performed twice for circular-staple-line recurrence after colorectal carcinoma surgery: report of a case. Surg Today. 2007;37:61–65. doi: 10.1007/s00595-006-3327-1. [DOI] [PubMed] [Google Scholar]
- 11.Futami R, Shimanuki K, Sugiura A, Tsuchiya Y, Kaneko M, Okawa K, Mineta S, Sugiyama Y, Akimaru K, Tajiri T. Recurrence of colonic cancer twice at the site of stapled colorectal anastomosis. J Nippon Med Sch. 2007;74:251–256. doi: 10.1272/jnms.74.251. [DOI] [PubMed] [Google Scholar]
- 12.Funahashi K, Koike J, Saito N, Shiokawa H, Shirasaka K, Teramoto T. A rare case of repeated anastomotic recurrence due to tumor implantation after curative surgery for sigmoid colon cancer. World J Surg Oncol. 2007;5:91. doi: 10.1186/1477-7819-5-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum. 1997;40:15–24. doi: 10.1007/BF02055676. [DOI] [PubMed] [Google Scholar]
- 14.Pietra N, Sarli L, Thenasseril BJ, Costi R, Sansebastiano G, Peracchia A. Risk factors of local recurrence of colorectal cancer: a multivariate study. Hepatogastroenterology. 1998;45:1573–1578. [PubMed] [Google Scholar]
- 15.Matsuda K, Hotta T, Takifuji K, Yokoyama S, Oku Y, Yamaue H. Clinicopathological features of anastomotic recurrence after an anterior resection for rectal cancer. Langenbecks Arch Surg. 2010;395:235–239. doi: 10.1007/s00423-009-0519-3. [DOI] [PubMed] [Google Scholar]
- 16.Umpleby HC, Fermor B, Symes MO, Williamson RC. Viability of exfoliated colorectal carcinoma cells. Br J Surg. 1984;71:659–663. doi: 10.1002/bjs.1800710902. [DOI] [PubMed] [Google Scholar]
- 17.Law WL, Chu KW. Local recurrence following total mesorectal excision with double-stapling anastomosis for rectal cancers: analysis of risk factors. World J Surg. 2002;26:1272–1276. doi: 10.1007/s00268-002-6560-9. [DOI] [PubMed] [Google Scholar]
- 18.Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- 19.Costi R, Azzoni C, Marchesi F, Bottarelli L, Violi V, Bordi C. Repeated anastomotic recurrence of colorectal tumors: genetic analysis of two cases. World J Gastroenterol. 2011;17:3752–3758. doi: 10.3748/wjg.v17.i32.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norgren J, Svensson JO. Anal implantation metastasis from carcinoma of the sigmoid colon and rectum--a risk when performing anterior resection with the EEA stapler? Br J Surg. 1985;72:602. doi: 10.1002/bjs.1800720806. [DOI] [PubMed] [Google Scholar]
- 21.Jenner DC, de Boer WB, Clarke G, Levitt MD. Rectal washout eliminates exfoliated malignant cells. Dis Colon Rectum. 1998;41:1432–1434. doi: 10.1007/BF02237063. [DOI] [PubMed] [Google Scholar]
- 22.Keighley MR, Hall C. Anastomotic recurrence of colorectal cancer--a biological phenomenon or an avoidable calamity? Gut. 1987;28:786–791. doi: 10.1136/gut.28.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donnell AF, O’Connell PR, Royston D, Johnston DH, Barnard R, Bouchier-Hayes D. Suture technique affects perianastomotic colonic crypt cell production and tumour formation. Br J Surg. 1991;78:671–674. doi: 10.1002/bjs.1800780611. [DOI] [PubMed] [Google Scholar]
- 24.McCue JL, Sheffield JP, Uff C, Phillips RK. Experimental carcinogenesis at sutured and sutureless colonic anastomoses. Dis Colon Rectum. 1992;35:902–909. doi: 10.1007/BF02047881. [DOI] [PubMed] [Google Scholar]
- 25.Mariani PP, van Pelt JF, Ectors N, Topal B, D’Hoore A, Penninckx F. Rectal washout with cytotoxic solution can be extended to the whole colon. Br J Surg. 2002;89:1540–1544. doi: 10.1046/j.1365-2168.2002.02224.x. [DOI] [PubMed] [Google Scholar]
- 26.Hurst PA, Prout WG, Kelly JM, Bannister JJ, Walker RT. Local recurrence after low anterior resection using the staple gun. Br J Surg. 1982;69:275–276. doi: 10.1002/bjs.1800690515. [DOI] [PubMed] [Google Scholar]
- 27.Docherty JG, McGregor JR, Akyol AM, Murray GD, Galloway DJ. Comparison of manually constructed and stapled anastomoses in colorectal surgery. West of Scotland and Highland Anastomosis Study Group. Ann Surg. 1995;221:176–184. doi: 10.1097/00000658-199502000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuto T, Tsukamoto T, Ohta Y, Takemura M, Ikebe T, Kinoshita H. Anastomotic recurrence due to tumor implantation using the double stapling technique. Hepatogastroenterology. 1999;46:2521–2522. [PubMed] [Google Scholar]
- 29.Di Vizio D, Insabato L, Conzo G, Zafonte BT, Ferrara G, Pettinato G. Sarcomatoid carcinoma of the colon: a case report with literature review. Tumori. 2001;87:431–435. doi: 10.1177/030089160108700615. [DOI] [PubMed] [Google Scholar]
- 30.Redmond HP, Austin OM, Clery AP, Deasy JM. Safety of double-stapled anastomosis in low anterior resection. Br J Surg. 1993;80:924–927. doi: 10.1002/bjs.1800800746. [DOI] [PubMed] [Google Scholar]
- 31.Nomdedeu-Guinot J, Giber-Gerez J, Reig IC, Sanchís JL, Planelles RC, Del Castillo JR. Suture materials and local recurrence in colorectal cancer: an experimental study. Eur J Surg. 2001;167:142–145. doi: 10.1080/110241501750070628. [DOI] [PubMed] [Google Scholar]
- 32.Hellinger MD, Santiago CA. Reoperation for recurrent colorectal cancer. Clin Colon Rectal Surg. 2006;19:228–236. doi: 10.1055/s-2006-956445. [DOI] [PMC free article] [PubMed] [Google Scholar]


