Abstract
Background
Skeletal metastases are the most common malignant tumor in bone. Certain types of cancer (e.g., of the prostate or breast) are particularly likely to give rise to skeletal metastases, with prevalences of up to 70%. The diagnosis of skeletal metastases has a major impact on the overall treatment strategy and is an important determinant of the course of illness and the quality of life. The goal of diagnostic imaging is to detect skeletal metastases early, whenever they are suspected on the basis of clinical or laboratory findings or in patients who are at high risk. Other important issues include assessment of the risk of fracture and the response to treatment.
Methods
This review is based on selected pertinent articles published up to December 2013.
Results
Projectional radiography (plain films) is still useful for the immediate investigation of symptomatic bone pain and for the assessment of stability. Skeletal scintigraphy, the classic screening test for patients with cancer who do not have bone pain (specificity 81%, sensitivity 86%), has now been supplemented—in some cases, replaced—by other techniques. CT, including low-dose CT, is used to detect changes in bone structure due to metastases of some types of primary tumor (specificity 95%, sensitivity 73%); whole-body MRI, to detect metastases in the bone marrow and extraosseous soft tissues, e.g., metastases compressing the spinal cord (specificity 95%, sensitivity 91%); PET-CT, to detect metabolically active tumors (specificity 97%, sensitivity 90%).
Conclusion
Different imaging modalities are often used in combination to detect bone metastases optimally. Owing to advances in modern tomographic imaging, the current trend is toward whole-body imaging in a single session. The choice of method is based on the clinical situation and the type of primary tumor. Further research should address the impact of these costly and labor-intensive imaging methods on treatment strategies and on the course of illness.
As the population ages, cancer is becoming more common (1). Metastases are most commonly found in the lungs, the liver, and the bones (2). In adults, metastases are the most common type of malignant tumor in bone. Certain types of cancer (e.g., of the prostate or breast) are particularly likely to give rise to skeletal metastases, with prevalences of up to 70% (3– 5). Any type of cancer that metastasizes via the bloodstream can infiltrate the bone marrow. Different types of bone metastases vary in their metabolic activity and in the reaction they induce in the surrounding bone; it is therefore important to choose the suitable imaging method(s) for each (Tables 1 and 2). Two typical diagnostic scenarios often arise in routine clinical practice:
Table 1. The most common types of cancer. the probability of bone metastases for each. and suitable variables to measure in imaging studies (31).
| Primary tumor | Probability of bone metastases |
Variables for imaging studies | ||||
|---|---|---|---|---|---|---|
| Bone morphology | Bone metabolism |
Marrow involvement |
Diffusion | Glucose metabolism |
||
| Men | ||||||
| Prostate | very high (>50%) | osteoplastic | + | + | # | |
| Lung | high (30–50%) | SCLC: osteoplastic _NSCLC: osteolytic | + | + | + | |
| Bowel | moderate (10–30%) | osteolytic | + | + | + | |
| Bladder | high (30–50%) | variable | + | + | (+) | |
| Women | ||||||
| Breast | very high (>50%) | mixed | + | + | (+) | + |
| Bowel | moderate (10–30%) | osteolytic | + | + | + | |
| Lung | high (30–50%) | SCLC: osteoplastic _NSCLC: osteolytic | + | + | + | |
| Uterus/cervix/ovary | low | osteoplastic | + | |||
| Melanoma | moderate (10–30%) | osteolytic | + | + | + | |
+. suitable variables; (+). variables of limited suitability; #. special case: measurement of choline metabolism / PSMA-PET to detect prostate-specific membrane antigen by PET-CT; SCLC. small-cell lung carcinoma; NSCLC. non-small-cell lung carcinoma.
Table 2. Imaging studies based on variables that indicate bone metastases (blue box = suitable). Note the high potential of the hybrid techniques. PET-CT and PET-MRI.
| Bone morphology | Bone metabolism | Marrow involvement | Diffusion | Glucose metabolism | |
|---|---|---|---|---|---|
| Projectional radiography | |||||
| CT | |||||
| SPECT(-CT) | |||||
| MRI | |||||
| PET-CT | |||||
| PET-MRI |
CT, computerized tomography; SPECT(-CT), single-photon-emission computerized tomography (combined with CT); MRI, magnetic resonance imaging;
PET-CT, positron-emission tomography combined with CT (hybrid technique); PET-MRI, positron-emission tomography combined with MRI (hybrid technique).
A patient not yet known to have cancer presents with bone pain or a pathological fracture, and the subsequent evaluation reveals metastatic disease.
A cancer patient undergoes a staging evaluation to detect or rule out bone metastases, because metastatic disease would have a major effect on the patient’s quality of life, course of disease, and prognosis, and on decisions about further treatment.
In this review, which is based on a selective, up-to-date review of the literature (as of December 2013), we describe the role of various radiological and nuclear medical imaging studies in the detection and exclusion of bone metastases in patients with certain types of cancer. The available studies generally deal with only one tumor type per study and involve comparisons across imaging modalities, because a gold standard, e.g., documented histological findings, is generally unavailable.
Projectional radiography
Conventional projectional radiography still plays an important role in the diagnostic evaluation of bone metastases. Depending on their radiological appearance, bone metastases are classified as osteoplastic (bone-building), osteolytic (bone-destroying), or mixed. Osteolytic changes in some parts of the skeleton can be seen on plain films only if 50% or more of the bone substance has been destroyed (5, 6). Metastases measuring up to 1 cm in the spongiosa of a vertebral body or in the marrow of a long bone can be missed on plain x-ray; on the other hand, pathological changes in cortical bone are detectable by plain x-ray even if they are only a few millimeters wide (5, 7).
The diagnostic utility of plain films of the skull, spine, and pelvis is limited by superposition effects. In these areas, the sensitivity of plain films for bone metastases is only in the range of 44–50% (8).
Plain films are thus not suitable for use as a screening test. They were once considered the gold standard of staging for multiple myeloma (a major element in the differential diagnosis of osteolytic bone lesions), according to the so-called Paris scheme, but have been largely abandoned for this purpose in favor of low-dose CT, which is more sensitive and three times as fast (9– 11).
On the other hand, classic plain films in two planes still play an important role in the evaluation of bone pain. In particular, they can immediately reveal osteolytic lesions where a pathological fracture is in danger of arising or is already present (Figures 1 and 2) (5, 12). Such fractures arise in ca. 9% of patients with bone metastases (12). Plain films can also be helpful for the further evaluation of suspect scintigraphic findings (13), although normal plain films do not rule out a metastasis in such cases.
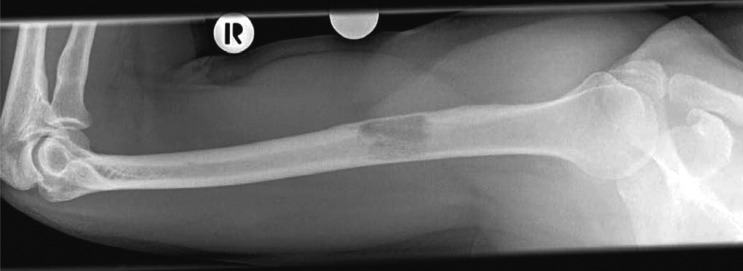
Figure 1.
Projectional radiography. The patient, a 58-year-old man, complained of focal pain in the right arm three months after the resection of a renal-cell carcinoma. The plain film reveals an osteolytic metastasis in the right humerus; the measuring sphere marks the site of pain. The image enables assessment of the risk of fracture.
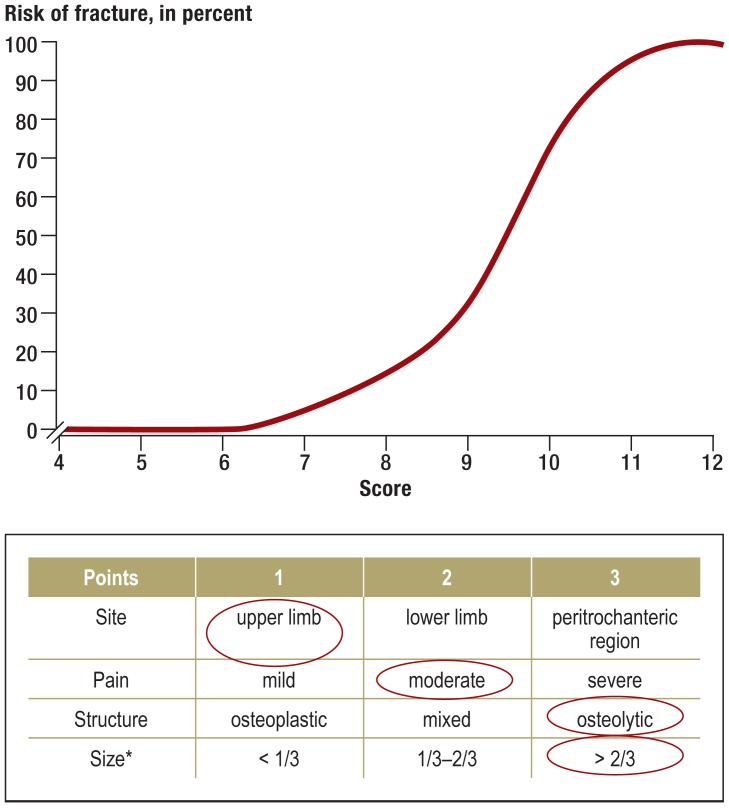
Figure 2.
Assessment of fracture risk. The Mirels score (4–12 points) is used to assess the risk of fracture in long-bone metastases (32). The metastasis shown in Figure 1 has a Mirels score of 9; in the cohort of patients that Mirels studied, the corresponding fracture risk was 33%. This robust scoring system provides a sound basis for judging whether a bone metastasis should be prophylactically treated to prevent fracture (33).
*This refers to the cortical circumference.
Computerized tomography (CT)
Multislice spiral CT enables imaging of every part of the skeleton without superposition effects and is thus more suitable than plain films for the detection of metastases in anatomically complex areas, such as the thoracic spine. CT is highly sensitive for osteolytic and osteoplastic bone lesions involving cortical bone (Figure 3), but less so for tumors restricted to the marrow space, which must be very extensive to be detectable. As a result, CT is of limited use as a screening test for bone metastases, despite its high specificity. Yang et al. compared the four main screening modalities in a large-scale meta-analysis and found that CT has a sensitivity of 73% and a pooled specificity (per patient) of 95% (14). Other authors made similar findings specifically with regard to metastatic breast cancer (15).
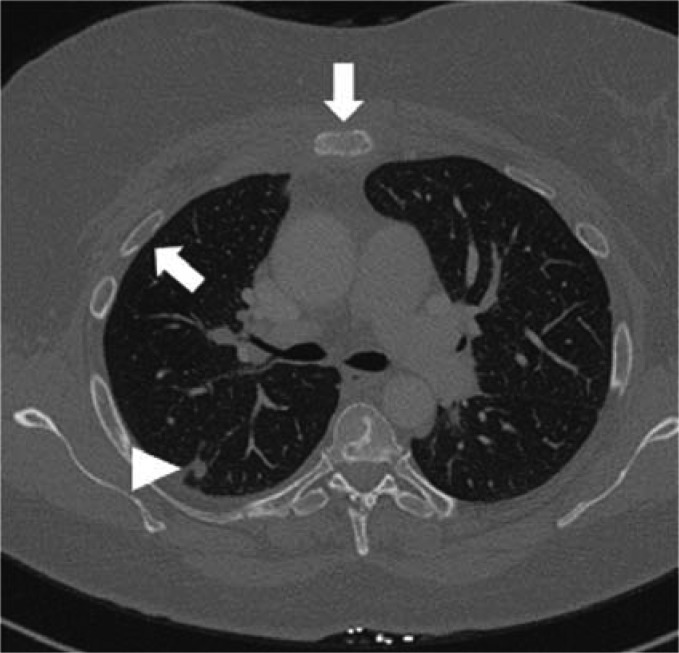
Figure 3.
CT. The image reveals metastases in the ribs and sternum (arrows), partly osteolytic and partly osteoplastic, and a suspect nodule in the right lung (arrowhead) in a woman with breast cancer.
For most types of cancer, CT is the modality of choice for staging in the chest and abdomen and for serial follow-up imaging. CT scans for these purposes encompass a large part of the axial skeleton and can thus detect, not just soft-tissue lesions, but osteoplastic or osteolytic bone metastases as well. CT is also used to assess the stability of bony structures that harbor metastases, particularly in areas of complex anatomy (5), and to obtain better structural definition of abnormal findings seen on scintigraphy or MRI. CT is the imaging method of choice in such situations because it enables the visualization of both trabecular and cortical bone with high resolution. Thus, for example, CT can be used to assess the risk of fracture arising from an already known spinal metastasis.
Skeletal scintigraphy, SPECT, and SPECT-CT
Skeletal scintigraphy (“bone scan”) with labeled phosphonates enables visualization of local bone metabolism (turnover), which is activated in an early phase of some types of cancer. It thus detects metastases best when they are associated with marked reactive hypermetabolism of bone (e.g., metastases of prostate cancer, breast cancer, and neuroendocrine tumors) or generate bone matrix themselves (osteosarcoma). In contrast, scintigraphy is relatively insensitive for tumors that cause areactive osteolysis or isolated bone-marrow infiltration (renal-cell carcinoma, lymphoma). Moreover, bone matrix regeneration after the successful treatment of a bone metastasis can induce metabolic activation, which is sometimes misinterpreted as progressive disease—the so-called flare phenomenon. Skeletal scintigraphy is obligatory before radionuclide treatment with phosphonates coupled to alpha- or beta-emitting isotopes, e.g., 223Ra (radium-223) treatment for prostate cancer.
Metastases in the axial skeleton that are not intensely hypermetabolic may escape detection in the planar images of conventional scintigraphy. The sensitivity and specificity of scintigraphy are both markedly increased with the use of contemporary types of SPECT and SPECT-CT apparatus (Figure 4) (16, 17). In a study with a mixed group of patients, the addition of SPECT to scintigraphy raised the negative predictive value of normal scintigraphic findings to 98% (18). The specificity is increased if the SPECT is immediately compared with, or acquired simultaneously with, a CT scan. The visualization by CT of degenerative processes in bone or (for example) osteoporotic vertebral body fractures enables better pathophysiologic assessment of any hypermetabolic areas that may appear on scintigraphy. When SPECT-CT is used, the sensitivity and specificity for metastases of certain types of cancer, e.g., prostate cancer, rises above 90% (19).
Figure 4.
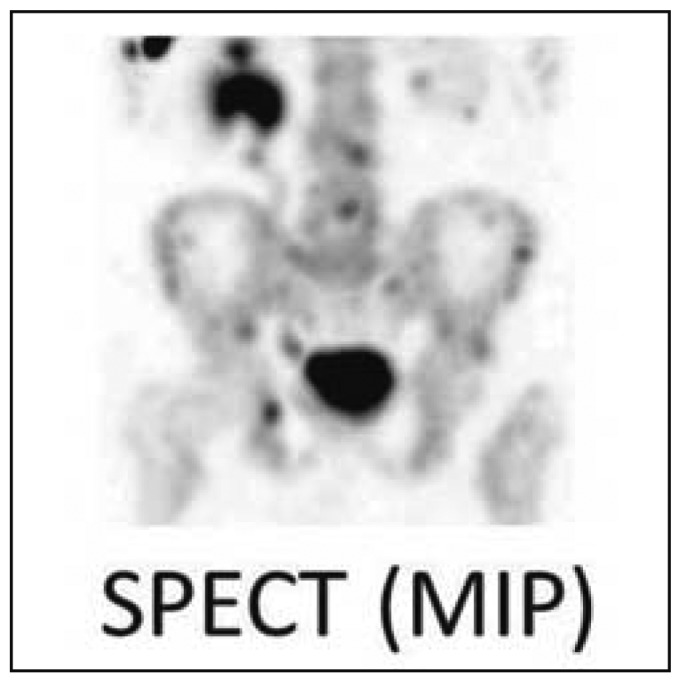
Skeletal scintigraphy and SPECT. In this patient with prostate cancer, 99mTc-MDP scintigraphy reveals bone metastases. The SPECT image is free of superposition effects and thus enables the precise localization of many metastases.
Magnetic resonance imaging
Magnetic resonance imaging (MRI), with its high soft-tissue contrast and high spatial resolution, reveals metastases in the bone marrow spaces early on, before any changes in internal bone structure arise that could be detected by CT. The use of T1-weighted and STIR sequences obviates the need for an intravenous contrast medium, so patients with poor renal function can also undergo MRI for this purpose. Whole-body MRI techniques for the detection of bone metastases are becoming widely available (Figure 5). A further advantage of MRI over CT for staging is that it does not involve any ionizing radiation. In the meta-analysis mentioned above, Yang et al. found that, on a per-patient basis, MRI is 91% sensitive and 95% specific (14). It is thus superior to both CT and planar skeletal scintigraphy and roughly as good as PET-CT. These findings have been confirmed in further studies, e.g., in one involving breast cancer (20). In another meta-analysis, MRI and PET-CT were both found to be more than 80% sensitive and more than 90% specific for the detection of bone metastases (21). A prospective, double-blind trial also showed MRI to be superior to planar skeletal scintigraphy for the detection of bone metastases of breast cancer (22). Further studies have shown that MRI is comparably useful for the detection of bone metastases of prostate cancer (23, 24). It should be noted, however, that these studies did not involve any comparison of MRI with SPECT or SPECT-CT, which represent the current state of the art.
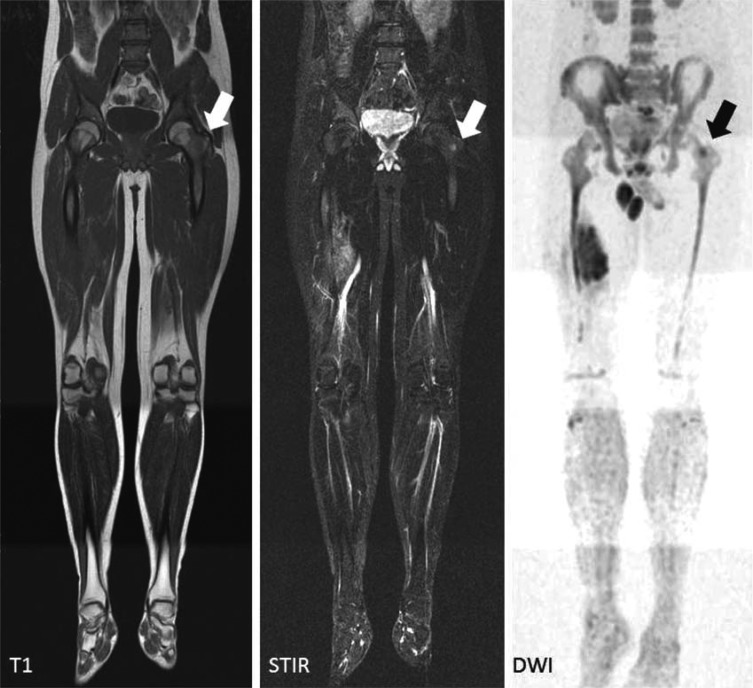
Figure 5.
Whole-body MRI. This 17-year-old boy has Ewing‘s sarcoma in the diaphysis of the right femur, with marked extension beyond the bone. Preoperative MRI clearly shows that the neighboring vessels are displaced, but not encased, by the tumor. The whole-body MRI reveals a PET-negative metastasis in the left trochanteric mass (hypointense on the T1-weighted image, hyperintense on the STIR and diffusion-weighted images [arrows]). This important finding for surgical planning was histologically confirmed.
Hybrid techniques (SPECT-CT, PET-CT, PET-MRI)
Unlike skeletal scintigraphy, which depicts bone metabolism, PET-CT with specific radiopharmaceuticals depicts tumor metabolism all over the body, including in bone. It is a type of molecular imaging.
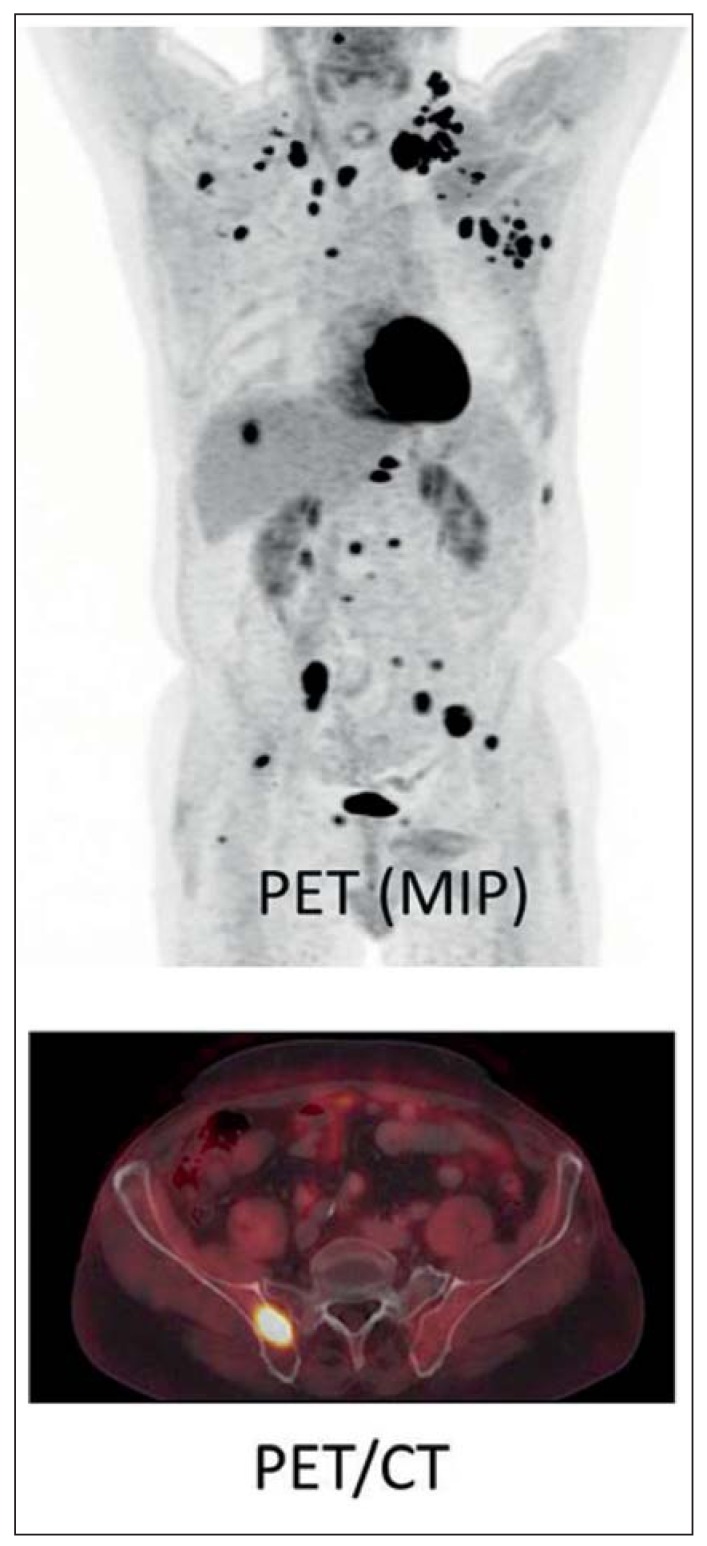
The visualization of glucose metabolism by positron-emission tomography with 18F-fluorodeoxyglucose, coupled with a simultaneously obtained CT (18F-FDG-PET-CT), is now a standard diagnostic technique in oncology. In patients with lung cancer or malignant melanoma, for example, PET-CT with FDG has replaced other techniques for the detection of bone metastases (Figure 6); as these are highly metabolically active tumor types, metastases can be detected with high sensitivity and specificity. Because of the high tumor contrast, metastases in other organ systems or in the soft tissues can be detected as well. 18F-FDG-PET-CT can thus be used for complete staging of these tumor types, among others (25).
Figure 6.
PET-CT. This 66-year-old man, in clinical remission after treatment for lung cancer, has numerous metastases to the lymph nodes, the liver, and the skeleton. The bone metastases are barely visible by CT; 18F-FDG-PET-CT demonstrates them well and confirms skeletal stability.
All imaging methods have strengths and weaknesses, depending on their underlying principles. Hybrid apparatus can be used to combine the strengths of the individual component techniques while compensating for their weaknesses (Table 2). SPECT-CT and PET-CT are two well-established examples in clinical practice (2, 26– 28). PET-MRI is the most recent development in the area of hybrid imagine techniques. Even without a single device for both modalities, the retrospective fusion of FDG-PET images with MRI is a promising method for tumor detection, as has been demonstrated for gynecological tumors in the pelvic area (29). A comparison of PET-MRI with PET-CT in cancer patients showed that 18% of patients had findings on PET-MRI that were clinically and therapeutically relevant but were missed by PET-CT (30). Further systematic study is needed to determine the role that PET-MRI might play in the detection of bone metastases in patients with various types of primary tumor.
Concluding assessment and discussion
The detection and evaluation of bone metastases is a matter of high clinical importance. Bone metastases are revealed by imaging studies either by anatomical visualization or by the detection of metabolic turnover in the metastasis itself or in the surrounding bone.
An analysis of the literature on bone metastases indicates a number of current trends:
The established imaging techniques—projectional radiography, skeletal scintigraphy, CT, MRI, and PET—have undergone further development in recent years, with a resulting improvement in their diagnostic yield.
As a complement to these, we now also have the hybrid techniques SPECT-CT, PET-CT, and, most recently, PET-MRT. The simultaneous performance of two techniques improves the overall diagnostic yield synergistically while shortening the duration of testing. Most comparative studies of imaging methods for detecting metastases use surrogate parameters as a reference standard, because universal biopsies for histology (the theoretical gold standard) would be neither practicable nor ethical. The results of many of these studies are, therefore, difficult to generalize.
The quality of the findings of imaging depends not only on the apparatus used, but also on the user’s experience in the diagnosis of musculoskeletal lesions.
For each patient, the optimal diagnostic technique should be chosen individually, by a joint decision of the imaging specialists and the treating physicians, on the basis of the tumor entity, the tumor biology, and the patient’s general condition.
Table 3. The sensitivity and specificity of various imaging techniques for the detection of bone metastases (according to Ref. 14).
| MRI | PET(-CT) | CT | Scintigraphy | |
|---|---|---|---|---|
| Sensitivity (%) | 91 | 90 | 73 | 86 |
| Specificity (%) | 95 | 97 | 95 | 81 |
CT, computerized tomography; SPECT(-CT), single-photon-emission computerized tomography (combined with CT); MRI, magnetic resonance imaging; PET-CT, positron-emission tomography combined with CT
(hybrid technique); PET-MRI, positron-emission tomography combined with MRI (hybrid technique).
Skey Messages.
For patients with certain types of primary tumor who are asymptomatic but have a moderate to high risk of metastasis, skeletal scintigraphy detects metastases with high sensitivity, particularly if SPECT or SPECT-CT is performed in addition.
Projectional radiography is the diagnostic method of choice to evaluate symptomatic bone lesions, to assess the risk of fracture, to investigate suspect scintigaphic findings, and to monitor the effects of treatment. If scintigraphy is positive but plain films are negative, a CT or MRI should be obtained.
CT is helpful if the findings of other imaging techniques are unclear (e.g., pathological vs. non-pathological rib fracture), and it is an important means of assessing stability in bone lesions. CT combined with SPECT enhances the specificity of scintigraphy by revealing degenerative changes.
Whole-body MRI and PET-CT are now the most sensitive and specific methods for the detection of skeletal metastases. Whole-body MRI is becoming more widely available; it enables the most sensitive detection of bone-marrow metastases and extraosseous tumor extension. For certain types of primary tumor, PET-CT often suffices as the sole imaging method for staging.
Hybrid techniques like SPECT-CT, PET-CT, and PET-MRI combine the strengths of their individual components while canceling out their weaknesses.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Schober, Prof. Heindel, Prof. Schäfers, Prof. Weckesser, and Dr. Vieth are authors of the book “PET-CT,” for which they have received honoraria.
Dr. Gübitz states that he has no conflict of interest.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Vassiliou V, Andreopoulos D, Frangos S, Tselis N, Giannopoulou E, Lutz S. Bone metastases: assessment of therapeutic response through radiological and nuclear medicine imaging modalities. Clin Oncol (R Coll Radiol) 2011;23:632–645. doi: 10.1016/j.clon.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Plunkett TA, Rubens RD. The biology and management of bone metastases. Crit Rev Oncol Hematol. 1999;31:89–96. doi: 10.1016/s1040-8428(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 4.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 5.Layer G. Skelettmetastasen In Muskuloskelettales System 2. In: Stäbler A, editor. Springer. Berlin: Heidelberg; 2005. [Google Scholar]
- 6.Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53–64. [PubMed] [Google Scholar]
- 7.Freyschmidt J. Springer. Berlin: Heidelberg; 2008. Primäre und sekundäre Knochengeschwulste Skeletterkrankungen. [Google Scholar]
- 8.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 9.Bannas P, Kroger N, Adam G, Derlin T. Moderene Bildgebung beim Multiplen Myelom [Modern imaging techniques in patients with multiple myeloma] Rofo. 2013;185:26–33. doi: 10.1055/s-0032-1325405. [DOI] [PubMed] [Google Scholar]
- 10.Horger M, Claussen CD, Bross-Bach U, et al. Whole-body low-dose multidetector row-CT in the diagnosis of multiple myeloma: an alternative to conventional radiography. Eur J Radiol. 2005;54:289–297. doi: 10.1016/j.ejrad.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Kropil P, Fenk R, Fritz LB, et al. Comparison of whole-body 64-slice multidetector computed tomography and conventional radiography in staging of multiple myeloma. Eur Radiol. 2008;18:51–58. doi: 10.1007/s00330-007-0738-3. [DOI] [PubMed] [Google Scholar]
- 12.Higinbotham NL, Marcove RC. The management of pathological fractures. J Trauma. 1965;5:792–798. doi: 10.1097/00005373-196511000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Costelloe CM, Rohren EM, Madewell JE, et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606–614. doi: 10.1016/S1470-2045(09)70088-9. [DOI] [PubMed] [Google Scholar]
- 14.Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: a meta-analysis comparing (1)(8)FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21:2604–2617. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- 15.Piccardo A, Altrinetti V, Bacigalupo L, et al. Detection of metastatic bone lesions in breast cancer patients: fused (18)F-Fluoride-PET/MDCT has higher accuracy than MDCT Preliminary experience. Eur J Radiol. 2012;81:2632–2638. doi: 10.1016/j.ejrad.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Haim S, Israel O. Breast cancer: role of SPECT and PET in imaging bone metastases. Semin Nucl Med. 2009;39:408–415. doi: 10.1053/j.semnuclmed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Beheshti M, Langsteger W, Fogelman I. Prostate cancer: role of SPECT and PET in imaging bone metastases. Semin Nucl Med. 2009;39:396–407. doi: 10.1053/j.semnuclmed.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Chua S, Gnanasegaran G, Cook GJ. Miscellaneous cancers (lung, thyroid, renal cancer, myeloma, and neuroendocrine tumors): role of SPECT and PET in imaging bone metastases. Semin Nucl Med. 2009;39:416–430. doi: 10.1053/j.semnuclmed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297. [PubMed] [Google Scholar]
- 20.Wu LM, Gu HY, Zheng J, et al. Diagnostic value of whole-body magnetic resonance imaging for bone metastases: a systematic review and meta-analysis. J Magn Reson Imaging. 2011;34:128–135. doi: 10.1002/jmri.22608. [DOI] [PubMed] [Google Scholar]
- 21.Duo J, Han X, Zhang L, Wang G, Ma Y, Yang Y. Comparison of FDG PET/CT and gadolinium-enhanced MRI for the detection of bone metastases in patients with cancer: a meta-analysis. Clin Nucl Med. 2013;38:343–348. doi: 10.1097/RLU.0b013e3182817af3. [DOI] [PubMed] [Google Scholar]
- 22.Ohlmann-Knafo S, Kirschbaum M, Fenzl G, Pickuth D. Diagnostischer Stellenwert der Ganzkörper-MRT und der Skelettszintigraphie in der ossären Metastasendetektion bei Mammakarzinompatienten Eine prospektive Doppelblindstudie an zwei Klinikzentren. [Diagnostic value of whole-body MRI and bone scintigraphy in the detection of osseous metastases in patients with breast cancer-A prospective double-blinded study at two hospital centers] Rofo. 2009;181:255–263. doi: 10.1055/s-0028-1109104. [DOI] [PubMed] [Google Scholar]
- 23.Ketelsen D, Rothke M, Aschoff P, et al. Nachweis ossärer Metastasen des Prostatakarzinoms Vergleich der Leistungsfähigkeit der Ganzkörper-MR und der Sklettszintigraphie.[Detection of bone metastasis of prostate cancer - comparison of whole-body MRI and bone scintigraphy] Rofo. 2008;180:746–752. doi: 10.1055/s-2008-1027479. [DOI] [PubMed] [Google Scholar]
- 24.Lecouvet FE, El Mouedden J, Collette L, et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol. 2012;62:68–75. doi: 10.1016/j.eururo.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Beheshti M, Vali R, Waldenberger P, et al. The use of F-18 choline PET in the assessment of bone metastases in prostate cancer: correlation with morphological changes on CT. Mol Imaging Biol. 2010;12:98–107. doi: 10.1007/s11307-009-0239-7. [DOI] [PubMed] [Google Scholar]
- 26.Grankvist J, Fisker R, Iyer V, et al. MRI and PET/CT of patients with bone metastases from breast carcinoma. Eur J Radiol. 2012;81:e13–e18. doi: 10.1016/j.ejrad.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 27.Niikura N, Costelloe CM, Madewell JE, et al. FDG-PET/CT compared with conventional imaging in the detection of distant metastases of primary breast cancer. Oncologist. 2011;16:1111–1119. doi: 10.1634/theoncologist.2011-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daldrup-Link HE, Franzius C, Link TM, et al. Whole-body MR imaging for detection of bone metastases in children and young adults: Comparison with skeletal scintigraphy and FDG PET. AJR. 2001 177:229–236. doi: 10.2214/ajr.177.1.1770229. [DOI] [PubMed] [Google Scholar]
- 29.Kitajima K, Suenaga Y, Ueno Y, et al. Value of fusion of PET and MRI in the detection of intra-pelvic recurrence of gynecological tumor: comparison with F-FDG contrast-enhanced PET/CT and pelvic MRI. Ann Nucl Med. 2014;28:25–32. doi: 10.1007/s12149-013-0777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catalano OA, Rosen BR, Sahani DV, et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: Initial experience in 134 patients—a hypothesis-generating exploratory study. Radiology. 2013;269:857–869. doi: 10.1148/radiol.13131306. [DOI] [PubMed] [Google Scholar]
- 31.Epidemiologisches Krebsregister NRW report 2012 mit Datenbericht 2010. www.krebsregister.nrw.de/fileadmin/user_upload/dokumente/veroeffentlichungen/Report_2012/EKRNRW_Report_2012_Internet_20-02-2013.pdf. (last accessed on 30 September 2014)
- 32.Mirels H. Metastatic disease in long bones A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989:256–264. [PubMed] [Google Scholar]
- 33.Mac Niocaill RF, Quinlan JF, Stapleton RD, Hurson B, Dudeney S, O‘Toole GC. Inter- and intra-observer variability associated with the use of the Mirels’ scoring system for metastatic bone lesions. Int Orthop. 2011;35:83–86. doi: 10.1007/s00264-009-0941-8. [DOI] [PMC free article] [PubMed] [Google Scholar]