Abstract
Congenital defects of neural tube closure (neural tube defects; NTDs) are among the commonest and most severe disorders of the fetus and newborn. Disturbance of any of the sequential events of embryonic neurulation produce NTDs, with the phenotype (e.g. anencephaly, spina bifida) varying depending on the region of neural tube that remains open. While mutation of more than 200 genes is known to cause NTDs in mice, the pattern of occurrence in humans suggests a multifactorial polygenic or oligogenic aetiology. This emphasises the importance of gene-gene and gene-environment interactions in the origin of these defects. A number of cell biological functions are essential for neural tube closure, with defects of the cytoskeleton, cell cycle and molecular regulation of cell viability prominent among the mouse NTD mutants. Many transcriptional regulators and proteins that affect chromatin structure are also required for neural tube closure, although the downstream molecular pathways regulated by these proteins is unknown. Some key signalling pathways for NTDs have been identified: over-activation of sonic hedgehog signalling and loss of function in the planar cell polarity (non-canonical Wnt) pathway are potent causes of NTD, with requirements also for retinoid and inositol signalling. Folic acid supplementation is an effective method for primary prevention of a proportion of NTDs, in both humans and mice, although the embryonic mechanism of folate action remains unclear. Folic acid-resistant cases can be prevented by inositol supplementation in mice, raising the possibility that this could lead to an additional preventive strategy for human NTDs in future.
Introduction
Neural tube defects (NTDs) are common, severe congenital malformations, affecting 0.5-2 per 1000 established pregnancies, world wide [1]. Failure of the embryonic process of neural tube closure yields a brain and/or spinal cord in which neural tissue is exposed to the extra-embryonic environment. This leads to neuro-degeneration in utero, with loss of neurological function at and below the level of the lesion. As perinatal and infant mortality has declined progressively, with improvements in prenatal, intra-partum and neonatal care, so congenital defects including NTDs have come to comprise an ever more significant proportion of the life-threatening conditions of infancy. Children with a severe birth defect have a 15-fold increased risk of death during the first year of life, with 9-10% of such children dying during this period [2]. NTDs affecting the brain (e.g. anencephaly and craniorachischisis) are invariably lethal perinatally, whereas open spina bifida is compatible with postnatal survival, but frequently results in serious handicap. Neurological impairment below the lesion leads to lack of sensation, inability to walk and incontinence. Associated conditions include hydrocephalus, which often requires cerebrospinal fluid shunting, vertebral deformities, and genitourinary and gastrointestinal disorders.
Neural tube formation: primary and secondary neurulation
Formation of the brain and spinal cord begins with development of the neural tube, through the embryonic process of neurulation. The neural plate originates as a thickening of the dorsal surface ectoderm that folds up and fuses in the midline to create the neural tube. In mammals, neural tube closure initiates sequentially at different levels of the body axis (Fig. 1A). The sequence in the mouse has been most intensively studied, where closure is initiated at the hindbrain/cervical boundary (Closure 1). Fusion then spreads bi-directionally into the hindbrain and along the spinal region. Separate closure initiation sites occur at the midbrain/forebrain boundary (Closure 2) and at the rostral extremity of the forebrain (Clos 3). Progression of fusion (‘zippering’) occurs along the spine, culminating in final closure at the posterior neuropore, at the level of the second sacral segment. This completes the process of ‘primary’ neurulation (by neural folding).
Figure 1. Diagrammatic representation of neural tube closure and the origin of NTDs in (A) mouse and (B) human embryos.
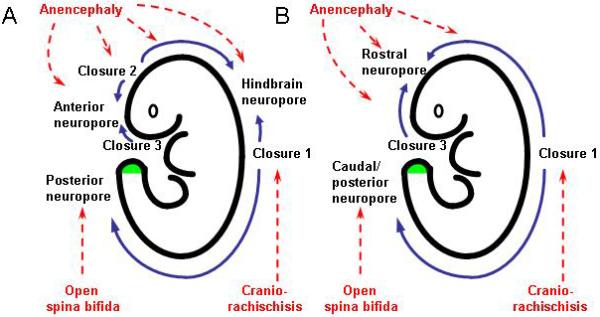
Initiation events (Closures 1, 2, 3) and completion events (at ‘neuropores’) are joined by unidirectional or bidirectional neural tube zippering (blue arrows). The affected events leading to NTDs (red labels) are indicated by red arrows. Secondary neurulation proceeds from the level of the closed posterior neuropore, as a result of canalisation within the tail bud (green). Modified from [6][64].
Formation of the spinal cord at lower sacral and caudal levels is accomplished by a different process, ‘secondary’ neurulation, in which a multipotential cell population in the tail bud (or caudal eminence: a derivative of the primitive streak at the caudal extremity; Fig. 1), differentiates to form cells of neural fate, which then organise themselves in the dorsal part of the tail bud to create a neuroepithelium surrounding a central cavity. This process, termed ‘canalisation’, yields a sacro-caudal neural tube whose lumen is continuous with the lumen of the primary neural tube more rostrally. The secondary neural tube forms a model around which lateral sclerotomal cells, also derived from the tail bud multipotential cell population, organise themselves to form the sacral and coccygeal vertebrae. The neural tube is underlain by the tail notochord and, more ventrally, by the tail-gut. During subsequent development, the caudal-most neural tube undergoes degeneration, by apoptosis.
The events of primary and secondary neurulation are conserved between mammalian species, although some differences exist in the precise sequence of closure events (Fig. 1B). For example, the human embryo appears to lack a de novo Closure 2 event at the midbrain/forebrain boundary. Instead, fusion progresses rostrally from Closure 1 and caudally from Closure 3 to close at a single anterior (rostral or cranial) neuropore [3]. Interestingly, mouse embryos that lack Closure 2 (the SELH/Bc inbred strain) achieve total cranial closure in around 80% of cases [4], indicating that Closure 2 is not obligatory for brain formation, even in mice.
Spectrum of NTDs, their natural history and prevention
In contrast to Closure 2, all other aspects of primary neurulation are essential for proper formation of the definitive brain and/or spinal cord. Failure of Closure 1 leads to the most severe NTD, craniorachischisis, which combines an open neural tube encompassing the midbrain, hindbrain and entire spinal region. If Closure 1 is completed, but closure of the cranial neural tube is incomplete, this leads to anencephaly with cases exhibiting either defects confined to the midbrain (meroanencephaly) or lesions extending into the hindbrain (holoanencephaly) [5]. Failure of Closure 3 is uncommon but when present yields split face with anencephaly. In the spinal region, failure of final closure at the posterior neuropore yields open spina bifida (myelomeningocele), in which the upper limit can be of varying axial level, depending on precisely when the progression of zippering becomes arrested [6].
Exposure of the open neural tube to the amniotic fluid environment in NTDs leads to neuroepithelial degeneration, with massive loss of neural tissue by the end of pregnancy. In mice, despite failed neural tube closure, neuronal differentiation and formation of functional nerve connections proceed, even below the level of an open spina bifida lesion [7]. However, neuro-degeneration later intervenes to destroy the functional nerves that have been formed. This finding provides a rationale for in utero surgery to cover spina bifida lesions in order to arrest the neurodegenerative process. However, this challenging surgical procedure, which is practised only in a few specialist centres, has so far been shown to offer at best palliation of the defect [8].
Primary preventive strategies, such as folic acid supplementation, by contrast offer complete rescue of neural tube closure, and have already made a major contribution to reducing the prevalence of NTDs in certain countries [9]. Remaining problems in maximising the preventive action of folic acid range from the public health challenge of ensuring widespread folate uptake throughout the population, to the scientific challenge of understanding how folic acid exerts its preventive action during neurulation. Further progress towards eradication of NTDs is likely to require significantly improved understanding of the factors, genetic and environmental, that predispose to NTDs, the embryonic pathogenesis that causes neurulation to fail in affected individuals, and how the underlying molecular and cellular events interact with folates and other exogenous factors.
Multifactorial causation of human NTDs
Both genetic and non-genetic factors are involved in the aetiology of NTDs. The recurrence risk for siblings of index cases is 2-5%, approximately 50-fold more than in the general population, with a further increase in risk after two or more affected pregnancies [10]. This and other lines of epidemiological evidence have led to the conclusion that up to 70% of the variance in NTD prevalence may be due to genetic factors [11]. On the other hand, analysis of NTD frequency in twins has suggested a somewhat lower genetic contribution, although the higher prevalence of NTDs in like-sex (presumed monozygotic) than unlike-sex twin pairs is consistent with a significant genetic component [12]. Previous twin analyses may have underestimated the genetic contribution to NTDs owing to possible connections between neurulation failure and the loss of one member of a twin pair during pregnancy [13]. In any event, it is accepted that the vast majority of NTDs show sporadic occurrence with relatively few reports of multi-generational families segregating the defects. This evidence, taken together with the high frequency of NTDs in almost all populations world wide, suggests a multifactorial polygenic or oligogenic pattern of inheritance, rather than a model based on single dominant or recessive genes with partial penetrance.
Over the past ten years, a large number of studies have investigated candidate genes in cohorts of patients with NTDs (see [14][15] for reviews). The primary focus has been on genes encoding proteins that participate in folate one-carbon metabolism, in view of the preventive action of folic acid. In addition, genes involved in glucose metabolism have been studied in order to understand the predisposition of diabetic pregnancy to NTDs. The human orthologues of genes that cause NTDs in mice have also been examined for possible causation of human NTDs (see next section). The most robust finding to emerge from this analysis has been two genetic polymorphisms (C677T and possibly A1298C) in the homocysteine remethylation gene MTHFR which are associated with increased risk of NTDs (approximately 1.8-fold). However, this predisposition appears to affect only certain human populations, whereas other groupings (e.g. Hispanics) do not show this association. Apart from MTHFR, very few other consistent findings have resulted from the candidate gene approach, although there is an emerging association between NTDs and genes of the non-canonical Wnt (planar cell polarity) pathway (see below).
It seems unlikely that major advances in our understanding of NTD genetics will result from a continued analysis of single candidate genes in relatively small patient cohorts. Rather, much larger studies are required that are sufficiently powered to detect significant associations with relatively weak risk factors, while analysis of multiple candidate genes in groups of well-genotyped individuals are necessary to detect possible gene-gene interactions. Further possibilities include use of high throughput genomic technology to determine the role of copy number variants and to detect regulatory mutations and those specific to particular individuals (‘private’ mutations), neither of which have been studied to date.
With regard to non-genetic factors, failure of neural tube closure can result from a wide variety of teratogenic agents in rodent models [16], whereas a smaller number of non-genetic factors have been definitively associated with the causation of NTDs in humans (Table 1). Valproic acid (VPA), a widely used anticonvulsant agent is well recognised to increase the risk of NTDs by approximately 10-fold, when taken during the first trimester of pregnancy. While many embryonic effects have been described for VPA, it seems increasingly likely that its potent histone deacetylase (HDAC) inhibitory activity may disturb the balance of protein acetylation/deacetylation, leading to failure of neural tube closure. This parallels the action of another HDAC inhibitor, trichostatin-A, which causes NTDs in mouse models [17]. Another group of non-genetic factors that increase risk of human NTDs are those pertaining to folate uptake (inhibited by the anticonvulsant carbamazepine, the fungal product fumonisin, and the antibiotic trimethoprim) or availability (conditions of metabolic folate or vitamin B12 deficiency). It seems likely that diminished access to folate metabolically exacerbates the risk of NTDs in a converse way to the preventive effect of exogenous folic acid. Another critical micronutrient for neural tube closure is inositol, which has been demonstrated to prevent mouse NTDs that fail to respond to folic acid (see below for further detail). Among the other non-genetic factors with a demonstrated link to NTDs (Table 1), diabetes mellitus is well known to predispose to a range of birth defects, including NTDs, while there is growing evidence to implicate maternal obesity as a NTD risk factor, probably via glycaemic dysregulation. Hyperthermia is a potent NTD-causing teratogen in rodents, and reports of NTDs following episodes of high maternal fever or extreme sauna usage in early pregnancy suggest that a similar relationship may exist in humans. Finally, a link between NTDs and zinc deficiency has been repeatedly claimed, although the underlying mechanism is unclear.
Table 1. Non-genetic factors linked to the causation of NTDs in human pregnancy.
| Category | Teratogenic agent | Proposed teratogenic mechanism | Refs |
|---|---|---|---|
| Folate antagonists | Carbamazepine | Inhibition of cellular folate uptake | [66] |
| Fumonisin | Inhibition of cellular folate uptake | [67][68] | |
| Trimethoprim | Disturbance of folate-related metabolism | [66] | |
|
| |||
| Glycaemic dysregulation | Hyperglycaemia (in poorly controlled maternal diabetes mellitus) | Increased cell death in neuroepithelium | [69][70] |
| Maternal obesity | Unknown | [71] | |
|
| |||
| Histone deacetylase inhibitors | Valproic acid | Disruption of key signalling pathways in neurulation | [72][73] |
|
| |||
| Micronutrient deficiencies | Folate | Disturbance of folate-related metabolism | [18][74] |
| Inositol | Disturbance of phosphorylation events downstream of protein kinase C | [55][57] | |
| Vitamin B12 | Disturbance of folate-related metabolism | [74] | |
| Zinc | Unknown | [57] | |
|
| |||
| Thermal dysregulation | Hyperthermia (e.g. maternal fever in weeks 3-4 of pregnancy) | Unknown | [75] |
In each of these cases, it is reasonable to assume a significant role for genotype, interacting with the teratogenic agent. For example, while folate and vitamin B12 deficiency are statistical risk factors in humans, a series of mouse studies have clearly demonstrated that folate deficiency causes NTDs only in the presence of a genetic predisposition. Mouse embryos with an entirely normal genotype do not develop NTDs, even under severe folate deficiency. On the other hand, littermates homozygous for the splotch (Pax3) mutation develop cranial NTDs at a significantly higher rate when also folate deficient [18]. Recent studies suggest that a combination of folate deficiency with a permissive genetic background may also be sufficient to induce cranial NTDs, even in the absence of a mutant gene [19]. This type of interaction may be particularly appropriate as a model of the gene-environment interactions likely to contribute to human NTDs. Hence, studies of possible teratogenic agents for human NTDs, must consider the effects of predisposing (and protective) genetic variants.
The mouse as a model for human NTDs - the example of PCP signalling
The close similarities between the embryonic processes of neurulation in humans and mice have been noted above. In contrast, neurulation in birds exhibits a different sequence of closure events [20], amphibia undergo neural tube closure almost simultaneously at all neuraxial levels, and fish form a neural tube by an embryonic process that does not involve bending and fusion of a neural plate. Hence, while these non-mammalian species provide attractive opportunities for experimental developmental biology analysis, they do not appear ideal as models for neural tube closure and NTD analysis, particularly where extrapolation to humans is an important goal.
Not only does the mouse embryo undergo human-like neurulation, but there is also a wealth of genetic and non-genetic models of NTD, including more than 200 mutant or targeted genes that cause NTDs [21]. These genetic mutants encompass all the main NTD phenotypes, including anencephaly, open spina bifida and craniorachischisis. When combined with the extensive catalogue of gene expression patterns in mouse embryos, the ready manipulation of the mouse genome by transgenesis, including conditional gene targeting, and the ability to culture whole mouse embryos for periods up to 48 hours throughout the period of neurulation, there is now very considerable scope for incisive experimental analysis of neurulation and NTD mechanisms in mice.
Perhaps the most valuable contribution that can be made by genetic approaches to NTDs in mice is the ability to combine different combinations of mutant alleles, and environmental influences, in order to study gene-gene and gene-environment interactions in normal and abnormal neural tube closure. This is particularly important in view of the likelihood that the key to understanding human NTDs lies in unravelling a myriad of genetic and gene-environment interactions. To illustrate this point, here we consider the genetic causation of craniorachischisis in mice.
In 2002, the first gene for craniorachischisis (Vangl2) was identified via positional cloning of the loop-tail (Lp) gene, for which homozygosity produces failure of Closure 1 [22][23]. Since this time, it has emerged that failure of mouse Closure 1 can result from defects in a number of different genes all of which function in non-canonical Wnt signalling: the planar cell polarity (PCP) pathway [24][25]. PCP signalling was defined originally in Drosophila, as a genetic cascade involving the receptor frizzled and the cytoplasmic protein disheveled, but without a requirement for β-catenin [26]. Subsequently, this pathway was found to specify polarity in the plane of epithelia, including the Drosophila wing and compound eye [27]. PCP signalling has since been implicated in gastrulation in vertebrate embryos, with key roles being defined for disheveled and strabismus (Vangl2) in Xenopus and zebrafish [28][29]. Hence, PCP signalling represents a highly conserved pathway critical for establishing and maintaining planar polarity during morphogenesis.
While homozygosity for each PCP pathway mutant produces craniorachischisis, it has also been found that compound heterozygotes for some pairs of the genes also produce this phenotype. For example, a proportion of Vangl2+/−; Celsr1+/− and Vangl2+/−; Scrb1+/− embryos fail in Closure 1 [30][31], demonstrating the potential of heterozygous gene interactions, as are more likely to occur in human populations. Intriguingly, however, Vangl2 can yield different NTD phenotypes if combined with other mutant genes. Hence, in double heterozygosity with the PCP gene Ptk7 and with the non-PCP gene Grhl3 (curly tail), open spina bifida is observed [32][33], whereas in combination with loss of the Cthrc1 gene, which encodes a canonical Wnt co-factor, exencephaly is the outcome [34]. These findings demonstrate that the precise combination of apparently closely-related gene defects can produce the whole range of NTDs. It is possible that a similar finding will ultimately apply to human NTDs and their genetic causation.
Linking genetic NTD causation to the developmental pathways of neural tube closure
In addition to identifying the individual genes and gene combinations that yield human NTDs, the next big challenge is to define the developmental requirement for those genes that are obligatory for neural tube closure, and whose disruption leads to NTDs. Since so many genes are known to be necessary for completion of one or more aspects of mouse neural tube closure, it is already possible to fit a number into well-established cell biological or molecular functions, or into specific signalling pathways. This is especially convincing where multiple NTD-causing genes cluster in a particular functional pathway, and these are the focus of the following discussion.
It should be noted, however, that the functionally-related gene groups described below are not mutually exclusive. They represent our current understanding and it seems likely that ultimately the pathways and functional groups will be shown to inter-link, to fit within a relatively small number of molecular-cellular integrated pathways that are essential for neural tube closure. Conversely, many other genes are known to cause NTDs but currently cannot be fitted easily into functional groupings. This represents the limitation on our knowledge, and eventually new key pathways may be identified that involve these and other genes. Thus, the current account must be considered an interim report.
Cell biological functions and NTDs
Critical cell biological functions for neural tube closure include: (i) cytoskeletal proteins which are essential for cranial closure, (ii) cell cycle/neurogenesis-related proteins which ensure a sufficient number of proliferating cells while avoiding premature neuronal formation that prevents neural tube closure, (iii) cell viability related proteins, without which apoptosis is excessive and NTDs result, and (iv) cell surface-extracellular matrix interactions, which are required via unknown mechanisms.
Cytoskeletal proteins
Table 2 lists the main genes encoding cytoskeletal proteins that have proven to be essential for neural tube closure. These include bona fide cytoskeletal protein components such as shroom and vinculin, and cytoskeleton-associated proteins such as cofilin, mena and palladin. Also included are signal transduction proteins such as the Abl1/2 tyrosine kinases, jun N-terminal kinases (JNKs), MARCKS and Mrp whose disturbance produces a cytoskeleton-associated phenotype. This genetic analysis complements studies of teratogenic agents, particularly the cytochalasins, which disassemble apical actin microfilaments within neuroepithelial cells, and which are well known to produce NTDs in experimental models [35]. Strikingly, while both genetic and teratogenic disturbances of the cytoskeleton produce the cranial NTD exencephaly, only in a few instances have spinal NTDs been observed (e.g. only in the Shroom and Mlp mutants). This argues for a greater dependence of cranial than spinal closure on cytoskeleton-mediated events. Indeed, it has been demonstrated that the events of midline neural plate bending, which is critical for Closure 1, and dorsolateral bending which is essential for completion of low spinal closure, are both resistant to cytochalasin D [36]. It will be interesting to determine which aspects of cranial neurulation are so highly dependent on the cytoskeleton and which specific functions in spinal closure require the cytoskeleton.
Table 2. Mouse genes causing NTDs via a mechanism involving disturbance of the cytoskeleton.
| Gene symbol(s) | Genes producing NTDs | NTD type * | Protein function | Refs |
|---|---|---|---|---|
| Abl1, Abl2 | Abl/ Arg double mutant | Ex | Non-receptor tyrosine kinases | [76] |
| Cofilin (n-) | n-cofilin | Ex | F-actin depolymerising factor | [77] |
| Grlfl | RhoGAP P190 | Ex | Glucocorticoid receptor DNA binding factor | [78] |
| Mapk8; Mapk9 | Jnk1/Jnk2 double mutant | Ex | c-Jun-N-terminal kinases | [79] |
| Mareks | MARCKS (myristoylated alanine rich C-kinase subtrate) | Ex | Cytoskeleton-related protein with function in signal transduction | [80] |
| Mena; Profilin | Mena/profilin I double mutant | Ex | Regulation of actin polymerisation | [81] |
| Mena; Vasp | Mena/Vasp double mutant | Ex | Member of Mena/Vasp/Evl family of cytoskeletal regulators | [82] |
| Mlp | MARCKS-like protein | Ex +/−Sb | Cytoskeleton-related protein with function in signal transduction | [83] |
| Palladin | Palladin | Ex | Actin cytoskeleton-associated protein | [84][85] [86] |
| Shrm | Shroom | Ex + Sb | PDZ-containing cytoskeletal protein | [87] |
| Vcl | Vinculin | Ex | Cytoskeletal protein | [88] |
Ex: exencephaly; Sb: spina bifida
Cell cycle and neurogenesis
The neurulation stage embryo is a rapidly proliferating system, with cell cycle times as short as 4-6 hours in the neuroepithelium. On the other hand, some neuroepithelial cells begin to exit the cell cycle and embark upon neuronal differentiation around the stage of neural tube closure, suggesting that the balance between continued proliferation and neuronal differentiation may be critical for successful closure. Indeed, a number of genes in which mutations cause NTDs appear to be essential for cell proliferation and/or prevention of premature neurogenesis (Table 3). Among the former are jumonji, which has homology to cell cycle-associated retinoblastoma-binding proteins, neurofibromin1 and nucleoporin, which are negative regulators of the cell cycle inhibitors p21 and p27 respectively, and Pax3 which is required for promotion of rapid dorsal neuroepithelial cell proliferation. In addition, NTDs result from defects in several genes encoding negative regulators of the Notch signalling pathway which is activated as neuroepithelial cells embark upon neuronal differentiation. Hence, Hes1, Hes3 and RBP-Jκ are all required to postpone neuronal differentiation until neural tube closure is complete. It remains to be determined precisely which steps in neural tube closure are compromised by premature neurogenesis, but one can speculate that bending of the dorsolateral neural plate and the adhesive events at the neural fold tips, necessary for fusion of the apposing neural folds, may be hampered.
Table 3. Mouse genes causing NTDs via a mechanism involving disturbance of cell proliferation or neuronal differentiation.
| Gene symbol(s) | Genes producing NTDs | NTD type * | Protein function | Refs |
|---|---|---|---|---|
| Brd2 | Brd2 | Ex | Double bromodomain containing protein | [89] |
| Hesl | Hairy and enhancer of split 1 | Ex | Transcription factor inhibiting Notch signalling | [90] |
| Hesl, Hes3 | Hes1/Hes3 double mutant | Ex | Transcription factors inhibiting Notch signalling | [91] |
| jmj | JumonJi | Ex | Retinoblastoma-binding protein homologue | [92] |
| Nfl | Neurofibromin | Ex | Negative regulator of p21ras | [93] |
| Nf1,Pax3 | Nf1/Pax3 double mutant | Ex +/−Sb | As Nf1; transcription factor | [93] |
| Notch3 | Notch 3 overexpression | Ex | Cell surface receptor for delta family ligands | [94] |
| Numb | Numb | Ex | Protein participating in asymmetric cell division | [95] |
| Nup50 | Nucleoporin | Ex | Nuclear pore protein binding to p27Kip1 | [96] |
| Pax3 | Splotch (Sp, Sp2H) mutant; Pax3-Cre strain | Ex + Sb | Transcription factor | [97][98] |
| Pax3; Pax7 | Pax3/Pax7 double mutant | Ex + Sb | Transcription factors | [99] |
| RBP-Jκ | RBP-Jκ | Ex | Transcription factor | [100] |
Ex: exencephaly; Sb: spina bifida
Cell death
Apart from cell cycle alterations, insufficient cells for neural tube closure may also result from excessive cell death. Indeed, increased apoptosis is one of the most frequent cellular changes observed in studies of gene targeted embryos that develop NTDs. As with all cellular abnormalities, it is crucial to demonstrate that increased apoptosis precedes failed neural tube closure, as later-appearing abnormalities could be a secondary consequence of the failed morphogenesis. Genetic defects in which excessive cell death occurs prior to or during neural tube closure (Table 4) include the anti-apoptotic Bcl-10 gene, Ikk factors that activate NFκB in an anti-apoptotic pathway, and the transcription factors AP-2 and Brca1, whose downstream gene functions are required for cell survival. Conversely, NTDs also result from genes including Apaf-1, and caspase 3 and 9 which are required for apoptosis. In these cases, diminished or abolished cell death is observed and NTDs are localised to the caudal midbrain and hindbrain. Neural tube closure in forebrain and spinal regions is completed normally in these mutants. Strikingly, mouse embryos in which apoptosis is blocked chemically, by inhibition of effector caspases with Z-vad-fmk or by inhibition of p53 with pifithrin-α, exhibit no defects of neural tube closure [37]. In particular, neural fold remodelling to create a continuous surface ectodermal layer over the neural tube roof plate, proceeds normally in the absence of apoptosis. This finding strongly suggests that mice with mutations in Apaf-1 or caspase 3/9 are unlikely to develop exencephaly solely due to diminution of programmed cell death. It is possible that development from fertilization to neurulation in the complete absence of apoptosis results in secondary tissue changes that are incompatible with mid/hindbrain closure, although the nature of such changes is unknown.
Table 4. Mouse genes causing NTDs with alterations in extent of neuroepithelial cell death.
| Gene(s) | Genotype producing NTDs | NTD type* | Protein function | Refs |
|---|---|---|---|---|
| AP2a | AP2 a (also called Tcfap2a) | Ex | Transcription factor | [101][102][103] |
| Apaf1 | Apaf1 andforebrain overgrowth (fog, mutation) | Ex | Protease-activating factor in the caspase-dependent apoptosis pathway | [104][105][106][ 107] |
| Bcl10 | Bcl10 | Ex | CARD-containing anti-apoptotic protein, activating IKKs and NFkB | [108] |
| Brca1 | Brca1 | Ex + Sb | Transcription factor | [109] |
| Brca1; Gadd45 | Brca1/ Gadd45 double mutant | Ex | Transcription factors | [110] |
| Casp3 | Caspase 3 | Ex | Enzyme of apoptosis pathway | [37][111] |
| Casp9 | Caspase 9 | Ex | Enzyme of apoptosis pathway | [112][113] |
| Chuk, Ikbkb | IKK1/IKK2 double mutant | Ex | Kinases activating NFkB in anti-apoptotic pathway | [114] |
| Mekk4 | Mekk4; Kinase-inactive mutant of MEKK4 | Ex ± Sb | MAP kinase enzyme, that phosphorylates JNK/p38 | [115][116] |
| Tcof1 | Treacle | Ex | Nucleolar phosphoprotein | [117] |
| Trx2 | Trx2 (Thioredoxin) | Ex | Mitochondrial redox protein | [118] |
Ex: exencephaly; Sb: spina bifida
Cell surface and extracellular matrix
Interactions between the extracellular matrix and cell surface receptors, particularly integrins, are well known to determine cell shape and polarisation, in part via cytoskeleton-mediated events. Genes that give rise to NTDs in this category include laminin-α5, perlecan and integrins-α3 and -α6 [38][39][40]. It remains to be determined how these cell-matrix interactions are involved in neural tube closure and, in particular, whether downstream signalling from integrins is necessary, or whether the involvement is purely one of adhesion of neural plate to its adjacent matrix. Similarly, there is evidence that cell-to-cell adhesion may also be essential to facilitate the initial process of fusion at the tips of the closing neural folds. For example, NTDs arise in mice lacking ephrinA5 or EphA7 [41], while disturbance of ephrinA-EphA interactions can delay neural tube closure [42].
Gene and protein regulatory functions and NTDs
Several groups of proteins have proven essential for neural tube closure, including those with functions in (i) transcriptional co-activation and chromatin remodelling, which are responsible for modulating key downstream signalling pathways, (ii) transcriptional regulation, leading to downstream effects which in many cases are largely unknown, and (iii) regulation of protein function, for example ubiquitin-mediated protein degradation complexes, which may participate in several cellular or signalling functions.
Table 5 lists a series of chromatin remodelling proteins and transcriptional co-activators whose genetic disruption leads to NTDs. These include members of the SWI/SNF chromatin remodelling complex (e.g. Cecr2, Smarcc1, Xnp), the polycomb proteins Rybp and Suz12, the histone acetyltransferase Gcm1, and a group of related transcriptional co-activators including Cart1, Cited2, CBP and p300. In addition, more than 20 different transcription factor genes give rise to NTDs when mutated (e.g. Grhl3, Zic2). Clearly, many gene expression pathways are required for neurulation in mice and these gene functions are probably necessary for activation of specific pathways or groups of pathways as neural tube closure proceeds. A major challenge for the coming years will be to decipher the gene regulatory networks that underlie neural tube closure, as has recently been initiated for a wide range of other developmental systems [43]. This will require analysis of many of the genetic NTD mutants (including gene-gene interactions), identification by RNA microarray of the genes whose expression is disturbed in such embryos, and integration of this information via analysis of gene expression patterns in affected embryos.
Table 5. Mouse genes causing NTDs via mechanisms involving transcriptional regulation and chromatin dynamics.
| Gene symbol(s) | Genes producing NTDs | NTD type * | Protein function | Refs |
|---|---|---|---|---|
| Adnp | Adnp | Ex | Activity dependent neuroprotective protein (ADNP), regulated by vasoactive intestinal peptide (VIP) | [119] |
| Cart1 | Cart1 (cartilage homeoprotein 1) | Ex | Transcription factor | [120] |
| Cecr2 | Cecr2 | Ex | Transcription factor that forms chromatin modelling complex with SNF2L | [121][122] |
| Cited2 | Cited2 | Ex | Transcriptional co-activator | [123][124] [125] |
| Crebbp | CBP | Ex | Transcriptional coactivator | [126] |
| Ep300 | p300 | Ex | Transcriptional integrator | [127] |
| Gcn5 | Gcn5 | Ex | Histone acetyltransferase | [128][129] [130] |
| Hipk1, Hipk2 | Hipk1/ Hipk2 double mutant | Ex | Homeodomain-interacting protein kinases, forming complex with AML1 and p300 | [131] |
| Nap1l2 | Nucleosome assembly protein-1-like 2 (Nap1l2) | Ex + Sb | Nucleosome assembly | [132] |
| Rybp | Ring1 and YY1 binding protein | Ex | Polycomb complex protein; binding partner for YY1, also a cause of NTDs | [133] |
| Smarca4 | Brg1 | Ex | Chromatin remodelling protein | [134] |
| Smarcc1 | Srg3 | Ex | Subunit of SWI/SNF complex, involved in chromatin remodelling | [135] |
| Suz12 | Suz12 | Sb | Polycomb protein | [136] |
| Xnp | ATRX | Ex | Member of SWI/SNF chromatin remodelling family | [137] |
Ex: exencephaly; Sb: spina bifida
An interesting group of genes whose disruption gives rise to NTDs encode the ubiquitin ligases, including Hectd1, Mib2 and Smurf1/2 [44][45][46]. These proteins function as part of multi-enzyme complexes to ubiquitinate and target proteins for degradation in the proteasome. Such targeted proteins could participate in any of the pathways that are essential for neural tube closure, with regulated protein degradation providing an alternative means of controlling pathway activity to transcriptional regulation. For example, Smurf1/2 have been shown to regulate the availability of the PCP protein prickle1, possibly serving to modulate PCP activity via this mechanism [44][45][46].
Specific signalling pathways and NTDs
Signalling pathways that are essential for neural tube closure include: (i) PCP signalling which is required for initiation of closure (reviewed above), (ii) Sonic hedgehog signalling, where over-activation leads to NTDs probably via prevention of DLHP formation, (iii) BMP signalling, which is necessary to prevent DLHPs, (iv) Notch signalling, via regulation of neurogenesis (reviewed above), (iv) retinoid signalling, via an unknown cellular mechanism, and (v) inositol metabolism with protein phosphorylation via protein kinase C.
Sonic hedgehog signalling
Sonic hedgehog (Shh), one of three mammalian homologues of Drosophila hedgehog, initiates a intracellular signalling pathway by binding to its transmembrane receptor, Patched1 (Ptc1). In the absence of Shh ligand, Ptc1 interacts with an associated membrane protein, Smoothened (Smo), to inhibit its activity. Shh binding to Ptc1 removes the inhibitory effect on Smo, allowing members of the Gli family of proteins to enter the nucleus and act as transcriptional activators. In addition to this core signal transduction machinery, a variety of other proteins have been identified that exert either positive or negative influences on Shh signalling. Strikingly, however, it is primarily genetic changes in proteins with a negative influence on the Shh pathway lead to NTDs in mice (Table 6). For example, mutations in Ptc1 that relieve the inhibition of Smo activity, and abolition of inhibitory phosphorylation sites for protein kinase A in Gli2, both lead to NTDs. Loss of function of other Shh inhibitory genes, including Fkbp8, Gli3, Rab23 and Tulp3, also produce NTDs. In contrast, loss of function of proteins that activate signalling, including Smo and Shh itself, does not produce NTDs [47][48], whereas over-expression of such proteins can compromise neural tube closure.
Table 6. Mouse genes causing NTDs via dysregulation of the sonic hedgehog signalling pathway.
| Gene symbol(s) | Genes producing NTDs | NTD type* | Protein function | Refs |
|---|---|---|---|---|
| Fkbp8 | FK506-binding protein 8 (also called Fkbp38) | Sb | Negative regulator of Shh signalling | [138][139][140] |
| Gli2 (P1-4) | Gli2 (with PKA phosphorylation sites mutated) | Ex | Shh signal transduction protein | [141] |
| Luzp | LUZP | Ex | Leucine zipper transcription factor expressed in brain. Possible negative regulator of Shh signalling | [142] |
| Prkaca; Prkacb | Protein kinase a subunits Cα/Cβl double mutant | Ex | Protein kinase A. Negative regulator of Shh signalling | [143] |
| Ptch1 | Patched1 | Ex or CRN | Transmembrane receptor for sonic hedgehog peptide. Negative regulator of Shh signalling | [144][145] |
| Rab23 | Open brain (opb) | Ex + Sb | Rab family of vesicle transport proteins; negative regulator of Shh signalling | [146][147][148] |
| Shh | Sonic hedgehog (over-expression) | Ex | Signalling peptide regulating nervous system dorso-ventral patterning | [149] |
| Sufu | Suppressor of fused | Ex | Shh signal transduction inhibitor | [150][151] |
| Tulp3 | Tubby-like protein 3 (knockout); hitchhiker (ENU mutant) | Ex + Sb | Negative regulator of Shh signalling | [152][153] |
CRN: craniorachischisis; Ex: exencephaly; Sb: spina bifida
These findings argue for an overall negative influence of Shh signalling on neural tube closure. This effect appears to be mediated via the prevention of neural plate bending in the dorsolateral region, which is an essential component of closure in both the midbrain and lower spinal region [49]. The function of Shh is to inhibit anti-BMP signalling (e.g. by noggin) from the dorsal neuroepithelium. At levels of the body axis where Shh production from the notochord is low, noggin expression dorsally is strong and BMPs are inhibited. This permits DLHP formation to proceed. Hence, any pathological increase in Shh signalling is expected to block DLHP formation, with spina bifida as a result. On the other hand, loss of Shh signalling allows DLHPs to form prematurely, ensuring neural tube closure along the entire body axis.
Retinoid signalling
Retinoic acid (RA) has long been studied as a potent teratogen in rodent systems, with NTDs among the malformations most often observed. It is now realised that endogenous synthesis of retinoids forms an integral part of early development, and that any disturbance in the balance between production and turnover of retinoids can adversely affect developmental events including neural tube closure. Hence, NTDs are observed in mice with loss of function of the genes encoding Raldh2, a principal enzyme of RA synthesis, Cyp26a1, a key RA metabolising enzyme and RA receptors α and γ, through which RA signalling is mediated [50][51][52]. While the existence of signalling pathways downstream of the RA receptors is well known, it remains unclear precisely how RA signalling participates in neural tube closure, at the cellular level.
Inositol metabolism
A series of studies have demonstrated that inositol can prevent a large proportion of the spinal NTDs in the curly tail mutant mouse model, whereas folic acid is ineffective [53][54][55]. This raises the possibility of using inositol supplementation, as an adjunct to folic acid therapy, to prevent of a greater proportion of NTDs than is possible with folic acid alone. Inositol deficiency during embryonic development can cause cranial NTDs in both rats and mice [56], and some human NTD pregnancies also have lower maternal inositol concentrations that unaffected pregnancies [57]. Moreover, targeted mutations in the Itpk1, PIP5KIγ and Inpp5e genes of mice produce NTDs via direct disturbance of inositol metabolism [58][59][60]. In the case of PIP5KIγ, the predicted effect is a reduction in protein kinase C (PKC) stimulation, a known requirement for prevention of NTDs by exogenous inositol [55]. Hence, it appears that inositol metabolism may lie upstream of key protein phosphorylation events, mediated by PKC, although the targets of such phosphorylation are currently unknown.
How does folic acid prevent NTDs?
The prevention of a proportion of NTDs by folic acid supplementation was confirmed in a randomised, double blind clinical trial published in 1991 [61]. Moreover, the effects of folate fortification in bread flour have illustrated this preventive effect, while demonstrating that not all cases of NTD are folate-preventable [62]. It is often assumed that NTDs are a vitamin-deficiency condition, but in fact the great majority of human pregnancies are not clinically folate-deficient. Moreover, frank folate deficiency in mouse models does not cause NTDs in the absence of a genetic predisposition [18]. It seems more likely, therefore, that exogenous folic acid is able to stimulate a cellular response, enabling the developing embryo to overcome the adverse effects of certain genetic and/or environmental disturbances that would otherwise lead to NTDs. These disturbances could involve abnormalities in folate-related pathways, but might also affect systems unrelated to folate metabolism. An important principle emerges, therefore: that folic acid may exert its preventive effect on an aetiologically diverse group of conditions (e.g. disorders resulting from different genetic anomalies), all of which converge on the process of neural tube closure to produce NTDs.
Folates are integral to intracellular one-carbon metabolism which produces pyrimidines and purines for DNA synthesis and s-adenosyl methionine, the universal methyl group donor for all macromolecules. Hence, cell proliferation and/or cell survival, which depend on continuation of DNA synthesis, are likely effects of folate supplementation, as is stimulation of DNA, protein or lipid methylation. Indeed, a beneficial effect of folate on cell proliferation can be readily understood as a possible means of preventing NTDs where increased apoptosis and/or reduced cell proliferation are observed. Nevertheless, this type of effect has not actually been demonstrated to date, in any folate-preventable NTD model system. Similarly, stimulation of specific gene methylation is also a possible outcome of folate supplementation, although no such examples have yet been reported. In a recent study of severe folate deficiency in mouse pregnancy, there was no decrease in embryonic global DNA methylation [18], raising questions as to the importance of this aspect of one-carbon metabolism in NTD prevention by folate.
Clearly, a priority for future research is to improve our understanding of the cellular mechanism(s) of folate’s preventive action. This could enable us to determine why some NTDs are folate-responsive and others folate-resistant, in turn focusing on the different aetiologies in each case, and leading to better prediction of the likely importance of folate supplementation in pregnancy, or the need for additional treatments, such as inositol (see above).
Conclusions and future perspectives
We have seen that the events of embryonic neural tube closure are highly conserved between mice and humans, making the mouse a valuable model system for studies of human neurulation. Moreover, the wealth of genetic and non-genetic sources of NTDs in mice provides an opportunity to probe the molecular and cellular mechanisms of failed neural tube closure, which may ultimately prove relevant to the corresponding defects in humans. A particularly valuable contribution of mouse models is the ability to study interactions between different genetic factors, via construction of animals with multiple mutations and, moreover, to determine the effects of interactions between genotype and environment. This type of multi-factor analysis seems essential if we are to improve our understanding of the aetiology of human NTDs. Other priorities for future research include an integration of the various signalling pathways that have been implicated in NTDs, and elucidation of their outputs at the level of cell movements, and in terms of the mechanical interactions between tissues that ultimately generate the physical forces underlying neural tube closure. The mechanics of neurulation have so far been studied only in non-mammalian species, although highly sophisticated computational models are now available (e.g. [63]). Hence, the use of analogous approaches to investigate and elucidate the mechanics of mammalian neurulation will be an exciting challenge for the near future.
Figure 2. Molecular regulation of dorsolateral bending in mouse neural tube closure.
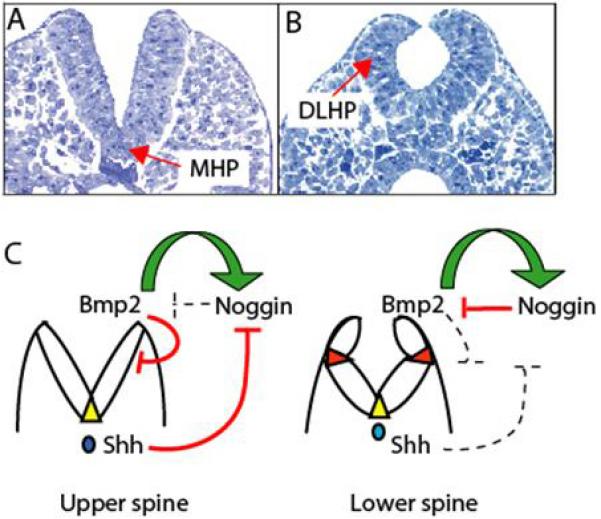
Neural tube closure changes in morphology from (A) high spinal to (B) low spinal regions of the mouse embryo. In the upper spine, the neural plate bends solely in the midline, at the median hinge point (MHP), whereas in the low spine bending occurs at dorsolateral hinge points (DLHPs). (C) Summary of the molecular interactions regulating DLHP formation. In the upper spine, DLHPs are absent because of unopposed inhibition by BMP2. Although Noggin expression is stimulated by BMP2 at all levels of the body axis, Shh expression from the notochord is strong in the upper spine, inhibiting Noggin. In the lower spine, Shh influence is reduced, Noggin expression is de-inhibited and antagonises the inhibitory influence of BMP2, allowing DLHPs to form. Yellow triangles: MHP; red triangles: DLHPs; green arrows: stimulatory interactions; red lines: inhibitory interactions; dashed lines: inactive influences. Modified from [65]
ACKNOWLEDGEMENTS
The authors thank the Wellcome Trust, Medical Research Council and SPARKS for funding of their research into neural tube defects.
References
- (1).Mitchell LE. Epidemiology of neural tube defects. Am J Med Genet C Semin Med Genet. 2005;135C:88–94. doi: 10.1002/ajmg.c.30057. [DOI] [PubMed] [Google Scholar]
- (2).Malcoe LH, Shaw GM, Lammer EJ, Herman AA. The effect of congenital anomalies on mortality risk in white and black infants. Am J Public Health. 1999;89:887–892. doi: 10.2105/ajph.89.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Copp AJ. Neurulation in the cranial region - normal and abnormal. J Anat. 2005;207:623–635. doi: 10.1111/j.1469-7580.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).MacDonald KB, Juriloff DM, Harris MJ. Developmental study of neural tube closure in a mouse stock with a high incidence of exencephaly. Teratology. 1989;39:195–213. doi: 10.1002/tera.1420390211. [DOI] [PubMed] [Google Scholar]
- (5).Moore CA. Classification of neural tube defects. In: Wyszynski DF, editor. Neural Tube Defects: From Origin to Treatment. Oxford University Press; Oxford: 2006. pp. 66–75. [Google Scholar]
- (6).Copp AJ, Greene NDE, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- (7).Stiefel D, Copp AJ, Meuli M. Fetal spina bifida: loss of neural function in utero. J Neurosurg. 2007;106:213–221. doi: 10.3171/ped.2007.106.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Johnson MP, Gerdes M, Rintoul N, Pasquariello P, Melchionni J, Sutton LN, et al. Maternal-fetal surgery for myelomeningocele: neurodevelopmental outcomes at 2 years of age. Am J Obstet Gynecol. 2006;194:1145–1150. doi: 10.1016/j.ajog.2006.01.072. [DOI] [PubMed] [Google Scholar]
- (9).Mills JL, Signore CC. Folic acid and the prevention of neural-tube defects. N Engl J Med. 2004;350:2209–2210. doi: 10.1056/NEJM200405203502120. [DOI] [PubMed] [Google Scholar]
- (10).Rampersaud E, Melvin EC, Speer MC. Nonsyndromic neural tube defects: genetic basis and genetic investigations. In: Wyszynski DF, editor. Neural Tube Defects: From Origin to Treatment. Oxford University Press; Oxford: 2006. pp. 165–75. [Google Scholar]
- (11).Leck I. Causation of neural tube defects: clues from epidemiology. Br Med Bull. 1974;30:158–163. doi: 10.1093/oxfordjournals.bmb.a071187. [DOI] [PubMed] [Google Scholar]
- (12).Elwood JM, Elwood JH. Epidemiology of anencephalus and spina bifida. Oxford University Press; Oxford: 1980. [Google Scholar]
- (13).Knox EG. Twins and neural tube defects. Br J prev soc Med. 1974;28:73–80. doi: 10.1136/jech.28.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Boyles AL, Hammock P, Speer MC. Candidate gene analysis in human neural tube defects. Am J Med Genet C Semin Med Genet. 2005;135C:9–23. doi: 10.1002/ajmg.c.30048. [DOI] [PubMed] [Google Scholar]
- (15).Greene NDE, Stanier P, Copp AJ. Genetics of human neural tube defects. Hum Mol Genet. 2009;18:R113–R129. doi: 10.1093/hmg/ddp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Copp AJ, Brook FA, Estibeiro JP, Shum ASW, Cockroft DL. The embryonic development of mammalian neural tube defects. Prog Neurobiol. 1990;35:363–403. doi: 10.1016/0301-0082(90)90037-h. [DOI] [PubMed] [Google Scholar]
- (17).Menegola E, Di Renzo F, Broccia ML, Prudenziati M, Minucci S, Massa V, et al. Inhibition of histone deacetylase activity on specific embryonic tissues as a new mechanism for teratogenicity. Birth Defects Res B Dev Reprod Toxicol. 2005;74:392–398. doi: 10.1002/bdrb.20053. [DOI] [PubMed] [Google Scholar]
- (18).Burren KA, Savery D, Massa V, Kok RM, Scott JM, Blom HJ, et al. Gene-environment interactions in the causation of neural tube defects: folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum Mol Genet. 2008;17:3675–3685. doi: 10.1093/hmg/ddn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Burren KA, Scott JM, Copp AJ, Greene NDE. The genetic background of the curly tail strain confers susceptibility to folate-deficiency-induced exencephaly. Birth Defects Res A Clin Mol Teratol. 2010;88:76–83. doi: 10.1002/bdra.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Van Straaten HWM, Janssen HCJP, Peeters MCE, Copp AJ, Hekking JWM. Neural tube closure in the chick embryo is multiphasic. Dev Dyn. 1996;207:309–318. doi: 10.1002/(SICI)1097-0177(199611)207:3<309::AID-AJA8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- (21).Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2007;79:187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- (22).Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nature Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- (23).Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–2601. doi: 10.1093/hmg/10.22.2593. [DOI] [PubMed] [Google Scholar]
- (24).Zohn IE, Chesnutt CR, Niswander L. Cell polarity pathways converge and extend to regulate neural tube closure. Trends Cell Biol. 2003;13:451–454. doi: 10.1016/s0962-8924(03)00173-9. [DOI] [PubMed] [Google Scholar]
- (25).Copp AJ, Greene NDE, Murdoch JN. Dishevelled: linking convergent extension with neural tube closure. Trends Neurosci. 2003;26:453–455. doi: 10.1016/S0166-2236(03)00212-1. [DOI] [PubMed] [Google Scholar]
- (26).McEwen DG, Peifer M. Wnt signaling: Moving in a new direction. Curr Biol. 2000;10:R562–R564. doi: 10.1016/s0960-9822(00)00611-4. [DOI] [PubMed] [Google Scholar]
- (27).Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–4513. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- (28).Park M, Moon RT. The planar cell-polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- (29).Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- (31).Murdoch JN, Henderson DJ, Doudney K, Gaston-Massuet C, Phillips HM, Paternotte C, et al. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- (32).Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- (33).Stiefel D, Shibata T, Meuli M, Duffy P, Copp AJ. Tethering of the spinal cord in mouse fetuses and neonates with spina bifida. J Neurosurg. 2003;99:206–213. doi: 10.3171/spi.2003.99.2.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- (35).Sadler TW, Greenberg D, Coughlin P, Lessard JL. Actin distribution patterns in the mouse neural tube during neurulation. Science. 1982;215:172–174. doi: 10.1126/science.7031898. [DOI] [PubMed] [Google Scholar]
- (36).Ybot-Gonzalez P, Copp AJ. Bending of the neural plate during mouse spinal neurulation is independent of actin microfilaments. Dev Dyn. 1999;215:273–283. doi: 10.1002/(SICI)1097-0177(199907)215:3<273::AID-AJA9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- (37).Massa V, Savery D, Ybot-Gonzalez P, Ferraro E, Rongvaux A, Cecconi F, et al. Apoptosis is not required for mammalian neural tube closure. Proc Natl Acad Sci U S A. 2009;106:8233–8238. doi: 10.1073/pnas.0900333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143:1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nature Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- (40).De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergistic activities of α3 and α6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126:3957–3968. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- (41).Holmberg J, Clarke DL, Frisén J. Regulation of repulsion versus adhesion by different splice forms of an Eph receptor. Nature. 2000;408:203–206. doi: 10.1038/35041577. [DOI] [PubMed] [Google Scholar]
- (42).Abdul-Aziz NM, Turmaine M, Greene ND, Copp AJ. EphrinA-EphA receptor interactions in mouse spinal neurulation: implications for neural fold fusion. Int J Dev Biol. 2009;53:559–568. doi: 10.1387/ijdb.082777na. [DOI] [PubMed] [Google Scholar]
- (43).Li E, Davidson EH. Building developmental gene regulatory networks. Birth Defects Res C Embryo Today. 2009;87:123–130. doi: 10.1002/bdrc.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zohn IE, Anderson KV, Niswander L. The Hectd1 ubiquitin ligase is required for development of the head mesenchyme and neural tube closure. Dev Biol. 2007;306:208–221. doi: 10.1016/j.ydbio.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Wu JI, Rajendra R, Barsi JC, Durfee L, Benito E, Gao G, et al. Targeted disruption of Mib2 causes exencephaly with a variable penetrance. Genesis. 2007;45:722–727. doi: 10.1002/dvg.20349. [DOI] [PubMed] [Google Scholar]
- (46).Narimatsu M, Bose R, Pye M, Zhang L, Miller B, Ching P, et al. Regulation of planar cell polarity by Smurf ubiquitin ligases. Cell. 2009;137:295–307. doi: 10.1016/j.cell.2009.02.025. [DOI] [PubMed] [Google Scholar]
- (47).Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- (48).Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105:781–792. [PubMed] [Google Scholar]
- (49).Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Sonic hedgehog and the molecular regulation of neural tube closure. Development. 2002;129:2507–2517. doi: 10.1242/dev.129.10.2507. [DOI] [PubMed] [Google Scholar]
- (50).Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nature Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- (51).Niederreither K, Abu-Abed S, Schuhbaur B, Petkovich M, Chambon P, Dollé P. Genetic evidence that oxidative derivatives of retinoic acid are not involved in retinoid signaling during mouse development. Nature Genet. 2002;31:84–88. doi: 10.1038/ng876. [DOI] [PubMed] [Google Scholar]
- (52).Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, et al. Function of the retinoic acid receptors (RARs) during development. (I) Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- (53).Greene NDE, Copp AJ. Inositol prevents folate-resistant neural tube defects in the mouse. Nature Med. 1997;3:60–66. doi: 10.1038/nm0197-60. [DOI] [PubMed] [Google Scholar]
- (54).Cogram P, Tesh S, Tesh J, Wade A, Allan G, Greene NDE, et al. D-chiro-inositol is more effective than myo-inositol in preventing folate-resistant mouse neural tube defects. Hum Reprod. 2002;17:2451–2458. doi: 10.1093/humrep/17.9.2451. [DOI] [PubMed] [Google Scholar]
- (55).Cogram P, Hynes A, Dunlevy LPE, Greene NDE, Copp AJ. Specific isoforms of protein kinase C are essential for prevention of folate-resistant neural tube defects by inositol. Hum Mol Genet. 2004;13:7–14. doi: 10.1093/hmg/ddh003. [DOI] [PubMed] [Google Scholar]
- (56).Cockroft DL, Brook FA, Copp AJ. Inositol deficiency increases the susceptibility to neural tube defects of genetically predisposed (curly tail) mouse embryos in vitro. Teratology. 1992;45:223–232. doi: 10.1002/tera.1420450216. [DOI] [PubMed] [Google Scholar]
- (57).Groenen PM, Peer PG, Wevers RA, Swinkels DW, Franke B, Mariman EC, et al. Maternal myo-inositol, glucose, and zinc status is associated with the risk of offspring with spina bifida. Am J Obstet Gynecol. 2003;189:1713–1719. doi: 10.1016/s0002-9378(03)00807-x. [DOI] [PubMed] [Google Scholar]
- (58).Wang Y, Lian L, Golden JA, Morrisey EE, Abrams CS. PIP5KI gamma is required for cardiovascular and neuronal development. Proc Natl Acad Sci U S A. 2007;104:11748–11753. doi: 10.1073/pnas.0700019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Wilson MP, Hugge C, Bielinska M, Nicholas P, Majerus PW, Wilson DB. Neural tube defects in mice with reduced levels of inositol 1,3,4-trisphosphate 5/6-kinase. Proc Natl Acad Sci U S A. 2009;106:9831–9835. doi: 10.1073/pnas.0904172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nature Genet. 2009;41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- (61).Wald N, Sneddon J, Densem J, Frost C, Stone R, MRC Vitamin Study Res Group Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- (62).Eichholzer M, Tonz O, Zimmermann R. Folic acid: a public-health challenge. Lancet. 2006;367:1352–1361. doi: 10.1016/S0140-6736(06)68582-6. [DOI] [PubMed] [Google Scholar]
- (63).Chen X, Brodland GW. Multi-scale finite element modeling allows the mechanics of amphibian neurulation to be elucidated. Phys Biol. 2008;5:015003. doi: 10.1088/1478-3975/5/1/015003. [DOI] [PubMed] [Google Scholar]
- (64).Greene ND, Copp AJ. Development of the vertebrate central nervous system: formation of the neural tube. Prenatal Diag. 2009;29:303–311. doi: 10.1002/pd.2206. [DOI] [PubMed] [Google Scholar]
- (65).Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene ND, et al. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of BMP signalling. Development. 2007;134:3203–3211. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- (66).Hernández-Díaz S, Werler MM, Walker AM, Mitchell AA. Neural tube defects in relation to use of folic acid antagonists during pregnancy. Am J Epidemiol. 2001;153:961–968. doi: 10.1093/aje/153.10.961. [DOI] [PubMed] [Google Scholar]
- (67).Missmer SA, Suarez L, Felkner M, Wang E, Merrill AH, Jr., Rothman KJ, et al. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environ Health Perspect. 2006;114:237–241. doi: 10.1289/ehp.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Sadler TW, Merrill AH, Stevens VL, Sullards MC, Wang E, Wang P. Prevention of fumonisin B1-induced neural tube defects by folic acid. Teratology. 2002;66:169–176. doi: 10.1002/tera.10089. [DOI] [PubMed] [Google Scholar]
- (69).Kucera J. Rate and type of congenital anomalies among offspring of diabetic women. J Reprod Med. 1971;7:73–82. [PubMed] [Google Scholar]
- (70).Chappell JH, Jr., Wang XD, Loeken MR. Diabetes and apoptosis: neural crest cells and neural tube. Apoptosis. 2009 doi: 10.1007/s10495-009-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Rasmussen SA, Chu SY, Kim SY, Schmid CH, Lau J. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198:611–619. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- (72).Robert E, Guibaud P. Maternal valproic acid and congenital neural tube defects. Lancet. 1982;2:937. doi: 10.1016/s0140-6736(82)90908-4. [DOI] [PubMed] [Google Scholar]
- (73).Gurvich N, Berman MG, Wittner BS, Gentleman RC, Klein PS, Green JB. Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J. 2005;19:1166–1168. doi: 10.1096/fj.04-3425fje. [DOI] [PubMed] [Google Scholar]
- (74).Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;86:703–708. [PubMed] [Google Scholar]
- (75).Moretti ME, Bar-Oz B, Fried S, Koren G. Maternal hyperthermia and the risk for neural tube defects in offspring: systematic review and meta-analysis. Epidemiology. 2005;16:216–219. doi: 10.1097/01.ede.0000152903.55579.15. [DOI] [PubMed] [Google Scholar]
- (76).Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, Miczek KA, et al. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- (77).Gurniak CB, Perlas E, Witke W. The actin depolymerizing factor n-cofilin is essential for neural tube morphogenesis and neural crest cell migration. Dev Biol. 2005;278:231–241. doi: 10.1016/j.ydbio.2004.11.010. [DOI] [PubMed] [Google Scholar]
- (78).Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Jr., Silver J, et al. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development. 2000;127:4891–4903. doi: 10.1242/dev.127.22.4891. [DOI] [PubMed] [Google Scholar]
- (79).Sabapathy K, Jochum W, Hochedlinger K, Chang LF, Karin M, Wagner EF. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev. 1999;89:115–124. doi: 10.1016/s0925-4773(99)00213-0. [DOI] [PubMed] [Google Scholar]
- (80).Stumpo DJ, Bock CB, Tuttle JS, Blackshear PJ. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc Natl Acad Sci USA. 1995;92:944–948. doi: 10.1073/pnas.92.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Lanier LM, Gates MA, Witke W, Menzies AS, Wehman AM, Macklis JD, et al. Mena is required for neurulation and commissure formation. Neuron. 1999;22:313–325. doi: 10.1016/s0896-6273(00)81092-2. [DOI] [PubMed] [Google Scholar]
- (82).Menzies AS, Aszodi A, Williams SE, Pfeifer A, Wehman AM, Goh KL, et al. Mena and vasodilator-stimulated phosphoprotein are required for multiple actin-dependent processes that shape the vertebrate nervous system. J Neurosci. 2004;24:8029–8038. doi: 10.1523/JNEUROSCI.1057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Wu M, Chen DF, Sasaoka T, Tonegawa S. Neural tube defects and abnormal brain development in F52-deficient mice. Proc Natl Acad Sci U S A. 1996;93:2110–2115. doi: 10.1073/pnas.93.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Luo HJ, Liu XS, Wang F, Huang QH, Shen SH, Wang L, et al. Disruption of palladin results in neural tube closure defects in mice. Mol Cell Neurosci. 2005;29:507–515. doi: 10.1016/j.mcn.2004.12.002. [DOI] [PubMed] [Google Scholar]
- (85).Liu XS, Luo HJ, Yang H, Wang L, Kong H, Jin YE, et al. Palladin regulates cell and extracellular matrix interaction through maintaining normal actin cytoskeleton architecture and stabilizing beta1-integrin. J Cell Biochem. 2007;100:1288–1300. doi: 10.1002/jcb.21126. [DOI] [PubMed] [Google Scholar]
- (86).Liu XS, Li XH, Wang Y, Shu RZ, Wang L, Lu SY, et al. Disruption of palladin leads to defects in definitive erythropoiesis by interfering with erythroblastic island formation in mouse fetal liver. Blood. 2007;110:870–876. doi: 10.1182/blood-2007-01-068528. [DOI] [PubMed] [Google Scholar]
- (87).Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99:485–497. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- (88).Xu WM, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–337. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- (89).Shang E, Wang X, Wen D, Greenberg DA, Wolgemuth DJ. Double bromodomain-containing gene Brd2 is essential for embryonic development in mouse. Dev Dyn. 2009;238:908–917. doi: 10.1002/dvdy.21911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Ishibashi M, Ang S-L, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- (91).Hirata H, Tomita K, Bessho Y, Kageyama R. Hes1 and Hes3 regulate maintenance of the isthmic organizer and development of the mid/hindbrain. EMBO J. 2001;20:4454–4466. doi: 10.1093/emboj/20.16.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Takeuchi T, Yamazaki Y, Katoh-Fukui Y, Tsuchiya R, Kondo S, Motoyama J, et al. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 1995;9:1211–1222. doi: 10.1101/gad.9.10.1211. [DOI] [PubMed] [Google Scholar]
- (93).Lakkis MM, Golden JA, O’Shea KS, Epstein JA. Neurofibromin deficiency in mice causes exencephaly and is a modifier for Splotch neural tube defects. Dev Biol. 1999;212:80–92. doi: 10.1006/dbio.1999.9327. [DOI] [PubMed] [Google Scholar]
- (94).Lardelli M, Williams R, Mitsiadis T, Lendahl U. Expression of the Notch 3 intracellular domain in mouse central nervous system progenitor cells is lethal and leads to disturbed neural tube development. Mech Dev. 1996;59:177–190. doi: 10.1016/0925-4773(96)00589-8. [DOI] [PubMed] [Google Scholar]
- (95).Zhong WM, Jiang MM, Schonemann MD, Meneses JJ, Pedersen RA, Jan LY, et al. Mouse numb is an essential gene involved in cortical neurogenesis. Proc Natl Acad Sci USA. 2000;97:6844–6849. doi: 10.1073/pnas.97.12.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Smitherman M, Lee K, Swanger J, Kapur R, Clurman BE. Characterization and targeted disruption of murine Nup50, a p27(Kip1)-interacting component of the nuclear pore complex. Mol Cell Biol. 2000;20:5631–5642. doi: 10.1128/mcb.20.15.5631-5642.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Epstein DJ, Vekemans M, Gros P. splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell. 1991;67:767–774. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- (98).Engleka KA, Gitler AD, Zhang MZ, Zhou DD, High FA, Epstein JA. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol. 2005;280:396–406. doi: 10.1016/j.ydbio.2005.02.002. [DOI] [PubMed] [Google Scholar]
- (99).Mansouri A, Gruss P. Pax3 and Pax7 are expressed in commissural neurons and restrict ventral neuronal identity in the spinal cord. Mech Dev. 1998;78:171–178. doi: 10.1016/s0925-4773(98)00168-3. [DOI] [PubMed] [Google Scholar]
- (100).Oka C, Nakano T, Wakeham A, De la Pompa JL, Mori C, Sakai T, et al. Disruption of the mouse RBP-Jkappa gene results in early embryonic death. Development. 1995;121:3291–3301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- (101).Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature. 1996;381:235–238. doi: 10.1038/381235a0. [DOI] [PubMed] [Google Scholar]
- (102).Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, et al. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature. 1996;381:238–241. doi: 10.1038/381238a0. [DOI] [PubMed] [Google Scholar]
- (103).Kohlbecker A, Lee AE, Schorle H. Exencephaly in a subset of animals heterozygous for AP-2a mutation. Teratology. 2002;65:213–218. doi: 10.1002/tera.10037. [DOI] [PubMed] [Google Scholar]
- (104).Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94:727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- (105).Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94:739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- (106).Harris BS, Franz T, Ullrich S, Cook S, Bronson RT, Davisson MT. Forebrain overgrowth (fog): A new mutation in the mouse affecting neural tube development. Teratology. 1997;55:231–240. doi: 10.1002/(SICI)1096-9926(199704)55:4<231::AID-TERA3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- (107).Honarpour N, Gilbert SL, Lahn BT, Wang XD, Herz J. Apaf-1 deficiency and neural tube closure defects are found in fog mice. Proc Natl Acad Sci USA. 2001;98:9683–9687. doi: 10.1073/pnas.171283198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Ruland J, Duncan GS, Elia A, del Barco Barrantes I, Nguyen L, Plyte S, et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-kappaB and neural tube closure. Cell. 2001;104:33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- (109).Gowen LC, Johnson BL, Latour AM, Sulik KK, Koller BH. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nature Genet. 1996;12:191–194. doi: 10.1038/ng0296-191. [DOI] [PubMed] [Google Scholar]
- (110).Wang XY, Wang RH, Li WM, Xu XL, Hollander MC, Fornace AJ, Jr., et al. Genetic interactions between Brca1 and Gadd45a in centrosome duplication, genetic stability, and neural tube closure. J Biol Chem. 2004;279:29606–29614. doi: 10.1074/jbc.M312279200. [DOI] [PubMed] [Google Scholar]
- (111).Leonard JR, Klocke BJ, D’Sa C, Flavell RA, Roth KA. Strain-dependent neurodevelopmental abnormalities in caspase-3-deficient mice. J Neuropathol Exp Neurol. 2002;61:673–677. doi: 10.1093/jnen/61.8.673. [DOI] [PubMed] [Google Scholar]
- (112).Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- (113).Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;94:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- (114).Li QT, Estepa G, Memet S, Israel A, Verma IM. Complete lack of NF-kappaB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev. 2000;14:1729–1733. [PMC free article] [PubMed] [Google Scholar]
- (115).Chi H, Sarkisian MR, Rakic P, Flavell RA. Loss of mitogen-activated protein kinase kinase kinase 4 (MEKK4) results in enhanced apoptosis and defective neural tube development. Proc Natl Acad Sci U S A. 2005;102:3846–3851. doi: 10.1073/pnas.0500026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).Abell AN, Rivera-Perez JA, Cuevas BD, Uhlik MT, Sather S, Johnson NL, et al. Ablation of MEKK4 kinase activity causes neurulation and skeletal patterning defects in the mouse embryo. Mol Cell Biol. 2005;25:8948–8959. doi: 10.1128/MCB.25.20.8948-8959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Dixon J, Brakebusch C, Fässler R, Dixon MJ. Increased levels of apoptosis in the prefusion neural folds underlie the craniofacial disorder, Treacher Collins syndrome. Hum Mol Genet. 2000;9:1473–1480. doi: 10.1093/hmg/9.10.1473. [DOI] [PubMed] [Google Scholar]
- (118).Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM, et al. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res. 2003;144:83–90. doi: 10.1016/s0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- (120).Zhao Q, Behringer RR, De Crombrugghe B. Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nature Genet. 1996;13:275–283. doi: 10.1038/ng0796-275. [DOI] [PubMed] [Google Scholar]
- (121).Banting GS, Barak O, Ames TM, Burnham AC, Kardel MD, Cooch NS, et al. CECR2, a protein involved in neurulation, forms a novel chromatin remodeling complex with SNF2L. Hum Mol Genet. 2005;14:513–524. doi: 10.1093/hmg/ddi048. [DOI] [PubMed] [Google Scholar]
- (122).Davidson CE, Li Q, Churchill GA, Osborne LR, McDermid HE. Modifier locus for exencephaly in Cecr2 mutant mice is syntenic to the 10q25.3 region associated with neural tube defects in humans. Physiol Genomics. 2007;31:244–251. doi: 10.1152/physiolgenomics.00062.2007. [DOI] [PubMed] [Google Scholar]
- (123).Bamforth SD, Braganτa J, Eloranta JJ, Murdoch JN, Marques FIR, Kranc KR, et al. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat. Genet. 2001;29:469–474. doi: 10.1038/ng768. [DOI] [PubMed] [Google Scholar]
- (124).Barbera JP, Rodriguez TA, Greene NDE, Weninger WJ, Simeone A, Copp AJ, et al. Folic acid prevents exencephaly in Cited2 deficient mice. Hum Mol Genet. 2002;11:283–293. doi: 10.1093/hmg/11.3.283. [DOI] [PubMed] [Google Scholar]
- (125).Yin Z, Haynie J, Yang XM, Han BG, Kiatchoosakun S, Restivo J, et al. The essential role of Cited2, a negative regulator for HIF-1a, in heart development and neurulation. Proc Natl Acad Sci USA. 2002;99:10488–10493. doi: 10.1073/pnas.162371799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Tanaka Y, Naruse I, Hongo T, Xu MJ, Nakahata T, Maekawa T, et al. Extensive brain hemorrhage and embryonic lethality in a mouse null mutant of CREB-binding protein. Mech Dev. 2000;95:133–145. doi: 10.1016/s0925-4773(00)00360-9. [DOI] [PubMed] [Google Scholar]
- (127).Yao TP, Oh SP, Fuchs M, Zhou ND, Ch’ng LE, Newsome D, et al. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93:361–372. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- (128).Bu P, Evrard YA, Lozano G, Dent SY. Loss of Gcn5 acetyltransferase activity leads to neural tube closure defects and exencephaly in mouse embryos. Mol Cell Biol. 2007;27:3405–3416. doi: 10.1128/MCB.00066-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Lin W, Zhang Z, Srajer G, Chen YC, Huang M, Phan HM, et al. Proper expression of the Gcn5 histone acetyltransferase is required for neural tube closure in mouse embryos. Dev Dyn. 2008;237:928–940. doi: 10.1002/dvdy.21479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Lin W, Zhang Z, Chen CH, Behringer RR, Dent SY. Proper Gcn5 histone acetyltransferase expression is required for normal anteroposterior patterning of the mouse skeleton. Dev Growth Differ. 2008;50:321–330. doi: 10.1111/j.1440-169X.2008.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Isono K, Nemoto K, Li Y, Takada Y, Suzuki R, Katsuki M, et al. Overlapping roles for homeodomain-interacting protein kinases hipk1 and hipk2 in the mediation of cell growth in response to morphogenetic and genotoxic signals. Mol Cell Biol. 2006;26:2758–2771. doi: 10.1128/MCB.26.7.2758-2771.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Rogner UC, Spyropoulos DD, Le Novère N, Changeux JP, Avner P. Control of neurulation by the nucleosome assembly protein-1-like. Nature Genet. 2000;25:431–435. doi: 10.1038/78124. [DOI] [PubMed] [Google Scholar]
- (133).Pirity MK, Locker J, Schreiber-Agus N. Rybp/DEDAF is required for early postimplantation and for central nervous system development. Mol Cell Biol. 2005;25:7193–7202. doi: 10.1128/MCB.25.16.7193-7202.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell. 2000;6:1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- (135).Kim JK, Huh SO, Choi H, Lee KS, Shin D, Lee C, et al. Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol Cell Biol. 2001;21:7787–7795. doi: 10.1128/MCB.21.22.7787-7795.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (136).Miro X, Zhou X, Boretius S, Michaelis T, Kubisch C, Avarez-Bolado G, et al. Haploinsufficiency of the murine polycomb gene Suz12 results in diverse malformations of the brain and neural tube. Dis Model Mech. 2009;2:412–418. doi: 10.1242/dmm.001602. [DOI] [PubMed] [Google Scholar]
- (137).Berube NG, Jagla M, Smeenk C, De Repentigny Y, Kothary R, Picketts DJ. Neurodevelopmental defects resulting from ATRX overexpression in transgenic mice. Hum Mol Genet. 2002;11:253–261. doi: 10.1093/hmg/11.3.253. [DOI] [PubMed] [Google Scholar]
- (138).Cho A, Ko HW, Eggenschwiler JT. FKBP8 cell-autonomously controls neural tube patterning through a Gli2- and Kif3a-dependent mechanism. Dev Biol. 2008;321:27–39. doi: 10.1016/j.ydbio.2008.05.558. [DOI] [PubMed] [Google Scholar]
- (139).Shirane M, Ogawa M, Motoyama J, Nakayama KI. Regulation of apoptosis and neurite extension by FKBP38 is required for neural tube formation in the mouse. Genes Cells. 2008;13:635–651. doi: 10.1111/j.1365-2443.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- (140).Wong RL, Wlodarczyk BJ, Min KS, Scott ML, Kartiko S, Yu W, et al. Mouse Fkbp8 activity is required to inhibit cell death and establish dorso-ventral patterning in the posterior neural tube. Hum Mol Genet. 2008;17:587–601. doi: 10.1093/hmg/ddm333. [DOI] [PubMed] [Google Scholar]
- (141).Pan Y, Wang C, Wang B. Phosphorylation of Gli2 by protein kinase A is required for Gli2 processing and degradation and the Sonic Hedgehog-regulated mouse development. Dev Biol. 2009;326:177–189. doi: 10.1016/j.ydbio.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Hsu CY, Chang NC, Lee MW, Lee KH, Sun DS, Lai C, et al. LUZP deficiency affects neural tube closure during brain development. Biochem Biophys Res Commun. 2008;376:466–471. doi: 10.1016/j.bbrc.2008.08.170. [DOI] [PubMed] [Google Scholar]
- (143).Huang Y, Roelink H, McKnight GS. Protein kinase A deficiency causes axially localized neural tube defects in mice. J Biol Chem. 2002;277:19889–19896. doi: 10.1074/jbc.M111412200. [DOI] [PubMed] [Google Scholar]
- (144).Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- (145).Milenkovic L, Goodrich LV, Higgins KM, Scott MP. controls body size determination and limb patterning. Development. 1999;126:4431–4440. doi: 10.1242/dev.126.20.4431. [DOI] [PubMed] [Google Scholar]
- (146).Günther T, Struwe M, Aguzzi A, Schughart K. open brain, a new mouse mutant with severe neural tube defects, shows altered gene expression patterns in the developing spinal cord. Development. 1994;120:3119–3130. doi: 10.1242/dev.120.11.3119. [DOI] [PubMed] [Google Scholar]
- (147).Eggenschwiler JT, Espinoza E, Anderson KV. Rab23 is an essential negative regulator of the mouse Sonic hedgehog signalling pathway. Nature. 2001;412:194–198. doi: 10.1038/35084089. [DOI] [PubMed] [Google Scholar]
- (148).Eggenschwiler JT, Bulgakov OV, Qin J, Li T, Anderson KV. Mouse Rab23 regulates hedgehog signaling from smoothened to Gli proteins. Dev Biol. 2006;290:1–12. doi: 10.1016/j.ydbio.2005.09.022. [DOI] [PubMed] [Google Scholar]
- (149).Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- (150).Cooper AF, Yu KP, Brueckner M, Brailey LL, Johnson L, McGrath JM, et al. Cardiac and CNS defects in a mouse with targeted disruption of suppressor of fused. Development. 2005;132:4407–4417. doi: 10.1242/dev.02021. [DOI] [PubMed] [Google Scholar]
- (151).Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, et al. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- (152).Ikeda A, Ikeda S, Gridley T, Nishina PM, Naggert JK. Neural tube defects and neuroepithelial cell death in Tulp3 knockout mice. Hum Mol Genet. 2001;10:1325–1334. doi: 10.1093/hmg/10.12.1325. [DOI] [PubMed] [Google Scholar]
- (153).Patterson VL, Damrau C, Paudyal A, Reeve B, Grimes DT, Stewart ME, et al. Mouse hitchhiker mutants have spina bifida, dorso-ventral patterning defects and polydactyly: identification of Tulp3 as a novel negative regulator of the Sonic hedgehog pathway. Hum Mol Genet. 2009;18:1719–1739. doi: 10.1093/hmg/ddp075. [DOI] [PMC free article] [PubMed] [Google Scholar]


