Abstract
Background
Measurement of cervical length (CL) by transvaginal ultrasound (TVU) is predictive of preterm birth (PTB). It is unclear if this screening test is effective for prevention of PTB.
Objectives
To assess the effectiveness of antenatal management based on TVU CL screening for preventing PTB.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (September 2008), MEDLINE (1966 to September 2008), and reviewed the reference list of all articles. We updated the search of the Cochrane Pregnancy and Childbirth Group’s Trials Register on 27 January 2012 and added the results to the awaiting classification section.
Selection criteria
Published and unpublished randomized controlled trials including pregnant women between the gestational ages of 14 to 32 weeks screened with TVU CL for risk of PTB. This review focuses exclusively on studies based on knowledge versus no knowledge of TVU CL results.
Data collection and analysis
All potential studies identified as in the search were assessed for inclusion by three independent review authors. We also analyzed studies for quality measures and extracted data.
Main results
Of 12 trials identified, five were eligible for inclusion (n = 507). Three included singleton gestations with preterm labor (PTL); one included singleton gestations with preterm prelabour rupture of membranes (PPROM); and one included twin gestations without or with PTL.
In the three trials of singleton gestations with PTL, 290 women were randomized; 147 to knowledge and 143 to no knowledge of TVU CL. Knowledge of TVU CL results was associated with a non-significant decrease in PTB at less than 37 weeks (22.3% versus 34.7%, respectively; risk ratio 0.59, 95% confidence interval (CI) 0.26 to 1.32). Delivery occurred at a later gestational age in the knowledge versus no knowlege groups (mean difference 0.64 weeks (CI 0.03 to 1.25)). All other outcomes for which there were available data (PTB at less than 34 or 28 weeks; birthweight less than 2500 grams; perinatal death; maternal hospitalization; tocolysis; and steroids for fetal lung maturity) were similar in the two groups.
The trial of singleton gestations with PPROM (n = 92) evaluated as its primary outcome safety of TVU CL in this population, and not its effect on management. The incidence of maternal and neonatal infections was similar in the TVU CL and no TVU CL groups.
In the trial of twin gestations with or without PTL (n = 125), PTB at less than 36, 34, or 30 weeks, gestational age at delivery, and other perinatal and maternal outcomes were similar in the TVU CL and the no TVU CL groups. Life table analysis revealed significantly less preterm birth at less than 35 weeks in the TVU CL group compared to the no TVU CL group (P = 0.02).
Authors’ conclusions
Currently there is insufficient evidence to recommend routine screening of asymptomatic or symptomatic pregnant women with TVU CL. Since there is a non-significant association between knowledge of TVU CL results and a lower incidence of PTB at less than 37 weeks in symptomatic women, we encourage further research. Future studies should look at specific populations separately (eg singleton versus twins; symptoms of PTL or no such symptoms), report on all pertinent maternal and perinatal outcomes, and include cost-effectiveness analyses. Most importantly, future studies should include a clear protocol for management of women based on TVU CL results, so that it can be easily evaluated and replicated.
[Note: The two citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Medical Subject Headings (MeSH): Cervical Length Measurement [*methods]; Cervix Uteri [*ultrasonography]; Pregnancy, Multiple; Premature Birth [*prevention & control; ultrasonography]; Randomized Controlled Trials as Topic; Twins
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Importance of preterm birth
Preterm birth is defined by the World Health Organization as birth between 20 and 36 6/7 weeks. Preterm birth can be spontaneous, and follow preterm labour (50%), or preterm premature rupture of membranes (30%). It can also be iatrogenic (caused by health worker intervention) (20%). Its incidence is about 5% to 8% in most developed and developing countries. This incidence is increasing in many countries, including developing countries, despite extensive research efforts. It has increased to 12.8% in 2006 in the USA (a greater than 20% increase in the last 10 years), representing more than 500,000 preterm births annually in the USA alone (National Vital Statistics Report 2006). Some of the reasons may include increases in the incidence of multiple gestations, assisted reproductive technologies, better dating and recording of gestational age, more fetal monitoring and iatrogenic deliveries, etc. Preterm birth is the main cause of neonatal morbidity and mortality in most countries, especially in developed countries. In the USA, 75% of perinatal mortality occurs in preterm babies; 60% of total perinatal mortality occurs in infants born before 32 weeks. Mortality and morbidities are inversely associated with gestational age at birth. Morbidities include respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage, necrotizing enterocolitis, sepsis, retinopathy, etc. The whole family suffers greatly in several aspects when a baby has been born prematurely, including medically, socially, psychologically, and financially.
Interventions to reduce preterm birth
Most of the interventions studied have been aimed at tertiary prevention, i.e. prevention once symptoms (e.g., preterm labour or premature preterm rupture of membranes) develop. Interventions based on risk factors, usually based on prior history, have generally been unsuccessful. Recently, a screening test, cervical ultrasound, has been associated with better prediction of preterm birth than previously available tests, and interventions based on this screening test have been tested in randomized trials.
Cervical assessment by ultrasound to predict and reduce preterm birth
Cervical assessment by ultrasound has been correlated with the prediction of spontaneous preterm birth (Berghella 2003). The most objective and effective ultrasound method is transvaginal. The most predictive and reproducible variable that can be measured is cervical length. The gestational age at which transvaginal ultrasound (TVU) cervical length (CL) is most predictive of preterm birth (PTB) is 14 to 34 weeks, but shortening at earlier and later gestational ages is also associated with PTB. The shorter the cervical length, the higher the risk of PTB becomes (Grimes-Dennis 2007). The earlier in gestation the shortening is detected, the higher the risk of PTB (Berghella 2007). This prediction has been confirmed in all populations screened with TVU CL so far, including singleton and multiple gestations, women with or without risk factors (e.g., prior PTB, mullerian anomalies, cervical surgery, etc.) for PTB, asymptomatic women as well as those with preterm labour or preterm premature rupture of membranes (Grimes-Dennis 2007). In fact, TVU CL is one of the best predictors of preterm birth in all populations studied so far. The overall sensitivity and specificity vary according to CL cutoff used (e.g., 25 mm versus 15 mm); gestational age at screening; population studied; prevalence of preterm birth; single versus serial screening; etc. Its positive predictive value also varies depending on the incidence of preterm labour in the population studied.
OBJECTIVES
To assess the effectiveness of antenatal management based on transvaginal ultrasound cervical length screening for preventing preterm birth.
METHODS
Criteria for considering studies for this review
Types of studies
Published and unpublished randomized controlled trials. We planned to include cluster-randomized and quasi-randomized trials, if available.
Types of participants
Pregnant women between the gestational ages of 14 to 34 weeks screened with transvaginal ultrasound (TVU) cervical length (CL) for risk of preterm birth. The population of main interest for primary analysis was symptomatic women with singleton gestations with signs and/or symptoms of preterm labour (PTL). We carried out analysis of other participants by type of population, as described under ’subgroup analyses’.
Types of interventions
A screening test such as TVU CL can only be considered effective if interventions based on screening results reduce the outcome of preterm birth. For this review, screening TVU CL modalities on which interventions were based were:
knowledge versus no knowledge of TVU CL results (i.e TVU CL is performed on all women, but women are randomized so that in 50% of them the result is available to the managing obstetrician, while in 50% the managing obstetrician is blind to the TVU CL result);
TVU CL versus no TVU CL (TVU CL screening is only done on half of the women).
Types of outcome measures
Primary outcomes
Preterm birth (less than 37 weeks for singleton gestations; less than 34 weeks for twin gestations)
Secondary outcomes
Preterm birth (less than 34 weeks)
Preterm birth (less than 32 weeks)
Preterm birth (less than 28 weeks)
Gestational age at delivery
Birthweight less than 2500 grams
Composite perinatal outcome (perinatal death, respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and sepsis)
Perinatal death (fetal death and neonatal death)
Fetal death
Neonatal death
Respiratory distress syndrome
Intraventricular hemorrhage
Necrotizing enterocolitis
Sepsis
Neonatal intensive care unit (NICU) admission
NICU days
Maternal hospitalization
Maternal wellbeing (e.g., stress level, etc)
Economic analysis (cost effectiveness, cost utility)
Tocolysis
Cervical cerclage
Steroids
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co-ordinator (September 2008). We updated this search on 27 January 2012 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched MEDLINE (January 1966 to September 2008) using the search strategy in Appendix 1.
Searching other resources
We reviewed the reference list of all articles, in particular trials and review articles. If necessary, we contacted researchers to provide further information. We contacted experts in the field for additional and ongoing trials.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
We assessed for inclusion all potential studies we identified as a result of the search strategy. Independently, all three review authors (V Berghella (VB), N Hendrix (NH), and JK Baxter (JB)) assessed all studies for inclusion in the review using the inclusion criteria. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. Three authors (VB, NH, JB) extracted the data using the agreed form. We resolved any disagreement through discussion. We used the Review Manager software (RevMan 2008) to double enter all the data or a subsample.
When information regarding any of the above was unclear, or to obtain additional data not published, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
We have assessed the validity of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We described the methods used for generation of the randomization sequence for each trial.
(1) Sequence generation (checking for possible selection bias)
We have described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We have assessed the method as:
adequate (any truly random process e.g. random number table; computer random number generator);
inadequate (any non-random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
adequate (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non-opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We have described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We have judged studies to be at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We have assessed blinding separately for different outcomes or classes of outcomes.
We have assessed the methods as:
adequate, inadequate, or unclear for participants;
adequate, inadequate, or unclear for personnel;
adequate, inadequate, or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We have described for each included study, and for each outcome or class of outcomes, the completeness of data, including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information has been reported, or been supplied by the trial authors, we have re-included missing data in our analyses. We have assessed methods as:
adequate (< 20%);
inadequate (>= 20%);
unclear.
(5) Selective reporting bias
We have described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We have assessed the methods as:
adequate (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed-effect meta-analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar. If heterogeneity was found, we explored this by sensitivity analysis, followed by random-effects analysis if required.
Dichotomous data
For dichotomous data, we presented results as summary relative risk with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardized mean difference to combine trials that measured the same outcome, but use different methods. If there was evidence of skewness, we reported this.
Unit of analysis issues
Cluster-randomized trials
We did not identify any cluster-randomized trials for inclusion in this review, but we may include trials of this type in future updates. If we do, we plan to include cluster-randomized trials in the analyses along with individually randomized trials. Their sample sizes will be adjusted using the methods described in Gates 2005 using an estimate of the intracluster correlation co-efficient (ICC) derived from the trial (if possible), or from another source. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster-randomized trials and individually randomized trials, we planned to synthesize the relevant information. We consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely. We will also acknowledged heterogeneity in the randomization unit and perform a separate meta-analysis; therefore, the meta-analysis will be performed in two parts as well if significant heterogeneity is found.
Dealing with missing data
We analyzed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analyzed in the group to which they were randomized, and there was sufficient information in the trial report, we attempted to restore them to the correct group.
Assessment of heterogeneity
We applied tests of heterogeneity between trials, if appropriate, using the I2 statistic. If we identified high levels of heterogeneity among the trials (exceeding 50%), we explored it by prespecified subgroup analysis and performed sensitivity analysis. We used a random-effects meta-analysis as an overall summary if this was considered appropriate.
Subgroup analyses
We planned the following subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001:
asymptomatic women with singleton gestations without signs and/or symptoms of preterm labour (PTL) or preterm pre-labor rupture of membranes (PPROM);
women with low-risk singleton gestations versus high-risk (e.g., prior preterm birth) singleton gestations;
timing of availability of results;
gestational age at screening for transvaginal ultrasound (TVU) cervical length (CL) (less than 22, 22 to 23, 24 to 28, more than 28 weeks);
degree of cervical shortening (e.g. 15 mm versus 25 mm as cutoff for the definition of a short TVU CL).
Sensitivity analysis
We carried out sensitivity analysis to explore the effect of trial quality. This involved analysis based on a ’yes, no, unclear’ rating of selection bias and attrition bias. We excluded studies of poor quality in the analysis (i.e. those with significant selection or attrition bias) in order to assess for any substantive difference to the overall result.
If quasi-randomized trials are included in the the future, we will perform a sensitivity analysis by trial quality.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Our search identified 12 trials, of which five were eligible for inclusion (n = 507). We identified no quasi-randomized trials. We excluded three trials because they compared history-indicated to ultrasound-indicated cerclage (Beigi 2005; Kassanos 2001; Shennan 2007); one because the TVU CL information was blinded and not used for management (Matijevic 2006); one because TVU information was not used for clinical care and no data on outcomes was provided (Owen 1999); and two because they utilized transabdominal - not transvaginal - ultrasound (Lorenz 1990; Van Dijken 1991).
The five included studies included symptomatic women with singleton gestations with signs and/or symptoms of PTL (three trials:Alfirevic 2007; Ness 2007; Palacio 2006); symptomatic women with singleton gestations with signs and/or symptoms of PPROM (one trial: Carlan 1997); asymptomatic women with twin gestations without or with signs and/or symptoms of PTL (one trial:Gordon 2006). We identified no trials including asymptomatic women with singleton gestations without signs and/or symptoms of PTL or PPROM. We requested patient-level databases from all authors, and obtained them from one trial (Ness 2007).
In the three trials of symptomatic women with singleton gestations with signs and/or symptoms of PTL, 290 women were randomized; 147 were randomized to knowledge and 143 to no knowledge of TVU CL. These numbers were 145 and 142, respectively, after exclusion of twin gestations from the Ness trial. Ness 2007 used knowledge of TVU CL mostly in its protocol for management, but for women with TVU CL 20 to 29 mm, fetal fibronectin (FFN) was used to discriminate management, as well.
In the one trial of symptomatic women with singleton gestations with PPROM, the analysis included 47 women who had TVU CL and 45 who did not.
In the one trial of symptomatic women with twin gestations without or with signs and/or symptoms of PTL, the analysis included 63 women who had TVU CL and 62 who did not.
See ’Characteristics of included studies’ and ’Characteristics of excluded studies’. (Two reports from an updated search in January 2012 have been added to Studies awaiting classification.)
Risk of bias in included studies
Risk of selection bias was not present in three studies (Alfirevic 2007; Carlan 1997; Ness 2007). The other two studies (Gordon 2006; Palacio 2006) were reported only as abstracts, with no information on allocation concealment.
Information regarding an intention-to-treat analysis was available for four of the five trials. In two (Alfirevic 2007; Ness 2007), all randomized women were included in the intention-to-treat analysis. In Carlan 1997, one out of 93 (1%) women randomized was excluded from analysis because she was delivered immediately. Attrition bias in terms of loss of data was present in Palacio 2006, since eight out of 157 (5%) women randomized were excludedfrom analysis because they were lost to follow up, and in Ness 2007 for some outcomes.
Risk of performance bias was present in all trials, as participants and researchers were aware of the arm to which they were randomized, but this was inevitable.
Effects of interventions
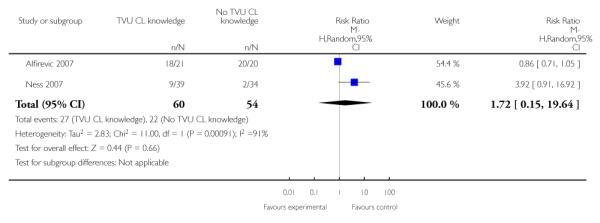
In symptomatic women with singleton gestations with signs and/ or symptoms of PTL, knowledge of TVU CL results was associated with a non-significant decrease in preterm birth at less than 37 weeks compared to no such knowledge (22.3% versus 34.7%, respectively; risk ratio (RR) 0.59, 95% confidence interval (CI) 0.26 to 1.32; 2 trials, n = 242). Delivery occurred at a later gestational age in the knowledge versus no knowlege groups (mean difference 0.64 weeks (CI 0.03 to 1.25)). These results were mostly determined by the Ness 2007 trial, which used FFN to determine management in women with TVU CL of 20 to 29 mm. All other outcomes for which there were available data (preterm birth less than 34 or 28 weeks; birthweight less than 2500 grams; perinatal death; maternal hospitalization; tocolysis; and steroids for fetal lung maturity) were similar in the two groups. Appropriateness of treatment in terms of steroids for fetal lung maturity was higher in the knowledge versus the no knowledge group in the one trial which evaluted this outcome (Alfirevic 2007). No other maternal or fetal outcome was available for meaningful analysis.
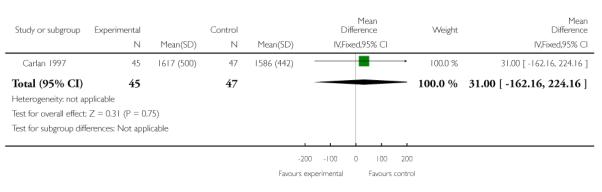
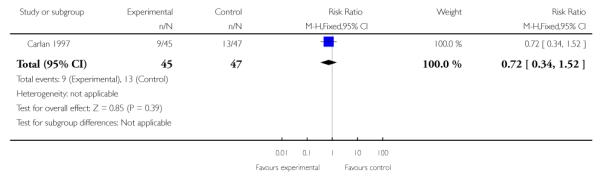
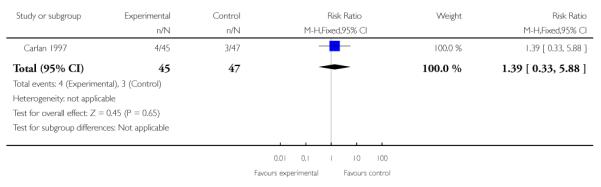
The one trial of symptomatic women with singleton gestations with PPROM (Carlan 1997) evaluted as its primary outcome safety of TVU CL in this population, and not its effect on management. Incidence of PTB or gestational age at delivery were not reported, while birth weight less than 2500 grams was similar in the two groups. The incidence of maternal (20% versus 28%) and neonatal (20% versus 17%) infections were similar in the TVU CL and no TVU CL groups, respectively.
In the one trial of twin gestations without or with signs and/or symptoms of PTL (Gordon 2006), preterm birth less than 36, 34, 32, or 30 weeks, gestational age at delivery, and other perinatal and maternal outcomes were similar in the TVU CL and the no TVU CL groups. Life table analysis revealed significantly less preterm birth at less than 35 weeks in the TVU CL group compared to the no TVU CL group (P = 0.02).
Heterogeneity was present in the analyses of preterm birth at less than 37 weeks, tocolysis, and steroids for fetal lung maturity.
DISCUSSION
Knowledge of TVU CL in management of women with singleton gestations and PTL is not associated with any significant effects in any maternal and perinatal outcomes evaluated, possibly due to the small number of trials. The effect of knowledge of TVU CL in the management of women with either PPROM or twin gestation cannot be determined, given that there is just one small trial on each of these populations, and no trial in asymptomatic women with singleton gestations. Further reseach is therefore necessary.
Furthermore, it is unclear which interventions are most efficacious once TVU CL results are known. The one study with the most promising results (Ness 2007) suggested use of FFN for management of women with TVU CL of 20 to 29 mm, and a protocol of no intervention for women with CL equal to or greater than 30 mm and intervention with steroids for fetal lung maturity and tocolysis for women with TVU CL less than 20 mm, but these results need to be replicated before widespread implementation.
Our review did not include, by design, assessment of effectiveness of interventions based on positive TVU CL screening (short CL), or negative TVU CL screening (normal or long CL).
AUTHORS’ CONCLUSIONS
Implications for practice
Currently there is insufficient evidence to recommend routine screening of asymptomatic or symptomatic pregnant women with TVU CL.
Implications for research
Since this review found a non-significant association between knowledge of TVU CL results and a lower incidence of preterm birth before 37 weeks in symptomatic women, the authors encourage further research. Future studies should look at specific populations separately (e.g., singleton versus twins; symptoms of PTL or no such symptoms), report on all pertinent maternal and perinatal outcomes, and include cost-effectiveness analyses. Most importantly, future studies should include a clear protocol for management of women based on TVU CL results, so that it can be easily evaluated and replicated.
[Note: The two citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
PLAIN LANGUAGE SUMMARY.
Cervical assessment by ultrasound for preventing preterm delivery
Preterm birth before 37 weeks is the main cause of death and disability for neonates. The lower part of the uterus, called the cervix, is the passage through which births, including preterm, occur. Ultrasound performed through the vagina can detect early changes of the cervix that predict preterm birth. This review assessed if knowledge of such changes can prevent preterm birth. Of the 12 trials identified, five (507 women) were eligible for inclusion. Currently the studies reported are insufficient to recommend ultrasound of the cervix for prevention of preterm birth. Since there is a tendency for knowledge of the results of the cervical ultrasound to be associated with a lower chance of preterm birth in women who have uterine contractions and preterm labor, further research should be encouraged.
ACKNOWLEDGEMENTS
Chris Harvey was co-author of the published protocol for this review. Jolene Seibel-Seamon helped with statistics.
As part of the pre-publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group’s Statistical Adviser.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | RCT. | |
| Participants | Singleton gestations; uterine contractions at < 34 weeks; and clinical decision to use steroids and tocolytics. N = 41 | |
| Interventions | TVU CL knowledge or not (the control group did not receive TVU CL) Time TVU CL results available: not specified. Protocol for TVU knowledge group: yes. |
|
| Outcomes | Primary: incidence of women still pregnant at 7 days. | |
| Notes | Intention-to-treat; only singletons; protocol for management of TVU CL group Short TVU CL (< 15mm): 7/21 (33%) in knowledge group; not done in other group |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated randomization. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Women and physicians knew which group was randomized to ‘knowledge’ or ‘no knowledge’ |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | No incomplete outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome was delivery within 7 days. |
| Methods | RCT. | |
| Participants | Singleton gestations; PPROM; 24 to 34 weeks. N = 92. | |
| Interventions | TVU CL or not (the control group did not receive TVU CL). Time TVU CL results available: not specified. Protocol for TVU knowledge group: no. |
|
| Outcomes | Primary: maternal infection. | |
| Notes | Intention to treat; only singletons; PPROM; no protocol (really a safety study for TVU CL in PPROM women) Short TVU CL ( < 25mm): 14/45 (31%) in knowledge group; not done in other group |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomly-generated assignment”. |
| Allocation concealment (selection bias) | Low risk | “Randomly-generated” assignments in sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Study group had weekly US while controls had none. |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 1% explained. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome was chorioamnionitis. |
| Methods | RCT. | |
| Participants | Twin gestations; asymptomatic and with PTL symptoms; 15 to 34 weeks. N = 125 | |
| Interventions | TVU CL screening at 15 to 28 weeks, and if PTL symptoms develop or not (the control group did not receive TVU CL) Time TVU CL results available: not specified. Protocol for TVU knowledge group: yes. |
|
| Outcomes | Primary: length of gestation. | |
| Notes | Only abstract published; unclear if intention to treat; only twins; protocol for management of TVU CL group Short TVU CL not available. |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Different protocols for study and control groups. |
| Incomplete outcome data (attrition bias) All outcomes |
Unclear risk | No incomplete outcomes mentioned. |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome was gestational age at delivery. |
| Methods | RCT. | |
| Participants | Singleton (and 3 twin) gestations; uterine contractions or symptoms suggestive of preterm labor at 24 to 33 6/7 weeks. N = 100 | |
| Interventions | TVU CL knowledge or not (the control group did receive TVU CL, but results were blinded to managing physicians) Time TVU CL results available: not specified. Protocol for TVU knowledge group: yes. |
|
| Outcomes | Primary: time from initial evaluation to discharge. | |
| Notes | Intention to treat; 97% singletons; protocol for management of TVU CL group, which included management based on FFN for women with CL 20 to 29 mm Short TVUCL ( < 20mm): 11/51 (22%) in knowledge group; 7/49 (15%) in the control group |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer-generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Numbered, sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Women and physicians knew which group was randomized to ‘knowledge’ or ‘no knowledge’ |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | Intention-to-treat analyses. |
| Selective reporting (reporting bias) | Low risk | Primary outcome time from evaluation to discharge. |
| Methods | RCT. | |
| Participants | Singleton gestations; preterm labor at 24 to 35 6/7 weeks. N = 149 | |
| Interventions | TVU CL knowledge or not (the control group did receive TVU CL, but results were blinded to managing physicians) Time TVU CL results available: not specified. Protocol for TVU knowledge group: yes. |
|
| Outcomes | Primary: hospital length of stay. | |
| Notes | Only abstract published; 7 women lost to follow up; only singletons; protocol for management of TVU CL group Short TVU CL ( < 25mm): 22/75 (29%) in knowledge group; 20/74 (27%) in the control group |
|
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes |
High risk | Women and physicians knew which group was randomized to ‘knowledge’ or ‘no knowledge’ |
| Incomplete outcome data (attrition bias) All outcomes |
Low risk | 5% of data removed from final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Length of hospital stay primary outcome. |
CL: cervical length
PPROM: preterm prelabour rupture of membranes
PTL: preterm labor
RCT: randomized controlled trial
TVU: transvaginal ultrasound
US: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beigi 2005 | Compared history-indicated to ultrasound-indicated cerclage. |
| Kassanos 2001 | Compared history-indicated to ultrasound-indicated cerclage. |
| Lorenz 1990 | Utilized transabdominal - not transvaginal - ultrasound. |
| Matijevic 2006 | The TVU CL information was blinded and not used for management |
| Owen 1999 | TVU information was not used for clinical care and no data on outcomes was provided |
| Shennan 2007 | Compared history-indicated to ultrasound-indicated cerclage. |
| Van Dijken 1991 | Utilized transabdominal - not transvaginal - ultrasound. |
CL: cervical length
TVU: transvaginal ultrasound Cervical
DATA AND ANALYSES
Comparison 1.
TVU CL knowledge versus no knowledge (singletons with PTL)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth < 37 weeks | 2 | 242 | Risk Ratio (M-H, Random, 95% CI) | 0.59 [0.26, 1.32] |
| 2 Preterm birth < 34 weeks | 3 | 256 | Risk Ratio (M-H, Fixed, 95% CI) | 0.55 [0.25, 1.20] |
| 3 Preterm birth < 28 weeks | 2 | 137 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 gestational age at delivery | 3 | 290 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [0.03, 1.25] |
| 5 Birth weight < 2500 grams | 1 | 70 | Risk Ratio (M-H, Fixed, 95% CI) | 0.71 [0.21, 2.44] |
| 6 Perinatal death | 1 | 97 | Risk Ratio (M-H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maternal hospitalization | 1 | 93 | Risk Ratio (M-H, Fixed, 95% CI) | 2.94 [0.85, 10.16] |
| 8 Tocolysis | 2 | 102 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.11, 6.58] |
| 9 Steroids for fetal lung maturity | 2 | 114 | Risk Ratio (M-H, Random, 95% CI) | 1.72 [0.15, 19.64] |
Comparison 2.
TVU CL Knowledge versus no knowledge (singletons with PPROM)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birth weight < 2500 grams | 1 | 92 | Mean Difference (IV, Fixed, 95% CI) | 31.0 [−162.16, 224.16] |
| 2 Chorioamnionitis | 1 | 92 | Risk Ratio (M-H, Fixed, 95% CI) | 0.72 [0.34, 1.52] |
| 3 Endometritis | 1 | 92 | Risk Ratio (M-H, Fixed, 95% CI) | 1.39 [0.33, 5.88] |
| 4 Neonatal infection | 1 | 92 | Risk Ratio (M-H, Fixed, 95% CI) | 1.18 [0.50, 2.78] |
Comparison 3.
TVU CL knowledge versus no knowledge (twins)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth < 36 weeks | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 1.27 [0.85, 1.90] |
| 2 Preterm birth < 34 weeks | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 0.62 [0.30, 1.25] |
| 3 Preterm birth < 32 weeks | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 0.56 [0.17, 1.83] |
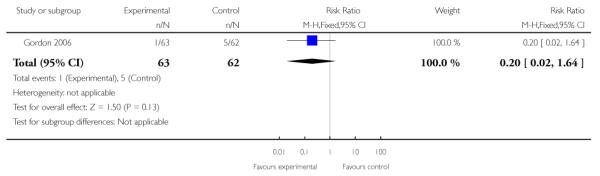
| 4 Preterm birth < 30 weeks | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 0.20 [0.02, 1.64] |
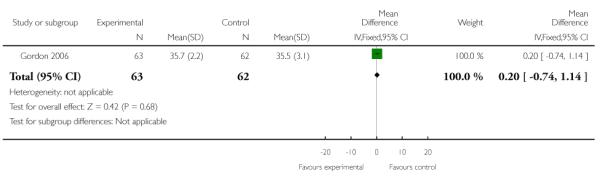
| 5 Gestational age at delivery | 1 | 125 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [−0.74, 1.14] |
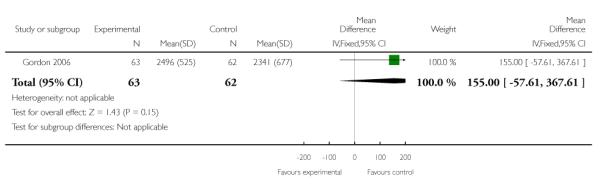
| 6 Birth weight | 1 | 125 | Mean Difference (IV, Fixed, 95% CI) | 155.0 [−57.61, 367.61] |
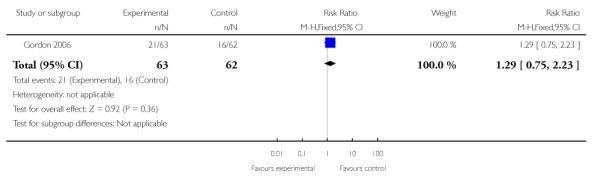
| 7 Maternal hospitalization (PTL) | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 1.29 [0.75, 2.23] |
| 8 Tocolysis | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 1.34 [0.74, 2.42] |
| 9 Steroids for fetal lung maturity | 1 | 125 | Risk Ratio (M-H, Fixed, 95% CI) | 0.79 [0.49, 1.26] |
Analysis 1.1. Comparison 1 TVU CL knowledge versus no knowledge (singletons with PTL), Outcome 1 Preterm birth < 37 weeks
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 1 TVU CL knowledge versus no knowledge (singletons with PTL)
Outcome: 1 Preterm birth < 37 weeks
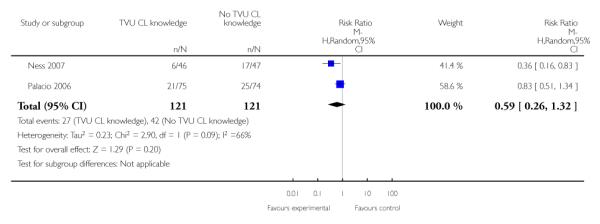 |
Analysis 1.2. Comparison 1 TVU CL knowledge versus no knowledge (singletons with PTL), Outcome 2 Preterm birth < 34 weeks
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 1 TVU CL knowledge versus no knowledge (singletons with PTL)
Outcome: 2 Preterm birth < 34 weeks
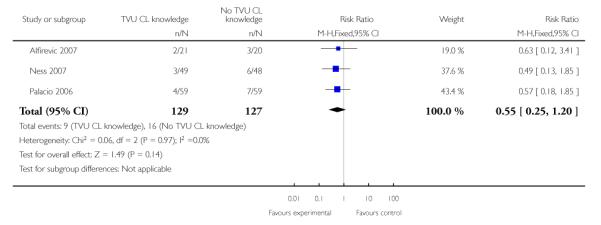 |
Analysis 1.3. Comparison 1 TVU CL knowledge versus no knowledge (singletons with PTL), Outcome 3 Preterm birth < 28 weeks
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 1 TVU CL knowledge versus no knowledge (singletons with PTL)
Outcome: 3 Preterm birth < 28 weeks
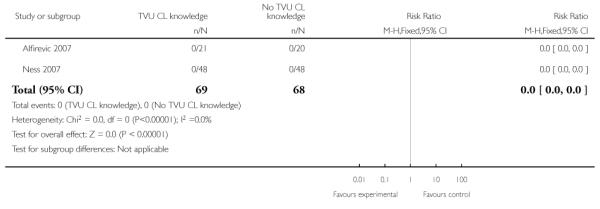 |
Analysis 1.4. Comparison 1 TVU CL knowledge versus no knowledge (singletons with PTL), Outcome 4 gestational age at delivery
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 1 TVU CL knowledge versus no knowledge (singletons with PTL)
Outcome: 4 gestational age at delivery
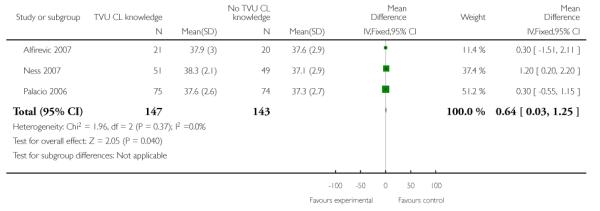 |
Analysis 1.5. Comparison 1 TVU CL knowledge versus no knowledge (singletons with PTL), Outcome 5 Birth weight < 2500 grams
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 1 TVU CL knowledge versus no knowledge (singletons with PTL)
Outcome: 5 Birth weight < 2500 grams
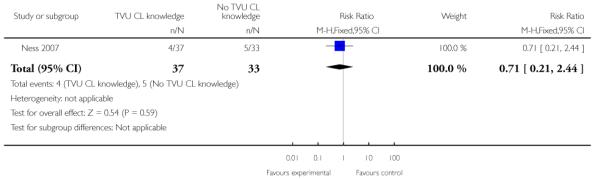 |
Analysis 1.6. Comparison 1 TVU CL knowledge versus no knowledge (singletons with PTL), Outcome 6 Perinatal death
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 1 TVU CL knowledge versus no knowledge (singletons with PTL)
Outcome: 6 Perinatal death
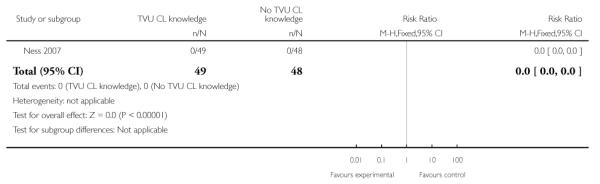 |
Analysis 1.7. Comparison 1 TVU CL knowledge versus no knowledge (singletons with PTL), Outcome 7 Maternal hospitalization
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 1 TVU CL knowledge versus no knowledge (singletons with PTL)
Outcome: 7 Maternal hospitalization
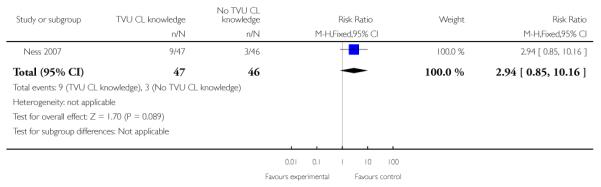 |
Analysis 1.8. Comparison 1 TVU CL knowledge versus no knowledge (singletons with PTL), Outcome 8 Tocolysis
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 1 TVU CL knowledge versus no knowledge (singletons with PTL)
Outcome: 8 Tocolysis
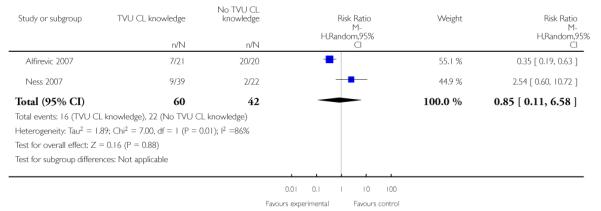 |
Analysis 1.9. Comparison 1 TVU CL knowledge versus no knowledge (singletons with PTL), Outcome 9 Steroids for fetal lung maturity
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 1 TVU CL knowledge versus no knowledge (singletons with PTL)
Outcome: 9 Steroids for fetal lung maturity
 |
Analysis 2.1. Comparison 2 TVU CL Knowledge versus no knowledge (singletons with PPROM), Outcome 1 Birth weight < 2500 grams
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 2 TVU CL Knowledge versus no knowledge (singletons with PPROM)
Outcome: 1 Birth weight < 2500 grams
 |
Analysis 2.2. Comparison 2 TVU CL Knowledge versus no knowledge (singletons with PPROM), Outcome 2 Chorioamnionitis
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 2 TVU CL Knowledge versus no knowledge (singletons with PPROM)
Outcome: 2 Chorioamnionitis
 |
Analysis 2.3. Comparison 2 TVU CL Knowledge versus no knowledge (singletons with PPROM), Outcome 3 Endometritis
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 2 TVU CL Knowledge versus no knowledge (singletons with PPROM)
Outcome: 3 Endometritis
 |
Analysis 2.4. Comparison 2 TVU CL Knowledge versus no knowledge (singletons with PPROM), Outcome 4 Neonatal infection
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 2 TVU CL Knowledge versus no knowledge (singletons with PPROM)
Outcome: 4 Neonatal infection
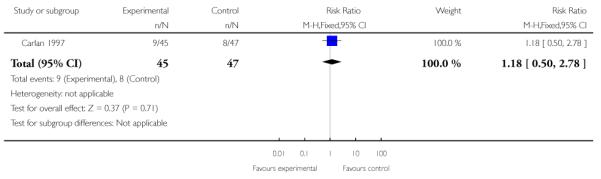 |
Analysis 3.1. Comparison 3 TVU CL knowledge versus no knowledge (twins), Outcome 1 Preterm birth < 36 weeks
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 3 TVU CL knowledge versus no knowledge (twins)
Outcome: 1 Preterm birth < 36 weeks
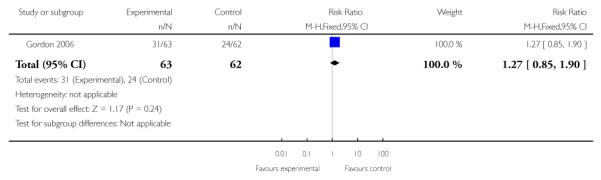 |
Analysis 3.2. Comparison 3 TVU CL knowledge versus no knowledge (twins), Outcome 2 Preterm birth < 34 weeks
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 3 TVU CL knowledge versus no knowledge (twins)
Outcome: 2 Preterm birth < 34 weeks
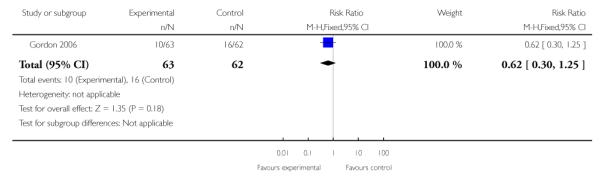 |
Analysis 3.3. Comparison 3 TVU CL knowledge versus no knowledge (twins), Outcome 3 Preterm birth < 32 weeks
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 3 TVU CL knowledge versus no knowledge (twins)
Outcome: 3 Preterm birth < 32 weeks
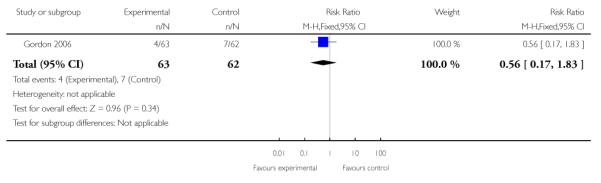 |
Analysis 3.4. Comparison 3 TVU CL knowledge versus no knowledge (twins), Outcome 4 Preterm birth < 30 weeks
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 3 TVU CL knowledge versus no knowledge (twins)
Outcome: 4 Preterm birth < 30 weeks
 |
Analysis 3.5. Comparison 3 TVU CL knowledge versus no knowledge (twins), Outcome 5 Gestational age at delivery
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 3 TVU CL knowledge versus no knowledge (twins)
Outcome: 5 Gestational age at delivery
 |
Analysis 3.6. Comparison 3 TVU CL knowledge versus no knowledge (twins), Outcome 6 Birth weight
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 3 TVU CL knowledge versus no knowledge (twins)
Outcome: 6 Birth weight
 |
Analysis 3.7. Comparison 3 TVU CL knowledge versus no knowledge (twins), Outcome 7 Maternal hospitalization (PTL)
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 3 TVU CL knowledge versus no knowledge (twins)
Outcome: 7 Maternal hospitalization (PTL)
 |
Analysis 3.8. Comparison 3 TVU CL knowledge versus no knowledge (twins), Outcome 8 Tocolysis
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 3 TVU CL knowledge versus no knowledge (twins)
Outcome: 8 Tocolysis
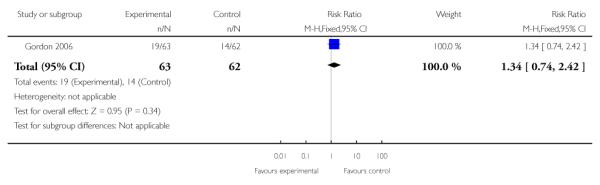 |
Analysis 3.9. Comparison 3 TVU CL knowledge versus no knowledge (twins), Outcome 9 Steroids for fetal lung maturity
Review: Cervical assessment by ultrasound for preventing preterm delivery
Comparison: 3 TVU CL knowledge versus no knowledge (twins)
Outcome: 9 Steroids for fetal lung maturity
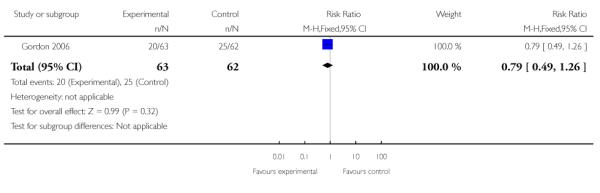 |
Appendix 1. MEDLINE search strategy
exp Ultrasonography, Prenatal/
(cervix or cervical or transvaginal$ or trans-vaginal$).mp.
exp Obstetric Labor, Premature/
1 and 2 and 3
HISTORY
Protocol first published: Issue 3, 2008
Review first published: Issue 3, 2009
Footnotes
WHAT’S NEW Last assessed as up-to-date: 21 February 2009.
| Date | Event | Description |
|---|---|---|
| 27 January 2012 | Amended | Search updated. Two trial reports added to Studies awaiting classification (Burwick 2011; Simcox 2009). |
DECLARATIONS OF INTEREST One of the authors of this Cochrane Review (V Berghella) is a co-author on one of the included trials (Ness 2007)
References to studies included in this review
- Alfirevic 2007 {published data only} .Alfirevic Z, Allen-Coward H, Molina F, Vinuesa CP, Nicolaides K. Targeted therapy for threatened preterm labor based on sonographic measurement of the cervical length: a randomized controlled trial. Ultrasound in Obstetrics & Gynecology. 2007;29(1):47–50. doi: 10.1002/uog.3908. [DOI] [PubMed] [Google Scholar]
- Carlan 1997 {published data only} .Carlan SJ, Richmond LB, O’Brien WF. Randomized trial of endovaginal ultrasound in preterm premature rupture of membranes. Obstetrics & Gynecology. 1997;89:458–61. doi: 10.1016/S0029-7844(97)00002-1. [DOI] [PubMed] [Google Scholar]
- Gordon 2006 {published data only} .Gordon M, Robbins A, McKenna D, Howard B, Barth W. Cervical length assessment as a resource to identify twins at risk for preterm delivery (clarity study) American Journal of Obstetrics and Gynecology. 2006;195(6 Suppl 1):S55. [Google Scholar]
- Ness 2007 {published data only} .Ness A, Visintine J, Ricci E, Berghella V. Does knowledge of cervical length and fetal fibronectin affect management of women with threatened preterm labor? A randomized trial. American Journal of Obstetrics and Gynecology. 2007;197(4):426.e1–426.e7. doi: 10.1016/j.ajog.2007.07.017. [DOI] [PubMed] [Google Scholar]; Ness A, Visintine J, Ricci E, Boyle K, Berghella V. Use of fetal fibronectin and transvaginal ultrasound cervical length to triage women with suspected preterm labor: a randomized trial. American Journal of Obstetrics and Gynecology. 2006;195(6 Suppl 1):S67. doi: 10.1016/j.ajog.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Palacio 2006 {published data only} .Palacio M, Sanchez M, Cobo T, Figueras F, Coll O, Cararach V. Uterine cervical length to reduce length of stay in patients admitted because of preterm labor. Prospective and randomized trial. Preliminary results. Ultrasound in Obstetrics & Gynecology. 2003;22(Suppl 1):47. [Google Scholar]; Palacio M, Sanchez M, Cobo T, Figueras F, Coll O, Cararach V, et al. Cervical length measurement to reduce length of stay in patients admitted because of preterm labor. Prospective and randomized trial. Final results. Ultrasound in Obstetrics & Gynecology. 2006;28(4):485. [Google Scholar]
References to studies excluded from this review
- Beigi 2005 {published data only} .Beigi A, Zarrinkoub F. Elective versus ultrasound-indicated cervical cerclage in women at risk for cervical incompetence. Medical Journal of the Islamic Republic of Iran. 2005;19(2):103–7. [Google Scholar]
- Kassanos 2001 {published data only} .Kassanos D, Salamalekis E, Vitoratos N, Panayotopoulos N, Loghis C, Creatsas C. The value of transvaginal ultrasonography in diagnosis and management of cervical incompetence. Clinical & Experimental Obstetrics & Gynecology. 2001;28:266–8. [PubMed] [Google Scholar]
- Lorenz 1990 {published data only} .Lorenz RP, Comstock CH, Bottoms SF, Marx SR. Randomized prospective trial comparing ultrasonography and pelvic examination for preterm labor surveillance. American Journal of Obstetrics and Gynecology. 1990;162:1603–10. doi: 10.1016/0002-9378(90)90926-x. [DOI] [PubMed] [Google Scholar]
- Matijevic 2006 {published data only} .Matijevic R, Grgic O, Vasilj O. Is sonographic assessment of cervical length better than digital examination in screening for preterm delivery in a low-risk population? Acta Obstetricia et Gynecologica Scandinavica. 2006;85(11):1342–7. doi: 10.1080/00016340600935722. [DOI] [PubMed] [Google Scholar]
- Owen 1999 {published data only} .Owen J, Neely C, Northen A. Transperineal versus endovaginal ultrasonographic examination of the cervix in the midtrimester: a blinded comparison. American Journal of Obstetrics and Gynecology. 1999;181(4):780–3. doi: 10.1016/s0002-9378(99)70300-5. [DOI] [PubMed] [Google Scholar]
- Shennan 2007 {published data only} .Shennan A, Maternal and Fetal Research Unit (MFRU) [accessed 25 February 2004];CIRCLE study: cerclage in relation to cervical length. Maternal and Fetal Research Unit. www.mfru.org.uk/CIRCLE.htm; Simcox R, Bennett F, Teoh TG, Shennan AH. A randomised controlled trial of cervical scanning vs history to determine cerclage in high risk women (circle trial) [abstract] Journal of Obstetrics and Gynaecology. 2007;27(Suppl 1):S18. doi: 10.1016/j.ajog.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Van Dijken 1991 {published data only} .Van Dijken DKE, Wolf H, Boer K, Treffers PE. Preliminary results of a randomized trial comparing abdominal ultrasonography and digital examination. Proceedings of 13th World Congress of Gynaecology and Obstetrics (FIGO); Singapore. 1991 Sept 15-20.1991. p. 289. [Google Scholar]
References to studies awaiting assessment
- Burwick 2011 {published data only} .Burwick RM, Zork NM, Lee GT, Ross MG, Kjos SL. Cervilenz assessment of cervical length compared to fetal fibronectin in the prediction of preterm delivery in women with threatened preterm labor. Journal of Maternal-Fetal & Neonatal Medicine. 2011;24(1):127–31. doi: 10.3109/14767058.2010.529201. [DOI] [PubMed] [Google Scholar]
- Simcox 2009 {published data only} .Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial) American Journal of Obstetrics and Gynecology. 2009;200(6):623.e1–6. doi: 10.1016/j.ajog.2009.03.010. [DOI] [PubMed] [Google Scholar]
Additional references
- Berghella 2003 .Berghella V, Bega G, Tolosa JE, Berghella M. Ultrasound assessment of the cervix. Clinical Obstetrics and Gynecology. 2003;46:947–62. doi: 10.1097/00003081-200312000-00026. [DOI] [PubMed] [Google Scholar]
- Berghella 2007 .Berghella V, Roman A, Daskalakis C, Ness A, Baxter JK. Gestational age at cervical length measurement and incidence of preterm birth. Obstetrics & Gynecology. 2007;110:311–7. doi: 10.1097/01.AOG.0000270112.05025.1d. [DOI] [PubMed] [Google Scholar]
- Deeks 2001 .Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. BMJ Books; London: 2001. [Google Scholar]
- Gates 2005 .Gates S. Methodological Guidelines. the Editorial Team. Pregnancy and Childbirth Group. About The Cochrane Collaboration (Collaborative Review Groups (CRGs)) 2005;(Issue 2) [Google Scholar]
- Grimes-Dennis 2007 .Grimes-Dennis J, Berghella V. Cervical length and prediction of preterm birth. Current Opinion in Obstetrics and Gynecology. 2007;19:191–5. doi: 10.1097/GCO.0b013e3280895dd3. [DOI] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. The Cochrane Collaboration. [updated February 2008]. 2008. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. Available from www.cochrane-handbook.org. [Google Scholar]
- National Vital Statistics Report 2006 .Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, et al. Births: final data for 2006. National Vital Statistics Report. 2009;57(7):1–102. [PubMed] [Google Scholar]
- RevMan 2008 .The Cochrane Collaboration . Review Manager (RevMan). 5.0. The Cochrane Collaboration; Copenhagen, The Nordic Cochrane Centre: 2008. [Google Scholar]; * Indicates the major publication for the study


