Background: The CpxR/CpxA system facilitates E. coli resistance to antimicrobial peptides. However, many CpxR-dependent mechanisms remain elusive.
Results: The CpxR/CpxA system activates mar transcription, subsequently facilitating E. coli resistance to protamine via tripartite multidrug transporters.
Conclusion: A CpxR/CpxA-dependent regulatory cascade controls MarRA for CAMP resistance in response to aromatic metabolites.
Significance: This is novel regulatory mechanism controlling bacterial resistance to CAMPs and antibiotics.
Keywords: Bacterial Signal Transduction, Multidrug Transporter, Transcription Factor, Transcription Promoter, Transcription Regulation, CpxR Box, The CpxR/CpxA Two-Component System, The mar Operon
Abstract
A genome-wide susceptibility assay was used to identify specific CpxR-dependent genes that facilitate Escherichia coli resistance to a model cationic antimicrobial peptide, protamine. A total of 115 strains from the Keio Collection, each of which contained a deletion at a demonstrated or predicted CpxR/CpxA-dependent locus, were tested for protamine susceptibility. One strain that exhibited high susceptibility carried a deletion of tolC, a gene that encodes the outer membrane component of multiple tripartite multidrug transporters. Concomitantly, two of these efflux systems, AcrAB/TolC and EmrAB/TolC, play major roles in protamine resistance. Activation of the CpxR/CpxA system stimulates mar transcription, suggesting a new regulatory circuit that enhances the multidrug resistance cascade. Tripartite multidrug efflux systems contribute to bacterial resistance to protamine differently from the Tat system. DNase I footprinting analysis demonstrated that the CpxR protein binds to a sequence located in the −35 and −10 regions of mar promoter. This sequence resembles the consensus CpxR binding site, however, on the opposite strand. aroK, a CpxR-dependent gene that encodes a shikimate kinase in the tryptophan biosynthesis pathway, was also found to facilitate protamine resistance. Specific aromatic metabolites from this pathway, such as indole, can stimulate expression of well studied CpxR-dependent genes degP and cpxP, which are not components of the tripartite multidrug transporters. Thus, we propose a novel mechanism for E. coli to modulate resistance to protamine and likely other cationic antimicrobial peptides in which the CpxR/CpxA system up-regulates mar transcription in response to specific aromatic metabolites, subsequently stimulating the multidrug resistance cascade.
Introduction
In enteric bacteria, the CpxR/CpxA two-component system regulates a vast number of genetic loci in response to periplasmic stress, including misfolded proteins, inner membrane disruptions, alkaline pH, starvation, and high osmolarity. The Escherichia coli CpxR/CpxA system responds to general periplasmic stress and has been used as a model two-component system for transcription regulation in bacteria (for review, see Ref. 1). This system has a global effect in a variety of signal transduction pathways and bacterial resistance as the vastness of its circuitries becomes evident as it continues to be implicated in virulence (1), biofilm formation (2), chemotaxis (3), and recently, resistance to antimicrobials (4–7). To date, there are >150 demonstrated or putative members of the CpxR/CpxA regulon. In a previous study we showed that the CpxR/CpxA system facilitates Salmonella and E. coli resistance to cationic antimicrobial peptides (i.e. CAMPs)3 including a model peptide protamine and α-helical CAMPs by activating transcription of two genetic loci, i.e. amiA and amiC, that encode two peptidoglycan amidases (4). On the other hand, activation of the CpxR/CpxA system in a ΔtatC mutant or ΔamiA ΔamiC double mutant could still facilitate resistance to protamine, suggesting that an additional gene(s) other than amiA and amiC is also required for the CpxR-dependent resistance.
The multidrug-resistant operon, marRAB, encodes a repressor, MarR, an activator, MarA, and an unknown function protein, MarB, which coordinately regulate the mar transcription to maintain intrinsic resistance to antimicrobial substances (8, 9). The natural signal for the marRAB operon has not been identified as it remains unknown whether plant-derived napthoquinones are natural inducers (10). It has been shown that the operon is activated by compounds such as salicylate, chloramphenicol, tetracycline, acetaminophen, sodium benzoate, 2,4-dinitrophenol, cinnamate, carbonyl cyanide m-chlorophenylhydrazone, menadione, and plumbagin (11, 12). In the presence of specific antibiotics (such as tetracycline and chloramphenicol; see Refs. 11 and 13), bile salts, and reactive oxygen species, MarA, in concert with global regulators Rob and SoxS, binds to the mar box sequence to activate transcription of the mar operon (14–17). In contrast, transcriptional repressor MarR, which binds at the operator region (marO), is only released once a compound such as salicylate binds, allowing for transcription (8, 9, 18). Interestingly, the marRAB operon can also be activated by sublethal concentrations of CAMPs via Rob (which is required for polymyxin B-induced up-regulation of micF) and facilitate resistance at least in part by overexpressing the AcrAB/TolC efflux pump (19, 20).
Although deletion of marA had no apparent phenotypic effect regarding susceptibility to CAMPs, constitutive expression of marA by either a point mutation in marR or a plasmid harboring a wild-type copy of marA raised bacterial resistance to multiple CAMPs, which represent various classes including α-helical and β-sheet CAMPs (such as LL-37 and HBD-1, respectively) as well as cyclic lipopeptide polymyxin B (19). However, mutation of acrAB or tolC eliminates this resistance in this constitutive marA strain (19). In addition, deletion of tolC in a marA constitutive expression strain causes a higher susceptibility to CAMPs than deletion of acrAB (19), suggesting that an additional TolC-dependent tripartite efflux system(s) other than AcrAB/TolC should contribute to this resistance. AcrAB and other resistance-nodulation-cell division superfamily efflux pumps probably use these export systems to mediate efflux of CAMPs because a loss of proton motive force in the presence of an uncoupler, i.e. carbonyl cyanide m-chlorophenyl hydrazone, causes an increased level of LL-37 susceptibility (19).
Here we investigated the role of those known CpxR-dependent loci in E. coli resistance to protamine. Our study revealed a new regulatory mechanism for bacterial resistance to this model CAMP. The CpxR/CpxA system can activate transcription of marRAB operon, thus facilitating multidrug-resistant regulator to enhance expression of TolC-dependent tripartite multidrug transporters. This E. coli resistance to protamine via tripartite multidrug efflux systems should represent a novel CpxR-mediated mechanism that is independent from the Tat system. Our results also suggest that the aroK gene is involved in the resistance by mediating synthesis of aromatic metabolites, which probably act as signal molecules to stimulate the CpxR/CpxA system and Mar regulators.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions
All bacterial strains used in this study are provided in Table 1. E. coli strains were obtained or derived from mutant strains in the Keio Collection (21) or derived from the wild-type strain BW25113 using the one-step gene deletion method (22). Bacteria were grown at 37 °C in Luria-Bertani broth (LB). When necessary, antibiotics were added at final concentrations of 50 μg/ml for ampicillin, 20 μg/ml for chloramphenicol, or 50 μg/ml for kanamycin. E. coli DH5α was used as the host for the preparation of plasmid DNA. E. coli BL21-Gold (Stratagene, Inc.) was used for protein expression. P1(vir) transduction was carried out for construction of E. coli multiple mutants from the Keio Collection.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| E. coli | ||
| Keio collection (parent strain, BW25113) | F−, DE(araD-araB)567 lacZ4787(del)::rrnB-3 LAM− rph-1 DE(rhaD-rhaB)568hsdR514 | (21) |
| YS Collection 7a | ΔemrB ΔacrB::KmR | This work |
| YS Collection 7b | ΔemrB ΔacrB ΔacrE::KmR | This work |
| YS Collection 7c | ΔemrB ΔacrB ΔemrY::KmR | This work |
| YS Collection 7d | ΔemrB ΔacrB ΔmacB::KmR | This work |
| YS14530 | ΔmarRA::CmR | This work |
| YS13007 | ΔtatC | (4) |
| YS15910 | ΔtolC ΔtatC | This work |
| YS15924 | ΔdegP ΔdsbA | This work |
| YS15925 | ΔtolC::KmR ΔdegP ΔdsbA | This work |
| YS15926 | ΔtatC::KmR ΔdegP ΔdsbA::KmR | This work |
| YS15537 | tolC-lac::KmR | This work |
| YS15538 | tolC-lac::KmR cpxR | This work |
| PND2000 | MC4100, kRS88[degP-lacZ] | (42) |
| SP1 | MC4100 ara +λplacMu53[cpxP-lacZ] | (43) |
| MG1301 | MC4100mgrB::λplacMu55 | (44) |
| DH5α | F− supE44 ΔlacU169 (φ80 lacZ ΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | (New England Biolabs) |
| BL21-Gold (DE3) | E. coli B F− ompT hsdSB (rB− mB−) dcm+ Tetr gal λ(DE3) endA Hte | (Agilent Technologies) |
| Plasmids | ||
| pKD4 | repR6K γApR FRT KmR FRT | (22) |
| pKD46 | reppSC101ts ApR ParaBAD γ β exo | (22) |
| pCP20 | reppSC101ts ApR CmR cI857 λPR | (22) |
| pKG136 | repR6Kγ KmR FRT lacZY this | (23) |
| pACYC184 | repp15A CmR TcR | (New England Biolabs) |
| pUHE21–2lacq | reppMB1 ApR lacIq | (24) |
| pYS2132 | reppMB1 ApR lacIq nlpE | (4) |
| pYS1755 | reppMB1 ApR lacIq tolC | This work |
| pYS1000 | repp15A CmR Plac1–6 lacZ this | (25) |
| pYS1736 | repp15A CmR Plac1–6 Pmar | This work |
| pET28a | repColE1 KmR lacI PT7 | EMD Biosciences |
| pYS2211 | repColE1 KmR lacI PT7 (Eco) cpxR-His6 | This work |
Construction of Strains with Chromosomal Deletions and lac Fusions
Simultaneous deletion of the marR and marA genes was carried out using primer pairs 1746 (5′-TGC AAC TAA TTA CTT GCC AGG GCA ACT AAT CAT ATG AAT ATC CTC CTT AG-3′) and 1747 (5′-ATT GCC TCA GTG ACG TTG TCA CGT TTT CAA GTG TAG GCT GGA GCT GCT TC-3′) to amplify the kanamycin resistance cassette (KmR) from plasmid pKD4 and integrate the resulting PCR product into the chromosome of wild-type harboring pKD46. The kanamycin-resistant cassette was removed using plasmid pCP20, and the lac transcriptional fusion plasmid pKG136 was integrated into the tyrosine DNA recombinase (FLP) recombination target sequence in the deleted locus (22, 23). P1 transduction was carried out to construct double and triple mutants.
Plasmid Construction
All plasmids used in this study are described in Table 1. pYS1755 (pUHE-tolC) was constructed using PCR fragments containing the full-length tolC coding region generated with primers 2126 (5′-CGG GAT CCA CAA GGA ATG CAA ATG-3′) and 2127 (5′-CCC AAG CTT GTC GTC ATC AGT TAC GG-3′) and wild-type BW25113 chromosomal template that were digested with BamH1 and HindIII and then ligated between BamH1 and HindIII sites of pUHE21–2lacIq (24). pYS1736 was constructed by cloning 120 bp of the marR promoter region amplified using primers 1731 (5′-CCG CTC GTT CAT TGA ACA GAT CGC TGG TAC TTT TCA C-3′) and 1736 (5′-GCG TCG ACC TTT AGC TAG CCT TGC ATC GCA TTG AAC AA-3′) and wild-type BW25113 chromosomal template into the SalI and XhoI sites of pYS1000 with a lacZ reporter (25). pYS2211 was constructed to produce the E. coli CpxR protein fused to a His6 tag at its N terminus by PCR amplification with the primers 1512 (5′-GTT TCA TAT GAA TAA AAT CCT G-3′) and 1714 (5′-ACG CGT CGA CTC ATG AAG CAG AAA CCA T-3′) and wild-type BW25113 chromosomal template. The PCR product was digested with NdeI and SalI and then ligated between the NdeI and SalI sites of pET28a.
Protamine Susceptibility Assay to Test CpxR-dependent Genes
Specific strains from the Keio Collection (21) were picked out to test their susceptibility to protamine (26). Bacterial cells were cultured overnight, re-inoculated (1:100) in LB broth, and grown for 4 h at 37 °C. Cultures were diluted, and ∼1 × 103 cells were dropped onto LB agar plates containing varying concentrations (0.5–1.5 mg/ml) of protamine sulfate (MP Biomedicals) and incubated overnight at 37 °C to determine the colony-forming units (cfu). The percentage survival of each strain was calculated by comparing cfu from plates supplemented with and without protamine.
β-Galactosidase Assay
β-Galactosidase assays (27) were carried out in triplicate, and the activity (Miller Unit) was determined using a VERSAmax plate reader (Molecular Device). Data correspond to three independent assays conducted in duplicate, and all values are the mean ± S.D.
Purification of CpxR-His6 Protein
The E. coli CpxR-His6 protein was purified from E. coli BL21-Gold with His-Select nickel affinity gel (Sigma) according to the manufacturer's instructions. After purification, the fractions containing CpxR-His6 protein were desalted and concentrated using an Amicon Ultra centrifugal filter (Millipore).
DNase I Footprinting Assay
DNase I footprinting assays were carried out using a DNA fragment whose non-coding strand was radioactively labeled. Primer 2051 (5′-CAG ATC GCT GGT ACT TTT CAC-3′) was labeled with [γ-32P]ATP (PerkinElmer Life Sciences) and T4 polynucleotide kinase (New England Biolabs). A 332-bp DNA fragment was amplified by PCR using BW25113 chromosomal DNA as template and primers 32P-labeled 2051 and 2050 (5′-TTG CCT GCC AGG CCA-3′). Approximately 25 pmol of labeled DNA and increasing amounts of CpxR-His6 protein were mixed in a 100-μl reaction containing 2 mm HEPES, pH 8.0, 10 mm KCl, 20 μm EDTA, 0.5 mg/ml BSA, 20 μg ml−1 poly(dI-dC), 2% glycerol (28). The reaction mixture was incubated at room temperature for 20 min. Then a DNase I solution (10 mm CaCl2, 10 mm MgCl2, and 0.01 units of DNase I (Fermentas)) was added, and the mixture was incubated at room temperature for 3 min. The DNase I digestion was stopped by phenol treatment, and the DNA was precipitated. Samples were analyzed by 6% polyacrylamide DNA sequencing electrophoresis supplemented with 8 m urea by comparison with a DNA sequence ladder generated with the same primers using a Maxam and Gilbert A+G reaction. The bands were detected by autoradiography.
RESULTS AND DISCUSSION
CpxR-regulated tolC Gene Contributes to E. coli Resistance to Protamine
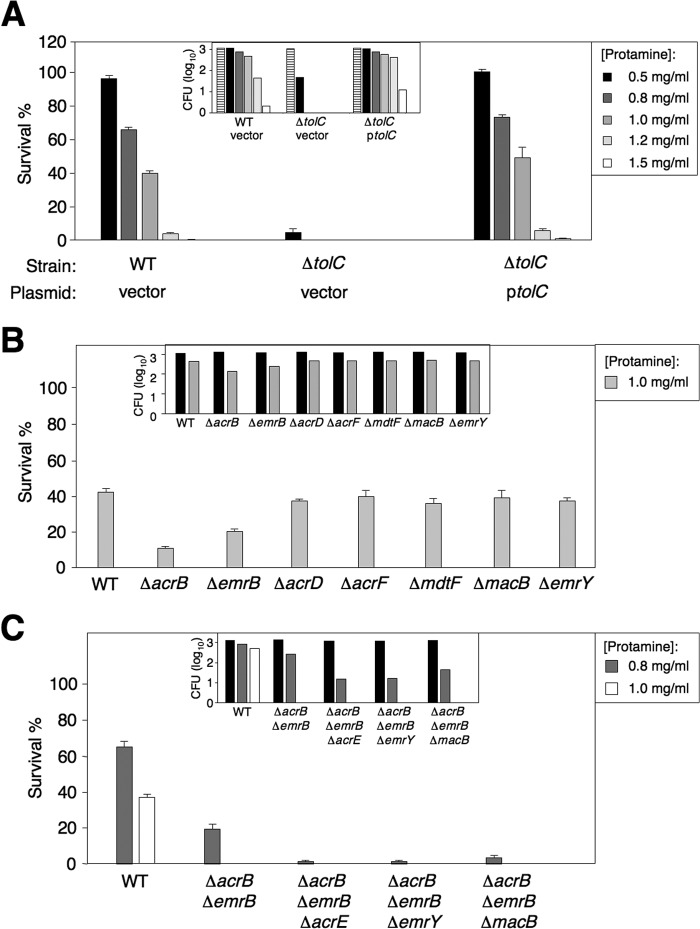
Our previous study has implied that the CpxR/CpxA system may control a Tat-independent mechanism that facilitates bacterial resistance to a model CAMP, protamine (4). To study this new mechanism, we carried out a susceptibility assay to compare protamine resistance of an E. coli wild type (BW25113) with particular isogenic strains from the Keio Collection, i.e. a mutant library carrying in-frame single-gene deletion (21). Protamine could kill this E. coli wild-type strain in a concentration-dependent manner because the percentage survival of BW25113 dropped when protamine concentration was gradually elevated (Fig. 1A). To genome-wide-characterize the CpxR-dependent loci that contribute to E. coli resistance to this CAMP, a total of 115 single mutants, each harboring a deletion at one demonstrated or predicted CpxR-regulated locus (29–31), were tested in our susceptibility assays. Bacterial cells were challenged by protamine at 1.0 mg/ml, which allowed ∼40% of wild-type cells to survive (Fig. 1A). Thus, a susceptible mutant should exhibit a survival rate significantly lower than the wild-type strain in the presence of this protamine. Individual strains were cultured in LB broth to log phase (4 h) and diluted with fresh medium. Bacterial cells (∼103) were inoculated onto LB agar plates with and without protamine and incubated at 37 °C overnight to develop colonies. Previous studies have suggested that the CpxR/CpxA system enhances the resistance of E. coli and Salmonella to protamine through activation of the Tat-dependent amiA and amiC loci as well as the yqjA gene, which is also regulated by the PhoP/PhoQ system (4, 32, 33). Consistently, the ΔamiA, ΔamiC, and ΔyqjA single mutants from the Keio Collection displayed increased susceptibility to protamine (Table 2 and data not shown).
FIGURE 1.
TolC-dependent multidrug transporters are required for E. coli resistance to protamine. A, protamine susceptibility assay for E. coli ΔtolC mutant and complementation. Plasmid harboring a wild-type copy of tolC restores resistance to protamine. Shown is the percentage survival and cfu in log10 (inlet) of wild type (BW25113) carrying plasmid pUHE21 (vector) and ΔtolC mutant (from the Keio Collection) carrying plasmid vector or pYS1755 (pUHE-tolC or ptolC) after incubation with protamine (0, 0.5, 0.8, 1.0, 1.2, or 1.5 mg/ml). B, protamine susceptibility assay for wild-type (BW25113) and selected single TolC-dependent multidrug transporter mutants (the Keio Collection) on LB plates containing protamine (0 or 1.0 mg/ml). Shown is the percentage survival and cfu in log10 (inlet) of wild type, ΔacrB, ΔemrB, ΔacrD, ΔacrF, ΔmdtF, ΔmacB, and ΔemrY single mutants (the Keio Collection) after incubation with protamine (0 or 1.0 mg/ml). C, percentage survival and cfu in log10 (inlet) of wild-type and ΔemrB ΔacrB double mutant (YS Collection 7a) as well as ΔemrB ΔacrB ΔacrE (YS Collection 7b), ΔemrB ΔacrB ΔemrY (YS Collection 7c), and ΔemrB ΔacrB ΔmacB (YS Collection 7d) triple mutants after incubation with protamine (0, 0.8 or 1.0 mg/ml). Data in these experiments correspond to mean values from at least two independent experiments performed in duplicate. Error bars, S.D. Percentage survival of the strains tested was calculated by comparing cfu from plates with protamine to that from input (i.e. the plate without supplementing protamine and shown as the column with light horizontal lines in A or column in black in B and C).
TABLE 2.
Susceptibility of E. coli mutants to protamine
The gene list was summarized from Refs. 8 and 29–31. All strains tested were derived from the Keio Collection. Highlighted mutants are characterized as E. coli strains susceptible to protamine.
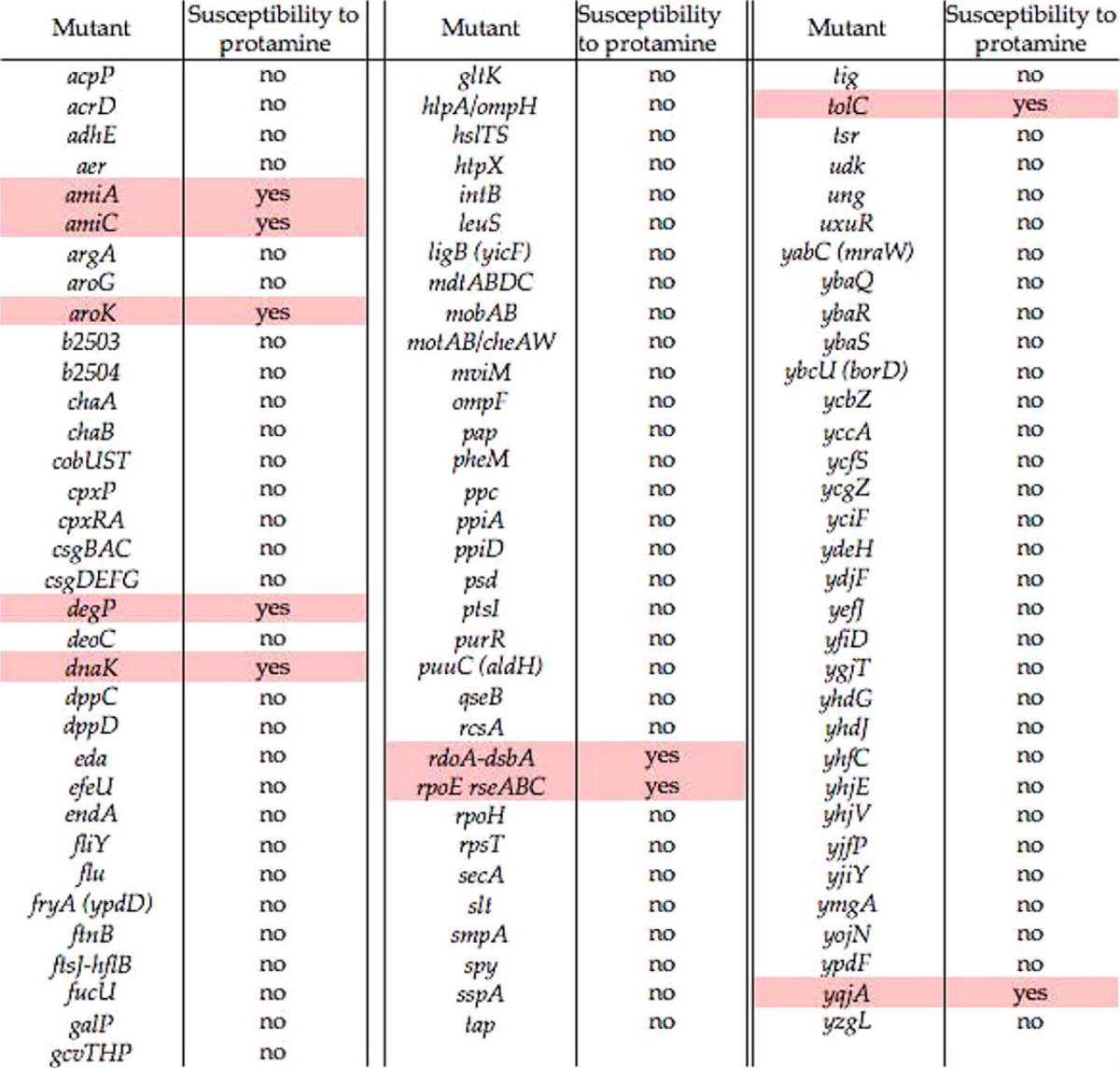
A susceptible mutant characterized from our susceptibility assay carried deletion at the tolC locus. The tolC gene encodes the surface component of tripartite multidrug efflux systems (34). The ΔtolC mutant cells exhibited hypersusceptibility to protamine and were killed completely (i.e. 0% survival) in the presence of 1 mg/ml protamine. Actually, only 4.4% of these mutant cells survived when challenged by protamine at a low concentration (0.5 mg/ml), which could not kill wild-type cells (Fig. 1A). The phenotype of the ΔtolC mutant was solely the result of a lack of the TolC protein because the survival rate under varied protamine concentrations was reversed to a level similar to wild-type by pYS1755, a plasmid containing an IPTG-inducible wild-type copy of the tolC gene (ptolC, Fig. 1A).
Multiple Tripartite Drug Efflux Transporters Play Major Roles in E. coli TolC-dependent Resistance to Protamine
TolC makes up tripartite multidrug efflux systems by pairing with AcrAB, AcrAD, AcrEF, MdtEF, MacAB, EmrAB, and EmrKY (35, 36). Susceptibility to protamine caused by tolC deletion suggests a role of drug efflux systems in bacterial resistance to protamine. Thus, we determined the protamine susceptibility of the Keio strains carrying a single deletion in these loci. In the presence of 1.0 mg/ml protamine, the percentage survival of the ΔacrB and ΔemrB mutants was 11 and 20%, respectively (Fig. 1B), suggesting that deletion of these genes rendered bacteria more susceptible to protamine when compared with the isogenic wild-type strain (∼42%), but not as susceptible as the ΔtolC mutant cells, which were actually killed completely (Fig. 1B). On the other hand, the survival rate of the ΔacrD, ΔacrE, ΔacrF, ΔmdtE, ΔmdtF, ΔmacA, ΔmacB, ΔemrK, and ΔemrY single mutants was similar to wild-type under the same conditions (Fig. 1B). Therefore, two tripartite drug efflux transporters, AcrAB-TolC and EmrAB-TolC, should play major roles in the protamine resistance. An ΔacrB ΔemrB double mutant was indeed more susceptible than either ΔacrB or ΔemrB single mutant and thus was killed completely by 1.0 mg/ml protamine (Fig. 1C). However, this double mutant was still more resistant than the ΔtolC mutant because 19.3% of the ΔacrB ΔemrB mutant cells survived when challenged by a lower concentration (0.8 mg/ml) of protamine (Fig. 1C), whereas the ΔtolC mutant cells were killed completely (Fig. 1A). This observation suggested that an additional TolC-dependent efflux pump(s) contributes to the resistance. We introduced the acrE, emrY, and macB mutations into the ΔacrB ΔemrB mutant, respectively, and found that all of these triple mutants became more susceptible than the ΔacrB ΔemrB mutant to protamine (Fig. 1C) regardless of a wild-type level resistance exhibited in their single mutants (Fig. 1B). Thus, multiple tripartite multidrug efflux systems, particularly AcrAB-TolC and EmrAB-TolC, should contribute to TolC-dependent protamine resistance. Taken together, we conclude that a CpxR/CpxA-dependent mechanism that controls E. coli resistance to protamine is to facilitate production, assembly, and action of multiple TolC-dependent tripartite drug efflux transporters.
Multiple Mechanisms Facilitate CpxR-dependent Resistance to Protamine in E. coli
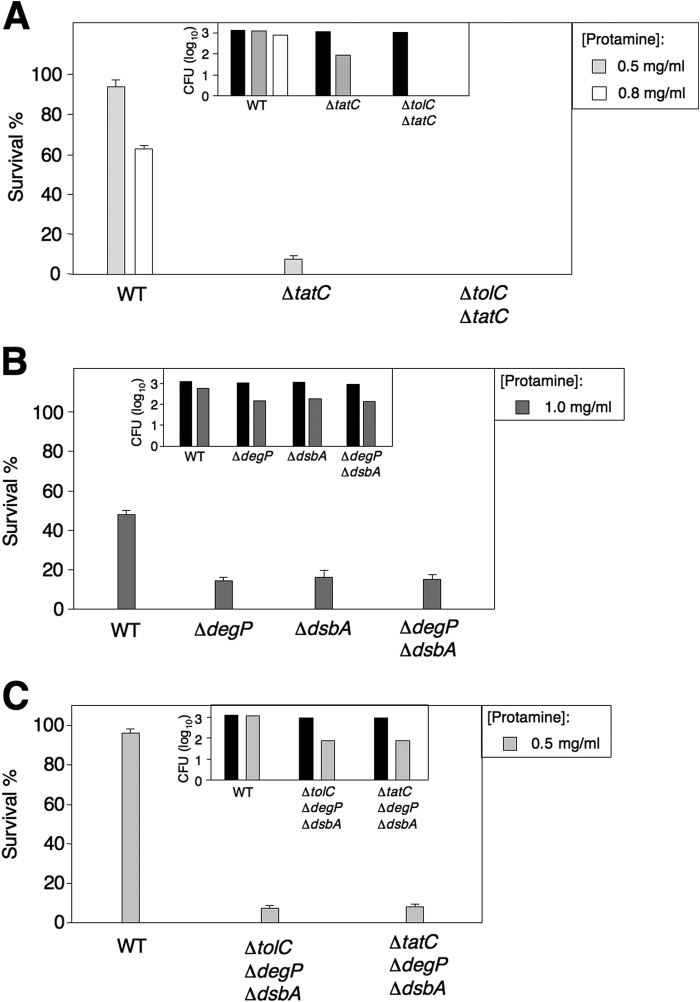
We constructed a ΔtolC ΔtatC double mutant to investigate whether the tripartite multidrug efflux systems were components of the Tat-mediated mechanism or functioned independently to confer resistance to protamine. In the presence of a low concentration of protamine (0.5 mg/ml), 4.4% and 7.5% of ΔtolC and ΔtatC single mutants survived, respectively, whereas all of the ΔtolC ΔtatC mutant cells were killed (Fig. 1A and Fig. 2A). Because this double mutant displayed an additive effect, we postulate that the tripartite multidrug efflux complexes function independently from the Tat system.
FIGURE 2.
Protamine resistance controlled by the CpxR/CpxA system in TolC-dependent and Tat-dependent manners. A, protamine susceptibility assay for wild type, ΔtatC single mutant (YS13007), and ΔtolC ΔtatC double mutant (YS15910) on LB plates containing protamine (0, 0.5, or 0.8 mg/ml). Shown is the percentage survival and cfu in log10 (inlet). B, percentage survival and cfu in log10 (inlet) of wild type, ΔdegP, and ΔdsbA single mutants (the Keio Collection) and ΔdegP ΔdsbA double mutant (YS15924) after incubation with protamine (0 or 1.0 mg/ml). C, percentage survival and cfu in log10 (inlet) of wild type, ΔtolC ΔdegP ΔdsbA (YS15925), and ΔtatC ΔdegP ΔdsbA (YS15926) triple mutants after incubation with protamine (0 or 0.5 mg/ml). The column in black is input cell number counted from the culture without protamine. Data in these experiments correspond to mean values from at least two independent experiments performed in duplicate. Error bars, S.D. Percentage survival of the strains tested was calculated by comparing cfu from plates with protamine to that from input (shown as the columns in black in A, B, and C).
Two more CpxR-regulated genes required for protamine resistance were degP and dsbA (Table 2). When challenged by 1.0 mg/ml protamine, the percentage survival of the ΔdegP and ΔdsbA single mutants was 14% and 16%, respectively (Fig. 2B). The degP and dsbA genes encode a heat shock response chaperone/protease and thiol disulfide oxidoreductase, respectively. They play important roles in the release of envelope stress and the maintenance of the outer membrane integrity by assisting in protein folding and assembly (37). Thus, the outer membrane of the ΔdegP and ΔdsbA mutants can be vulnerable and more susceptible to the membrane-acting antimicrobial peptides. DsbA is required for formation of one disulfide bond in the DegP protein, whose nascent form is unstable and thus becomes absent in a dsbA null mutant (38). Thus, the susceptibility of the ΔdsbA mutant to protamine could result from a lack of functional DegP proteins. Indeed, a ΔdegP ΔdsbA double mutant was as susceptible as individual single mutants (percentage survival, 14.8%, Fig. 2B). We constructed the ΔtolC ΔdegP ΔdsbA and ΔtatC ΔdegP ΔdsbA triple mutants and found that their susceptibility was similar to the ΔtolC and ΔtatC single mutants, respectively (Fig. 2C). Thus, we conclude that the Tat system and tripartite multidrug efflux complexes may require contain components related to DegP and DsbA for E. coli resistance to protamine.
The CpxR/CpxA System Up-regulates Transcription of the mar Operon
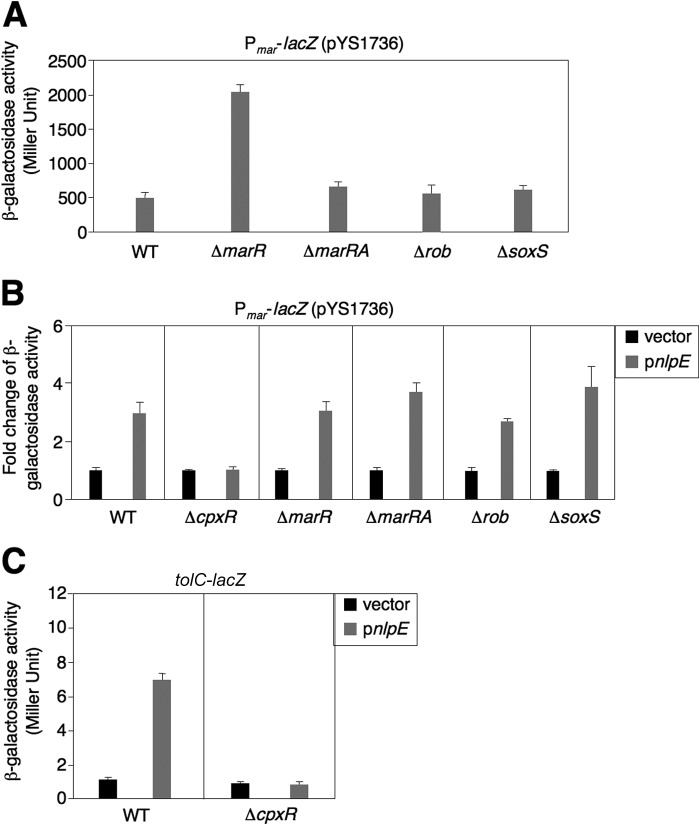
We postulated that the CpxR/CpxA system indirectly regulated the tolC gene because the consensus CpxR binding site (i.e. CpxR box, GTAAA-N5-GTAAA; see Ref. 3) was not present in its promoter region. It is known that the marA gene encodes MarA, a regulator that activates transcription of both tolC as well as the pump component genes, including acrAB (19). Therefore, we investigated whether the CpxR/CpxA system could activate transcription of the marRAB operon (marked as mar, hereinafter). We constructed a reporter plasmid, pYS1736, in which lacZ was fused with a DNA fragment covering 112 bp upstream of the mar transcription start site including both the marbox and MarR binding site characterized previously (18) and introduced it into E. coli strains. β-Galactosidase activity from a mutant harboring a deletion of the repressor gene marR was 4.1-fold higher than that from wild-type (Fig. 3A). This transcription activation caused by the marR mutation was eliminated when the activator gene marA was also deleted, and β-galactosidase activity in a ΔmarR ΔmarA double mutant was reversed to a level comparable to a basal level in wild-type (Fig. 3A). We postulate that MarA functions as an activator required for mar transcription but not as an antirepressor to antagonize MarR. These results demonstrated that the mar promoter region present in pYS1736 is sufficient for the MarR and MarA-dependent transcription regulation. Without supplementing inducer molecules, the mar operon remained at a basal level of expression in the wild-type strain when grown in LB. Furthermore, the level of mar transcription in a Δrob mutant or ΔsoxS mutant grown in LB was similar to that in a wild-type strain (Fig. 3A), implying that its expression is also activated by a factor(s) other than these known regulators.
FIGURE 3.
Activation of the CpxR/CpxA system by nlpE overexpression up-regulates mar transcription. A, a cloned mar promoter region including 112 bp of DNA upstream of the transcription start site is sufficient for the MarR and MarA-dependent regulation. Shown is β-galactosidase activity of wild type and ΔmarR (Keio) and ΔmarRA (YS14530) mutants harboring pYS1736 (Pmar-lacZ) grown in LB for 4 h. B, the CpxR/CpxA system activates mar transcription in different genetic backgrounds. -Fold change in β-galactosidase activity of wild type and ΔcpxR (the Keio Collection), ΔmarR (the Keio Collection), and ΔmarRA (YS14530) mutants harboring pYS1736 and either pUHE21 (vector) or pYS2132 (pnlpE) grown in LB supplemented with IPTG (0.1 mm) for 4 h. -Fold change was calculated: β-galactosidase activity of a strain grown with IPTG divided by that without. C, the CpxR/CpxA system activates tolC transcription. Shown is β-galactosidase activity of wild-type and cpxR harboring chromosomal tolC-lac fusion (YS15537 and YS15538, respectively) and either pUHE21 or pYS2132, grown in LB supplemented with IPTG (0.1 mm) for 4 h. All assays were conducted in triplicate. Error bars, S.D.
To study transcriptional regulation of mar controlled by the CpxR/CpxA system, we constructed a set of strains carrying pYS1736 and a compatible plasmid, pYS2132, that carried a wild-type copy of a IPTG-inducible nlpE gene (4). Overexpression of nlpE induced by IPTG will stimulate the regulatory activity of the CpxR/CpxA system (47). β-Galactosidase activity in wild-type strain was 3.1-fold higher when IPTG (0.1 mm) was supplemented (Fig. 3B). On the contrary, β-galactosidase activity from a cpxR deletion mutant remained at similar levels when grown in the medium with or without IPTG as that in the wild-type strain without IPTG induction (Fig. 3B). These results demonstrate that the CpxR/CpxA system is a regulator that activates mar transcription through the mar promoter fragment in pYS1736. To investigate whether the CpxR/CpxA system could regulate the mar transcription indirectly through a known mar regulator (14–17), we determined pYS1736-directed lacZ expression in the strains harboring a deletion at one of these regulator genes. Overexpression of nlpE could still activate mar transcription in the absence of these regulators because in the presence of IPTG, β-galactosidase activity in ΔmarR, ΔmarRA, Δrob, and ΔsoxS mutants was raised by 3.1-, 3.7-, 2.8-, and 3.9-fold, respectively (Fig. 3B). We conclude that the CpxR/CpxA system can activate mar expression by bypassing or overcoming these regulators. Concomitantly, percent survival of these mutants was similar to the wild-type strain when challenged by 1.0 mg/ml protamine (data not shown).
We also examined the effect of CpxR/CpxA system on the transcription of tolC gene. Supplementing IPTG raised β-galactosidase activity by ∼6.8-fold in a strain with a chromosomal tolC-lac fusion carrying pYS2132 (Fig. 3C). On the contrary, when the cpxR gene was deleted, β-galactosidase activity became unchanged regardless of IPTG (Fig. 3C). Taken together, we propose a transcriptional regulatory cascade that controls E. coli TolC-dependent resistance to protamine in which the CpxR/CpxA system up-regulates transcription of mar operon to facilitate expression of MarA-dependent loci such as tolC.
The CpxR Protein Binds to the mar Promoter
An electrophoretic mobility shift assay suggests that the CpxR binding site is present in the mar promoter because a CpxR-His6 fusion protein binds to the mar promoter (data not shown). To reveal the nucleotide sequence recognized by CpxR, we carried out a DNase I footprinting assay using a 32P-labeled 332-bp DNA fragment containing the mar promoter region present in pYS1736. We found that CpxR was able to protect DNA from DNase I cleavage in a region covering the nucleotides from −48 to −20 upstream of the +1 site (i.e. the transcription start site) (Fig. 4A and illustrated in Fig. 4B). We observed that this CpxR-protected region possessed a sequence, 5′-TTGACttataCTTGC-3′, which is located in −37 and −23 upstream of +1, whose sequence on the opposite strand, 5′-GCAAGtataaGTCAA-3′, resembled a consensus CpxR binding site (the CpxR box, 5′-GTAAA-N5-ATAAA-3′; see Ref. 29). Similar to a reverse CpxR box characterized from the amiC promoter (4), the CpxR binding site is located between the −35 and −10 regions (illustrated in Fig. 4B), which bind σ70 to initiate mar transcription. On the other hand, the marbox is located far upstream, approximately two helical turns apart from this CpxR box, providing a region for MarA/Rob/SoxS to interact with α subunits of RNA polymerase. Because of this, CpxR can act on the mar promoter in a manner independent from other regulators. Thus, we conclude that CpxR regulates mar transcription by directly binding to its promoter.
FIGURE 4.
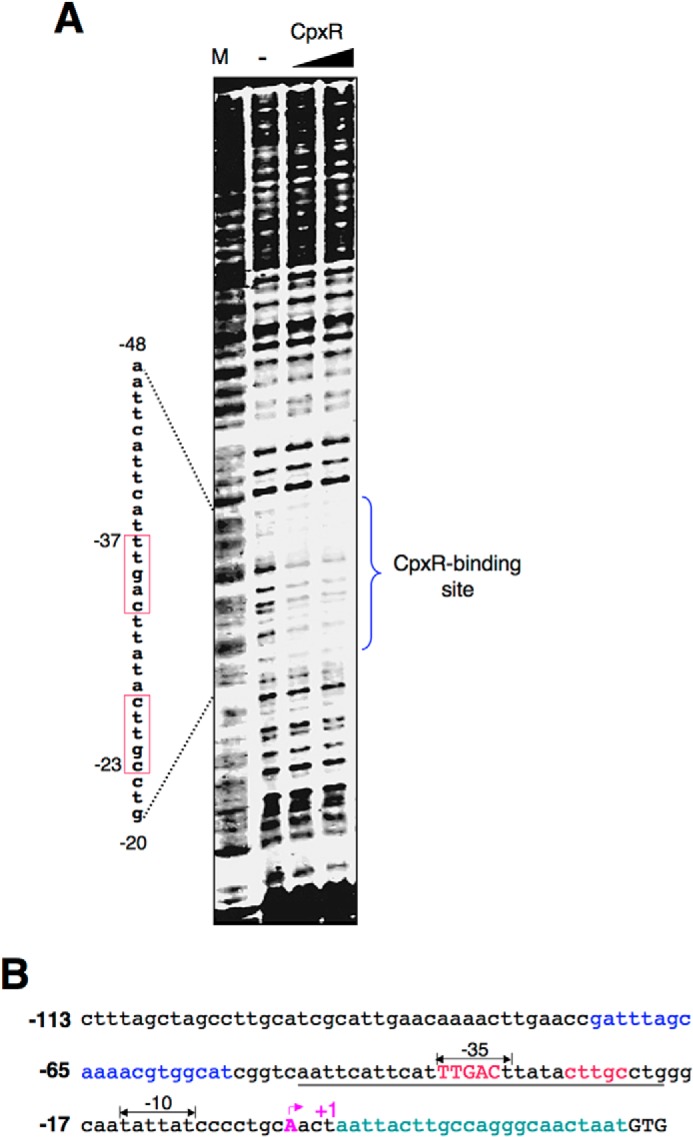
The CpxR protein binds to the mar promoter. A, DNase I footprinting analysis of the wild-type mar promoter with 32P-labeled probe for the noncoding strand and increasing amounts of CpxR-His6 protein (100 and 200 pmol). Products were subjected to polyacrylamide DNA sequencing electrophoresis, and the bands were detected by autoradiography. The right bracket corresponds to the CpxR binding site, and the sequence represents the DNA region protected by CpxR-His6 protein. Red boxes are the CpxR box sequence. B, DNA sequence of the mar promoter region. The underlines correspond to the CpxR-protected region. Sequences in red resemble a reverse consensus CpxR box. Double arrows indicate the putative −35 and −10 sequences, respectively. The nucleotide A in purple with arrow is the transcription start site (+1). Sequences in blue and green are the marbox and MarR binding site, respectively. Numbering in A and B is from the +1.
Activation of the CpxR/CpxA System through Aromatic Metabolites
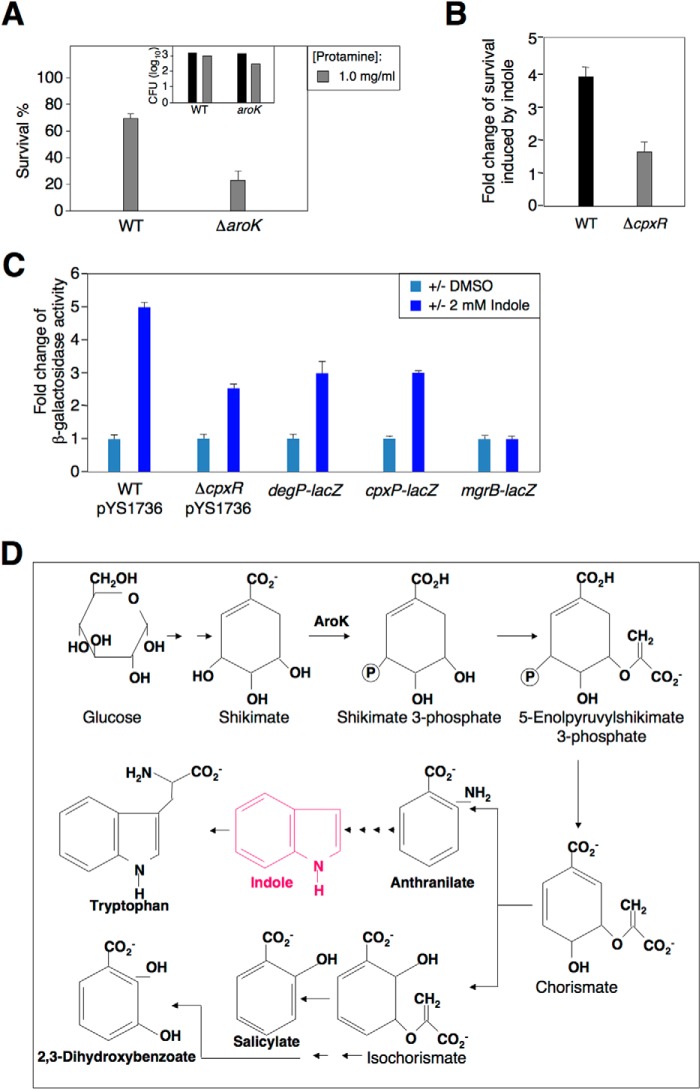
We wanted to know how the CpxR/CpxA system could activate mar transcription in wild type (Fig. 3B) when MarR was present and presumably bound to the promoter. We postulate that the CpxR/CpxA system actually facilitates production of a signal molecule(s) to destabilize MarR binding to the mar promoter, thus allowing an RNA polymerase to pass the MarR binding site. In fact, salicylate and 2,3-dihydroxybenzoate activate the mar promoter by directly binding to MarR with a similar affinity (40). We screened the aroK locus as a protamine-resistant gene because the ΔaroK mutant displayed increased susceptibility when challenged by 1.0 mg/ml protamine (23% survived; Fig. 5A). The aroK gene has been demonstrated as a CpxR-dependent gene (29) which encodes a kinase that generates shikimate 3-phosphate in chorismate biosynthesis in E. coli. As illustrated in Fig. 5D, chorismate is a precursor for biosynthesis of aromatic amino acids including phenylalanine, tyrosine, and tryptophan and also for the synthesis of specific aromatic metabolites, indole and salicylate, as well as salicylate-like compounds including 2,3-dihydroxybenzoate and anthranilate. Therefore, CpxR-activated aroK expression should enhance production of aromatic metabolites to derepress transcription of mar, resulting in activation of the tolC gene.
FIGURE 5.
A possible signal transduction required for resistance to protamine. A, percentage survival and cfu in log10 (inlet) of wild-type and ΔaroK mutant (Keio) after incubation with protamine (0 or 1.0 mg/ml). Percentage survival was calculated by comparing cfu from plates with protamine to that from input (the column in black). B, -fold change of percentage survival of wild-type and ΔcpxR mutant (Keio) after incubation in the presence of protamine (1.0 mg/ml) supplemented with indole (2 mm) or without. -Fold change was calculated as follows: percentage survival of the strains with indole divided by that without. C, -fold change in β-galactosidase activity of wild-type harboring pYS1736 and strains carrying chromosomal lacZ fusions at degP (PND2000), cpxP (SP1), and mgrB (MG1301) grown in LB for 4 h supplemented with or without 2 mm indole or solvent dimethyl sulfoxide (DMSO). -Fold change was calculated as follows: β-galactosidase activity of strains with indole or DMSO divided by that without. D, AroK is a kinase that mediates biosynthesis of chorismate, a precursor for the synthesis of aromatic metabolites including anthranilate, salicylate, 2,3-dihydroxybenzoate as well as indole (the structure in red).
Investigation of Indole as a Signal Molecule to Activate the CpxR-dependent Genes
Indole is able to induce the BaeR/BaeS two-component system to activate multidrug exporters including acrD and mdtABC (41). We found that indole (2 mm) induced a higher level of resistance to protamine in wild type (∼3.8-fold) than that in ΔcpxR mutant (∼1.6-fold) (Fig. 5B). Meanwhile, this indole could also facilitate mar transcription in wild type harboring pYS1736 to a higher level (∼4.8-fold) than the ΔcpxR mutant (∼2.5-fold) (Fig. 5C). To determine if indole could directly act on the CpxR/CpxA system, we constructed strains harboring a chromosomal lacZ fusion at degP and cpxP genes that have been demonstrated previously as CpxR-activated loci (4, 42, 43). Indole was shown to activate transcription of these Mar-independent genes because β-galactosidase activity in degP-lacZ and cpxP-lacZ strains was ∼3- and 2.9-fold higher, respectively, when 2 mm indole was supplemented to the culture. As a negative control, transcription of a PhoP-dependent gene, mgrB (44), could not be activated by indole (Fig. 5C). Taken together, we propose that indole may be one of the physiological signals for the CpxR/CpxA system, thus facilitating resistance to protamine.
Conclusion
The results presented in this study extend our understandings of molecular mechanisms for the CpxR-dependent resistance to CAMPs. Many CAMPs are not only membrane-active, thus causing damages of bacterial membranes, but are also membrane-permeable, thus entering the cytoplasm to target-specific cytoplasmic components (for reviews, see Refs. 45 and 46). A damaged bacterial envelope would be changed in membrane permeability or become leaky, resulting in uncontrollable transmembrane exchange of varied harmful substances. On the other hand, bacteria can probably release this stress by removing malicious substances from the cytoplasm through varied tripartite multidrug transporters. CAMPs that entered into the cytoplasm might be recognized as such noxious substances, subsequently exported by these transporters. This is probably a reason why loss of the TolC-dependent efflux can activate the BaeRS and CpxRA systems (39). Some cytoplasmic components, such as indole, could be facilitated to release out of the cytoplasm from a damaged bacterial envelope. We postulate that this extracytoplasmic indole can interact with the periplasmic domain of the CpxA sensor and subsequently activate this two-component system. Therefore, our findings provide new insights into a mechanism of action of the CpxR/CpxA system.
Antimicrobial peptides, rather than conventional antibiotics, are a natural component in the host environments. Thus, it is more likely that expression of the tripartite multidrug efflux systems actually is to facilitate bacterial resistance to antimicrobial peptides when bacteria are located in certain host niches. As illustrated in Fig. 6, we propose a model that outlines the new mechanism we uncovered in this study. The CpxR/CpxA system activates transcription of mar operon, thus facilitating generation of tripartite multidrug efflux transporters; meanwhile, it also activates transcription of the aroK gene, thus enhancing production of aromatic metabolites including indole, salicylate, and 2,3-dihydroxybenzoate, etc. Salicylate and 2,3-dihydroxybenzoate can act directly on MarR to release it from the marO site. Indole released into the extracytoplasm stimulates the CpxA sensor to phosphorylate CpxR, which subsequently activates the mar transcription.
FIGURE 6.
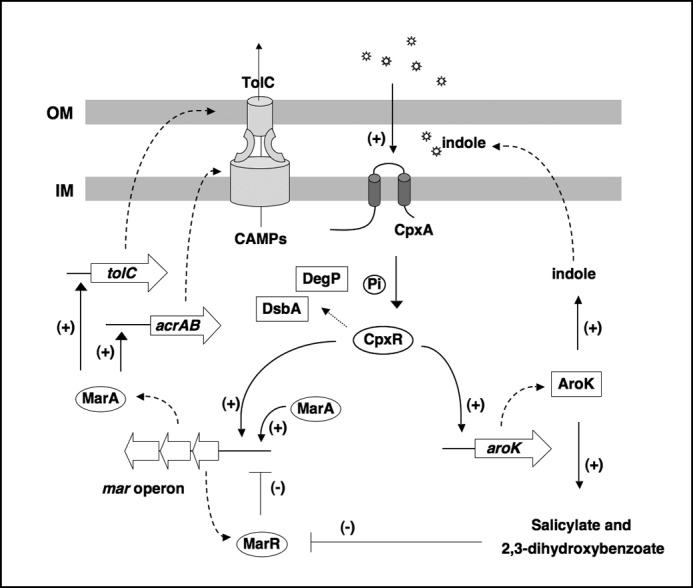
A proposed role for CpxR-dependent regulation in E. coli resistance to CAMP. The CpxR/CpxA response induces the expression of a variety of genes encoding MarA, MarR, AroK, and other envelope folding factors DsbA, DegP, etc. CpxR-induced MarA activates the genes encoding TolC and other components of tripartite multidrug transporters. DegP and DsbA affect assembly of these transporters, which mediate efflux of the CAMPs into the cytoplasm. AroK mediates production of indole, which is exported and stimulates the CpxR/CpxA system, resulting in activation of mar transcription. In the meantime, AroK also mediates production of salicylate and 2,3-dihydroxybenzoate, which bind to MarR to derepress mar transcription. OM and IM are bacterial outer and inner membranes, respectively.
Acknowledgments
We thank Dr. Morigen for strain construction, technical support, and thoughtful discussions. We thank Dr. Thomas Silhavy for strains PND2000 and SP1 and Dr. Akinori Kato for the strain MG1301. We also thank Cori Leonetti for technical aid and Melissa Ellingboe, Jacob Hrkal, Analissa Sarno, Kirk Schmitz, Kayla Wright, and Connie Zuo for editing.
This work was supported in part by Basic Research Grant of Major Open Topics from Science and Technology Department of Inner Mongolia Autonomous Region, China 20110905 (To Y. S.).
- CAMP
- cationic antimicrobial peptide
- mar
- the marRAB operon
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside.
REFERENCES
- 1. Raivio T. L. (2005) Envelope stress responses and Gram-negative bacterial pathogenesis. Mol. Microbiol. 56, 1119–1128 [DOI] [PubMed] [Google Scholar]
- 2. Ma Q., Wood T. K. (2009) OmpA influences Escherichia coli biofilm formation by repressing cellulose production through the CpxRA two-component system. Environ. Microbiol. 11, 2735–2746 [DOI] [PubMed] [Google Scholar]
- 3. De Wulf P., Kwon O., Lin E. C. (1999) The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J. Bacteriol. 181, 6772–6778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weatherspoon-Griffin N., Zhao G., Kong W., Kong Y., Morigen, Andrews-Polymenis H., McClelland M., Shi Y. (2011) The CpxR/CpxA two-component system up-regulates two Tat-dependent peptidoglycan amidases to confer bacterial resistance to antimicrobial peptide. J. Biol. Chem. 286, 5529–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirakawa H., Nishino K., Hirata T., Yamaguchi A. (2003) Comprehensive studies of drug resistance mediated by overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J. Bacteriol. 185, 1851–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishino K., Yamasaki S., Hayashi-Nishino M., Yamaguchi A. (2010) Effect of NlpE overproduction on multidrug resistance in Escherichia coli. Antimicrob. Agents Chemother. 54, 2239–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahoney T. F., Silhavy T. J. (2013) The Cpx stress response confers resistance to some, but not all, bactericidal antibiotics. J. Bacteriol. 195, 1869–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alekshun M. N., Levy S. B. (1999) The mar regulon: multiple resistance to antibiotics and other toxic chemicals. Trends Microbiol. 7, 410–413 [DOI] [PubMed] [Google Scholar]
- 9. Seoane A. S., Levy S. B. (1995) Characterization of MarR, the repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli. J. Bacteriol. 177, 3414–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roldán M. D., Pérez-Reinado E., Castillo F., Moreno-Vivián C. (2008) Reduction of polynitroaromatic compounds: the bacterial nitroreductases. FEMS Microbiol. Rev. 32, 474–500 [DOI] [PubMed] [Google Scholar]
- 11. Sulavik M. C., Gambino L. F., Miller P. F. (1995) The MarR repressor of the multiple antibiotic resistance (mar) operon in Escherichia coli: prototypic member of a family of bacterial regulatory proteins involved in sensing phenolic compounds. Mol Med 1, 436–446 [PMC free article] [PubMed] [Google Scholar]
- 12. Randall L. P., Woodward M. J. (2002) The multiple antibiotic resistance (mar) locus and its significance. Res. Vet. Sci. 72, 87–93 [DOI] [PubMed] [Google Scholar]
- 13. Hächler H., Cohen S. P., Levy S. B. (1991) marA, a regulated locus which controls expression of chromosomal multiple antibiotic resistance in Escherichia coli. J. Bacteriol. 173, 5532–5538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin R. G., Gillette W. K., Rhee S., Rosner J. L. (1999) Structural requirements for marbox function in transcriptional activation of mar/sox/rob regulon promoters in Escherichia coli: sequence, orientation and spatial relationship to the core promoter. Mol. Microbiol. 34, 431–441 [DOI] [PubMed] [Google Scholar]
- 15. Rosenberg E. Y., Bertenthal D., Nilles M. L., Bertrand K. P., Nikaido H. (2003) Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Mol. Microbiol. 48, 1609–1619 [DOI] [PubMed] [Google Scholar]
- 16. Martin R. G., Jair K. W., Wolf R. E., Jr., Rosner J. L. (1996) Autoactivation of the marRAB multiple antibiotic resistance operon by the MarA transcriptional activator in Escherichia coli. J. Bacteriol. 178, 2216–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller P. F., Gambino L. F., Sulavik M. C., Gracheck S. J. (1994) Genetic relationship between soxRS and mar loci in promoting multiple antibiotic resistance in Escherichia coli. Antimicrob. Agents Chemother. 38, 1773–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin R. G., Rosner J. L. (1995) Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc. Natl. Acad. Sci. U.S.A. 92, 5456–5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Warner D. M., Levy S. B. (2010) Different effects of transcriptional regulators MarA, SoxS and Rob on susceptibility of Escherichia coli to cationic antimicrobial peptides (CAMPs): Rob-dependent CAMP induction of the marRAB operon. Microbiology 156, 570–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oh J. T., Cajal Y., Skowronska E. M., Belkin S., Chen J., Van Dyk T. K., Sasser M., Jain M. K. (2000) Cationic peptide antimicrobials induce selective transcription of micF and osmY in Escherichia coli. Biochim. Biophys. Acta 1463, 43–54 [DOI] [PubMed] [Google Scholar]
- 21. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio Collection. Mol. Syst. Biol. 2, 0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Datsenko K. A., Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellermeier C. D., Janakiraman A., Slauch J. M. (2002) Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290, 153–161 [DOI] [PubMed] [Google Scholar]
- 24. Soncini F. C., Véscovi E. G., Groisman E. A. (1995) Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J. Bacteriol. 177, 4364–4371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cromie M. J., Shi Y., Latifi T., Groisman E. A. (2006) An RNA sensor for intracellular Mg2+. Cell 125, 71–84 [DOI] [PubMed] [Google Scholar]
- 26. Ando T., Yamasaki M., Suzuki K. (1973) Protamines. Isolation, characterization, structure and function. Mol. Biol. Biochem. Biophys. 12, 1–114 [PubMed] [Google Scholar]
- 27. Miller J. H. (1972) Experiments in Molecular Genetics, pp. 352–355, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 28. Kato A., Latifi T., Groisman E. A. (2003) Closing the loop: the PmrA/PmrB two-component system negatively controls expression of its posttranscriptional activator PmrD. Proc. Natl. Acad. Sci. U.S.A. 100, 4706–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Wulf P., McGuire A. M., Liu X., Lin E. C. (2002) Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277, 26652–26661 [DOI] [PubMed] [Google Scholar]
- 30. Bury-Moné S., Nomane Y., Reymond N., Barbet R., Jacquet E., Imbeaud S., Jacq A., Bouloc P. (2009) Global analysis of extracytoplasmic stress signaling in Escherichia coli. PLoS Genet. 5, e1000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Price N. L., Raivio T. L. (2009) Characterization of the Cpx regulon in Escherichia coli strain MC4100. J. Bacteriol. 191, 1798–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sikdar R., Simmons A. R., Doerrler W. T. (2013) Multiple envelope stress response pathways are activated in an Escherichia coli strain with mutations in two members of the DedA membrane protein family. J. Bacteriol. 195, 12–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi Y., Cromie M. J., Hsu F. F., Turk J., Groisman E. A. (2004) PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol. Microbiol. 53, 229–241 [DOI] [PubMed] [Google Scholar]
- 34. Gerken H., Misra R. (2004) Genetic evidence for functional interactions between TolC and AcrA proteins of a major antibiotic efflux pump of Escherichia coli. Mol. Microbiol. 54, 620–631 [DOI] [PubMed] [Google Scholar]
- 35. Koronakis V., Eswaran J., Hughes C. (2004) Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annu. Rev. Biochem. 73, 467–489 [DOI] [PubMed] [Google Scholar]
- 36. Yamanaka H., Kobayashi H., Takahashi E., Okamoto K. (2008) MacAB is involved in the secretion of Escherichia coli heat-stable enterotoxin II. J. Bacteriol. 190, 7693–7698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gerken H., Leiser O. P., Bennion D., Misra R. (2010) Involvement and necessity of the Cpx regulon in the event of aberrant β-barrel outer membrane protein assembly. Mol. Microbiol. 75, 1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hiniker A., Bardwell J. (2004) In vivo substrate specificity of periplasmic disulfide oxidoreductases. J. Biol. Chem. 279, 12967–12973 [DOI] [PubMed] [Google Scholar]
- 39. Rosner J. L., Martin R. G. (2013) Reduction of cellular stress by TolC-dependent efflux pumps in Escherichia coli indicated by BaeSR and CpxARP activation of spy in efflux mutants. J. Bacteriol. 195, 1042–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chubiz L. M., Rao C. V. (2010) Aromatic acid metabolites of Escherichia coli K-12 can induce the marRAB operon. J. Bacteriol. 192, 4786–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hirakawa H., Inazumi Y., Masaki T., Hirata T., Yamaguchi A. (2005) Indole induces the expression of multidrug exporter genes in Escherichia coli. Mol. Microbiol. 55, 1113–1126 [DOI] [PubMed] [Google Scholar]
- 42. Danese P. N., Snyder W. B., Cosma C. L., Davis L. J., Silhavy T. J. (1995) The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9, 387–398 [DOI] [PubMed] [Google Scholar]
- 43. Danese P. N., Silhavy T. J. (1998) CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180, 831–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kato A., Tanabe H., Utsumi R. (1999) Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J. Bacteriol. 181, 5516–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yeaman M. R., Yount N. Y. (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55, 27–55 [DOI] [PubMed] [Google Scholar]
- 46. Brogden K. A. (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250 [DOI] [PubMed] [Google Scholar]
- 47. Snyder W. B., Davis L. J., Danese P. N., Cosma C. L., Silhavy T. J. (1995) Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177, 4216–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]