Background: Green tea polyphenol (−)-epigallocatechin-3-O-gallate (EGCG) inhibits melanoma proliferation in a cancer-specific manner through 67-kDa laminin receptor (67LR).
Results: Identified protein phosphatase 2A (PP2A) as a critical downstream factor of 67LR.
Conclusion: Targeting 67LR/PP2A elicits activation of tumor suppressor Merlin and inhibition of mTOR pathway overcoming drug resistance.
Significance: 67LR/PP2A may be a promising therapeutic target for melanomas.
Keywords: Adenylate Cyclase (Adenylyl Cyclase), Cancer Chemoprevention, Melanoma, Membrane Protein, Polyphenol, Protein Phosphatase 2 (PP2A), Second Messenger
Abstract
The Ras/Raf/MEK/ERK pathway has been identified as a major, druggable regulator of melanoma. Mutational activation of BRAF is the most prevalent genetic alteration in human melanoma, resulting in constitutive melanoma hyperproliferation. A selective BRAF inhibitor showed remarkable clinical activity in patients with mutated BRAF. Unfortunately, most patients acquire resistance to the BRAF inhibitor, highlighting the urgent need for new melanoma treatment strategies. Green tea polyphenol (−)-epigallocatechin-3-O-gallate (EGCG) inhibits cell proliferation independently of BRAF inhibitor sensitivity, suggesting that increased understanding of the anti-melanoma activity of EGCG may provide a novel therapeutic target. Here, by performing functional genetic screening, we identified protein phosphatase 2A (PP2A) as a critical factor in the suppression of melanoma cell proliferation. We demonstrated that tumor-overexpressed 67-kDa laminin receptor (67LR) activates PP2A through adenylate cyclase/cAMP pathway eliciting inhibitions of oncoproteins and activation of tumor suppressor Merlin. Activating 67LR/PP2A pathway leading to melanoma-specific mTOR inhibition shows strong synergy with the BRAF inhibitor PLX4720 in the drug-resistant melanoma. Moreover, SET, a potent inhibitor of PP2A, is overexpressed on malignant melanoma. Silencing of SET enhances 67LR/PP2A signaling. Collectively, activation of 67LR/PP2A signaling may thus be a novel rational strategy for melanoma-specific treatment.
Introduction
Melanoma is the deadliest form of skin cancer and is notorious for its resistance to therapy. Nevertheless, recent targeted therapy trials have been promising (1, 2). The Ras/Raf/MEK/ERK pathway was identified as a major, druggable regulator of melanoma (1). Mutational activation of BRAF (mutated in 50 to 70% of melanomas) is the most prevalent genetic alteration in human melanomas, resulting in constitutive melanoma hyperproliferation (3). A selective BRAF inhibitor showed remarkable clinical activity in patients with mutated BRAF (4). Unfortunately, most patients rapidly acquired resistance to the BRAF inhibitor (5). The increase of drug resistant melanomas highlights the urgent need for new melanoma treatment strategies.
The mammalian target of rapamycin (mTOR) pathway is aberrantly activated in melanoma and is the major pathway contributing to drug resistance (6, 7) and may be a novel combination therapy target to treat drug-resistant melanoma. However, most mTOR inhibitors are associated with toxicity, and the related side effects may diminish patient quality of life (8).
(−)-Epigallocatechin-3-O-gallate (EGCG)3 is a major polyphenol component of green tea that can induce tumor-selective anti-melanoma activity through a cell surface receptor, 67-kDa laminin receptor (67LR) (9–11). However, the downstream target of 67LR is still unknown. Therefore, clarification of the underlying molecular mechanisms of EGCG signaling may lead to rational therapeutic targets for melanoma treatment.
A genetic suppressor element (GSE) methodology allows the identification of dominant negative peptides corresponding to different functional domains of a protein (12). GSEs, short fragments of cDNA encoding either inhibitory antisense RNA or dominant negative peptides, are isolated from expression libraries made from short random fragments of a target cDNA by selecting for inhibition of function (12). In this study, we applied GSE methodology to determine systematically the functional consequences of genes essential for the action of EGCG in melanoma. We selected GSEs conferring resistance to EGCG and isolated a GSE that encoded protein phosphatase 2A (PP2A), which protected cells from EGCG-induced anti-melanoma activity.
We here demonstrate that PP2A plays a critical role in EGCG-elicited anti-melanoma activity and 67LR-dependent PP2A activation suppressed melanoma cell proliferation in a cancer-specific manner. PP2A directly interacts with p70S6 kinase (p70S6k) and negatively regulates mTOR signaling (13). To investigate whether activated 67LR/PP2A signaling shows synergy with the selective BRAF inhibitor PLX4720, we compared the anti-melanoma activity of EGCG, PLX4720, or EGCG/PLX4720 in combination in PLX4720 resistant cell line Hs294T in vitro and in vivo. Moreover, we also identified SET, a potent inhibitor of PP2A, was overexpressed in malignant melanoma. By using shRNA, we examined a role of SET in 67LR/PP2A pathway. Our studies present new evidence here that activation of the 67LR/PP2A pathway may be an ideal target to overcome unresponsiveness to BRAF inhibition in drug-resistant melanoma.
EXPERIMENTAL PROCEDURES
Materials and Antibodies
EGCG, catalase, and the anti-β-actin antibody were purchased from Sigma-Aldrich. PLX4720 was purchased from Synkinase Pty., Ltd. (San Diego, CA). Anti-phospho-MRLC (Thr-18/Ser-19), anti-MLC2 (FL-172), anti-MYPT1 (H-130), anti-AKt1/2 (N-19), anti-phospho-ERK (E-4), anti-ERK1 (C-16), and anti-Merlin (NF2) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-phospho-MYPT1 (Thr-696) antibodies were purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Anti-SET, anti-phospho-CPI-17 (Thr-38), and anti-CPI-17 antibodies were purchased from Abcam (Cambridge, MA). Alexa Fluor 555 goat anti-IgG antibody was purchased from Invitrogen. Anti-PP2A A subunit, anti-p70S6k, anti-phospho-p70S6k (Thr-389), anti-S6, anti-phospho-S6 antibodies was obtained from Cell Signaling Technology (Beverly, MA). Anti-phospho-Merlin (Ser-518) was purchased from Rockland (Gilbertsville, PA). Aspartate transaminase and Alanine aminotransferase kit was purchased from Wako (Osaka, Japan).
Cell Culture
Primary normal human melanocyte (NHEM) cells in CSF-4HM-500D culture medium supplemented with human melanocyte growth supplements were obtained from DS Pharma Biomedical (Osaka, Japan). Mouse melanoma (B16) cells, human melanoma A375, Hs294T (BRAF-mutated) and MeWo (BRAF wild-type) cells, obtained from the American Type Culture Collection (ATCC, Manassas, VA), were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 5% (for B16 cells) or 10% (for other cells) fetal bovine serum (FBS). All cells were in a state of logarithmic growth at 37 °C in a humidified chamber with 5% CO2. To assess cell proliferation, cells were plated in 24-well plates at 1 × 104 cells/ml and were treated with EGCG at the indicated concentrations for the indicated time periods in DMEM supplemented with 1% FBS, 200 units/ml catalase, and 5 units/ml superoxide dismutase (Sigma).
RNA Interference by shRNA
Lentiviral vectors expressing non-targeting control shRNA and shRNAs targeting PP2A and SET were purchased from Sigma-Aldrich. Lentivirus production, transduction and selection were performed according to the manufacturer's instructions.
Generation of Doxycycline-inducible 67LR Cell Lines
Transduction and selection were performed as described by the Knock-outTM Single Vector Inducible RNAi System User Manual (Clontech; Mountain View, CA).
cAMP Assays
Measurement of cAMP was performed using the AlphaScreen® cAMP assay kit (PerkinElmer Life Science). The assays were performed according to the manufacturer's instructions. Cells were treated with reagents for 3 h in 96-well plates. Plates were read using the EnVision Plate Reader (PerkinElmer Life Science) at an excitation wavelength of 680 nm and emission wavelength of 615 nm.
PP2A Phosphatase Activity Assay
PP2A activity was determined using the human/mouse/rat active PP2A DuoSet IC (R&D Systems) malachite green/molybdate-based PP2A activity assay according to the manufacturer's instructions.
Long Term Colony Formation Assay
Hs294T cells were grown in the absence or presence of PLX4720 at the indicated concentrations alone or combined with EGCG (5 μm) or rapamycin (10 nm) for 14 days. Then, cells were fixed at the same time, stained with thiazolyl blue tetrazolium bromide (Research Organics, Inc.; Cleveland, OH), and images were captured.
Western Blot Analyses and Immunofluorescent Staining
Cells were lysed in lysis buffer containing 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% Triton X-100, 1 mm ethylenediamine tetra-acetic acid (EDTA), 50 mm sodium fluoride (NaF), 30 mm sodium pyrophosphate (Na4P2O7), 1 mm phenylmethanesulfonyl fluoride, 2 mg/ml aprotinin, and 1 mm pervanadate. Approximately 50 μg of protein was suspended in Laemmli sample buffer (0.1 m Tris-HCl buffer, pH 6.8, 1% SDS, 0.05%-mercaptoethanol, 10% glycerol, and 0.001% bromphenol blue), boiled, and electrophoresed on 8% SDS-polyacrylamide gels. Gels were then electroblotted onto Trans-Blot nitrocellulose membranes (Bio-Rad). Incubation with the indicated antibodies was performed in Tween 20/PBS (TPBS) containing 1% bovine serum albumin (BSA). Blots were washed with TPBS and incubated with anti-rabbit or anti-mouse horseradish peroxidase (HRP) conjugates. After washing, specific proteins were detected using an enhanced chemiluminescence system according to the manufacturer's instructions (Amersham Biosciences). Patient tissue samples were purchased from U. S. Biomax (Rockville, MD). Patients provided written informed consent, and the study was undertaken in accordance with the Declaration of Helsinki. Samples were incubated overnight at 4 °C with the primary antibody.
Anti-PP2A and anti-SET antibodies (Abcam) were used at 1:200 dilution. Slides were then treated with Alexa Fluor 555-conjugated secondary antibody at 1:100 dilution and incubated for 1 h. Anti-67LR Alexa Fluor 488-conjugated antibody was produced following the manufacturer's instructions, and slides were treated with Alexa Fluor 488-conjugated anti-67LR (5 μg/ml). Images were acquired with a Nikon Confocal Laser Microscope (A1, Nikon; Tokyo, Japan) using a PlanApo 60× oil objective lens.
Real-time Quantitative RT-PCR
Total RNA from cells was extracted with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The RNA was treated with RNase-free DNase I (Takara Bio) to remove any contaminating DNA. The RNA concentration was quantified using a spectrophotometer at A260 and A280 (Amersham Biosciences) and stored at −80 °C preanalysis. Real-time quantitative PCR was performed using the relative standard curve method to quantify the target gene expression. In brief, 1 μl of cDNA product was used as the template for RT-PCR, which was performed on the Thermal Cycler Dice Real Time System (Takara Bio) using SYBR Premix Ex Taq (Takara Bio) according to the manufacturer's instructions. Sequences for the PCR primers were as follows: PP2A, 5′-GCACTCGATCGCCTACAGGAA-3′ (sense) and 5′-GCATCTGACCACAGCAAGTCACA-3′ (antisense); for β-actin, 5′-CATCCGTAAAGACCTCTATGCCAA-3′ (sense) and 5′-ATGGAGCCACCGATCCACA-3′ (antisense). The mRNA levels were obtained from the value of cycle threshold for each specific gene and normalized against the cycle threshold of β-actin.
Animals
All animal studies were done in accordance with the law (no. 105) and notification (no. 6) of the Japanese government for the welfare of experimental animals. All procedures were approved by the Animal Care and Use Committee of Kyushu University (Fukuoka, Japan) and undertaken in strict accordance with institutional guidelines for handling laboratory animals.
Five-week-old C57BL/6N mice were obtained from Kyudo (Saga, Japan). Mice were inoculated subcutaneously in the interscapular area with 5 × 105 B16 cells. Following the appearance of palpable tumors, mice were divided randomly into groups with an even distribution of tumor sizes (six mice per group). They were then injected intraperitoneally with vehicle alone or EGCG (20 mg/kg) every 2 days. Five-week-old C57BL/6N mice were inoculated subcutaneously in the interscapular area with 1 × 107 B16 cells. Following the appearance of tumors, mice were given single intraperitoneal injections of or given single intraperitoneal injections with vehicle alone or EGCG (20 mg/kg). After 24 h, tumors were excised and evaluated for PP2A activity. Five-week-old female BALB nu/CrlCrlj mice (six mice/group) were obtained from Charles River Laboratories (Yokohama, Japan). They were inoculated with 2 × 106 Hs294T cells, and the injection regimens described above were followed. They were then injected intraperitoneally daily with vehicle, EGCG (20 mg/kg), PLX4720 (10 mg/kg), or EGCG/PLX4720 in combination every 2 days. Tumor growth was measured with calipers, and the tumor volume was calculated as volume = length × width2/2.
Statistical Analysis
Data were analyzed with GraphPad Prism (version 4) using Student's t tests when comparing two conditions, Dunnett's test when comparing with controls, or Tukey's test for multiple comparisons. Data for tumor growth in vivo were analyzed by Mann-Whitney U test or a two-way analysis of variance test. Values of p < 0.05 were considered significant. Statistical analyses of survival curves were performed using log-rank analyses of Kaplan-Meier curves. All of the in vitro data are representations of more than three independent experiments.
RESULTS
EGCG-induced Melanoma Growth Inhibition Is Attributed to PP2A Activation through 67LR
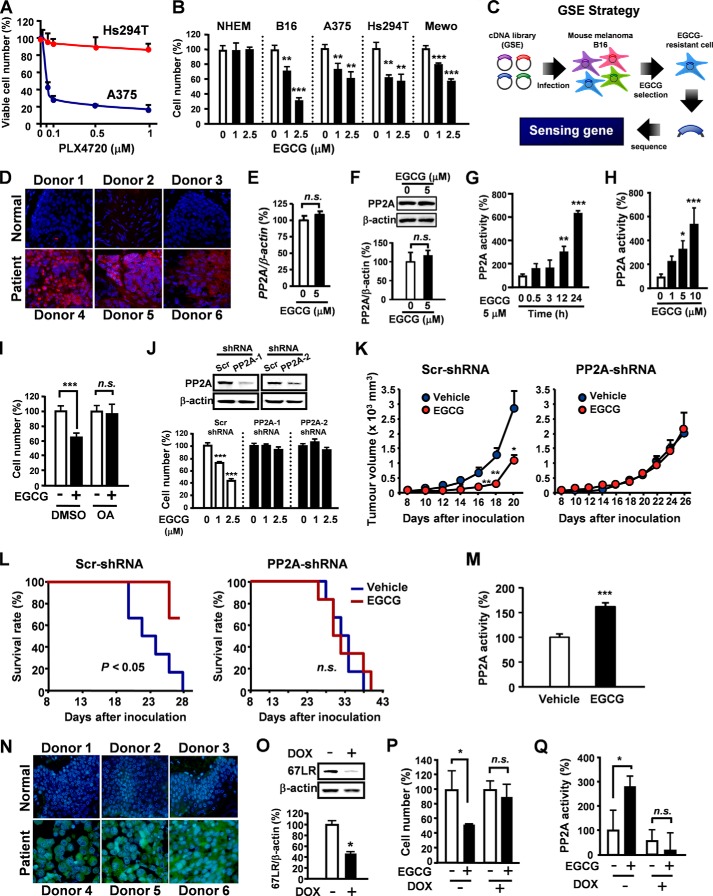
Although BRAF inhibitors have excellent clinical activity in BRAF mutant melanomas, clinical responses are limited by acquisition of drug resistance (5). Interestingly, green tea polyphenol EGCG inhibited cell proliferation without adverse effects on NHEM even in melanoma cells (Hs294T) resistant to PLX4720 and non-BRAF mutant cells (MeWo) (Fig. 1, A and B). To elucidate the molecular mechanisms involved in the cancer-specific anti-melanoma encoding dominant negative peptides or antisense RNAs inhibiting gene expression were used (12).
FIGURE 1.
EGCG-induced melanoma growth inhibition is attributed to PP2A activation through 67LR. A, melanoma cells (BRAF mutant) were treated with PLX4720 for 96 h. B, cells were treated with EGCG for 96 h. C, a flow chart illustrating the strategy used to select GSEs conferring EGCG-resistance to B16 cells from a normalized cDNA fragment library. D, PP2A expression was evaluated by immunofluorescence staining on malignant melanoma tissues and normal skin tissues. E and F, the effect of EGCG on PP2A mRNA (E) and protein (F) levels (24 h). G, B16 cells were treated with 5 μm EGCG, and PP2A phosphatase activity was assessed using the PP2A activity assay kit. H, B16 cells were treated with indicated concentration of EGCG for 24 h, and PP2A phosphatase activity was assessed. I, B16 cells were treated with EGCG for 96 h in the presence or absence of 5 nm OA. J, PP2A silencing on EGCG-induced anti-proliferation in B16 cells (n = 6). K and L, EGCG effects on tumor growth and survival. M, C57BL/6N mice were inoculated subcutaneously with B16 cells. Following appearance of tumors, the mice were treated intraperitoneally with vehicle or 20 mg/kg EGCG. After 24 h, the PP2A activities on tumors were measured (n = 8). N, 67LR expression was evaluated by immunofluorescence staining on malignant melanoma tissues and normal skin tissues. O–Q, used doxycycline (DOX)-induced 67LR gene silencing cells. Cells were treated with doxycycline (5 μg/ml) for 12 h, and 67LR expression was evaluated by Western blot analysis. O, cells were treated with doxycycline (1 μg/ml) for 12 h and cultured in medium containing of EGCG 5 μm for 96 h. P, cells were treated with doxycycline (5 μg/ml) for 12 h and cultured in medium containing of EGCG 5 μm for 24 h. PP2A activity was evaluated after cells treated with 5 μm EGCG for 24 h. Error bars, S.D. in vitro or S.E. in vivo (n = 3 per group in vitro). *, p < 0.5; **, p < 0.1; ***, p < 0.01; n.s., not significant; scr, scrambled.
To identify inhibitors of EGCG-induced cell growth in melanoma cells, GSEs (11) prepared from mouse embryo were screened for genes whose inhibition conferred EGCG resistance in B16 mouse melanoma cells. B16 cells were infected with GSEs and cultured in the presence of EGCG for 1 month (Fig. 1C). Among genetic elements protecting cells from EGCG-induced cell growth inhibition, we isolated a GSE corresponding to PP2A, a critical tumor suppressor gene that regulates multiple oncogenic signal transduction pathways (14, 15). PP2A was overexpressed in clinical tissue specimens compared with normal skin tissues, respectively (Fig. 1D). Thus, high PP2A expression may be important in the cancer-selective effect of EGCG.
To determine the effect of EGCG on PP2A, B16 cells were treated with EGCG, and the mRNA levels, protein levels, and activity of PP2A were assessed. EGCG did not change the expression of PP2A mRNA (Fig. 1E) or protein (Fig. 1F) in B16 cells. However, EGCG time- and dose-dependently enhanced PP2A activity in B16 cells (Fig. 1, G and H). Additionally, treatment with a specific PP2A inhibitor, okadaic acid (OA), abolished EGCG-elicited cell growth inhibition (Fig. 1I), indicating PP2A activation is critical for EGCG-induced anti-cell proliferative activity. To confirm the involvement of PP2A in EGCG anti-melanoma activity, B16 cells were transfected with lentivirus encoding PP2A shRNA or a scrambled control (Fig. 1J). PP2A silencing attenuated EGCG-elicited inhibitory effects on cell proliferation. To confirm these findings in vivo, we performed xenograft studies with PP2A knockdown B16 cells (Fig. 1, K and L). Tumor growth was significantly inhibited in EGCG-administered mice implanted with B16 cells containing control shRNA, whereas tumor growth was not affected by EGCG in mice implanted with PP2A knockdown B16 cells. Log-rank analyses of Kaplan-Meier survival curves showed increased survival of mice treated with EGCG compared with saline-treated mice implanted with control shRNA-expressing B16 cells. There was no statistically significant difference between groups implanted with PP2A knockdown B16 cells. Furthermore, EGCG also induced PP2A activation on tumors (Fig. 1M). Therefore, PP2A may be indispensable for EGCG-induced anti-melanoma activity.
67LR is a laminin-binding protein overexpressed in various cancers, including bile duct carcinoma, colorectal carcinoma, cervical cancer, and breast carcinoma. 67LR is critical for growth and metastasis of tumor cells and resistance to chemotherapy (16). Overexpression of 67LR correlates with tumor progression; thus, 67LR is considered a novel target for tumor-selective cancer therapy (17). Interestingly, EGCG acts as a natural ligand of 67LR, resulting in tumor growth inhibition and cancer-specific apoptotic cell death (11, 18–20). Therefore, the highly specific activity of EGCG against melanoma might be due to differences in 67LR expression levels. We found that 67LR expression was significantly increased in human melanoma tissue specimens compared with normal skin tissue (Fig. 1N). To determine the impact of 67LR on EGCG anti-melanoma activity, we constructed a doxycycline-inducible vector expressing 67LR shRNA. B16 cells were transfected with the vector and treated with doxycycline for 12 h (Fig. 1O). Silencing of 67LR induced by doxycycline treatment attenuated EGCG-mediated inhibition of cell proliferation and blocked EGCG-elicited PP2A activation (Fig. 1, P and Q), suggesting EGCG-elicited PP2A activation is mediated by 67LR. Taken together, abnormally expressed 67LR allows EGCG to act as a melanoma-specific inhibitor by activating PP2A without affecting NHEM cells.
EGCG Functions as a Merlin Activator by 67LR/cAMP/PKA/PP2A-mediating CPI-17 Inhibition
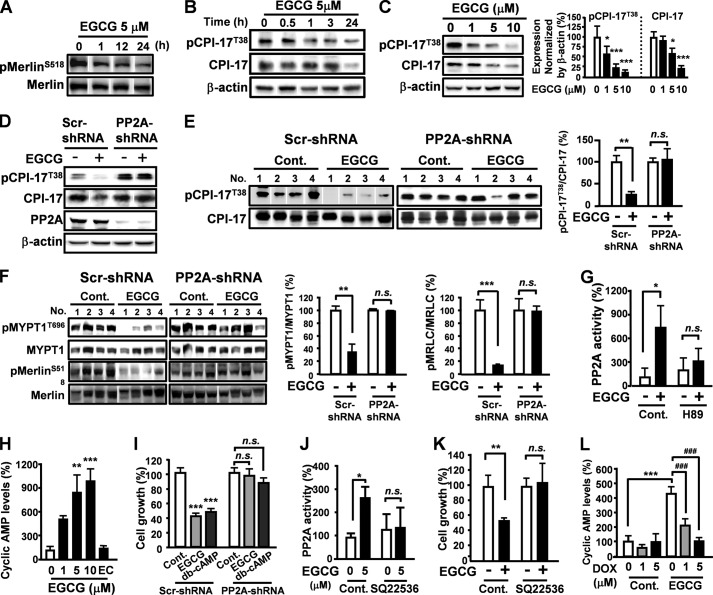
The tumor suppressor protein Merlin (encoded by neurofibromatosis type 2 gene, NF2) is an important regulator of proliferation in many cells and tissue types (21). Merlin is activated by myosin phosphatase (MYPT-1-PP1δ)-mediated dephosphorylation at Ser-518. However, the tumor suppressor cascade is hindered by protein kinase C-potentiated myosin phosphatase inhibitor (CPI-17) (22). PP2A induces inactivation of CPI-17 by promoting dephosphorylation at Thr-38 (23). Although Merlin was previously shown to be important for inhibition of melanoma cell growth, the promotion of Merlin activity has not been reported (24). Treatment with EGCG induced dephosphorylation of Merlin in a time-dependent manner (Fig. 2A). To determine whether EGCG could inhibit oncoprotein CPI-17, B16 cells were treated with EGCG, and phosphorylation levels of CPI-17 were assessed by Western blot analysis. EGCG dephosphorylated CPI-17 in a time- and dose-dependent manner (Fig. 2, B and C), nevertheless, the effect was abolished in PP2A knockdown B16 cells (Fig. 2D). Additionally, EGCG-induced dephosphorylation of CPI-17, MYPT1, and Merlin were observed in tumor cells transfected with control shRNA, whereas EGCG had no effect on phosphorylation of CPI-17, MYPT1, and Merlin in tumor cells grown from PP2A knockdown B16 cells (Fig. 2, E and F). Thus, EGCG may function as a Merlin activator by targeting PP2A activation.
FIGURE 2.
EGCG functions as a Merlin activator by 67LR/cAMP/PP2A-mediating CPI-17 inhibition. A, phosphorylation levels of Merlin in B16 cells treated with EGCG. B and C, phosphorylation levels of CPI-17 in EGCG-treated B16 cells. D, phosphorylation levels of CPI-17 and PP2A expression were evaluated in PP2A knockdown cells after treated with 5 μm EGCG for 24 h. E, effect of EGCG through PP2A on the phosphorylation levels of CPI-17 in tumor cells. F, effect of PP2A on EGCG-induced dephosphorylation of MYPT1 and Merlin in tumor cells (n = 4). EGCG activated MYPT-1/Merlin in tumor cells but not PP2A knockdown B16 cells (n = 4). G, B16 cells were treated with EGCG (5 μm) for 24 h in the presence or absence of H-89 (1 μm). PP2A phosphatase activity was assessed using the PP2A activity assay kit. H, B16 cells treated with indicated concentrations of EGCG or epicatechin (EC) (10 μm) for 30 min. cAMP production was evaluated using AlphaLISA. I, B16 cells or PP2A knockdown B16 cells were treated with 2.5 μm EGCG or 2 mm dibutyryl-cAMP for 96 h. J and K, B16 cells were treated with EGCG (5 μm) for 24 h in the presence or absence of SQ22536 (300 μm). L, cells were treated with indicated concentrations of doxycycline (DOX) for 12 h and cultured in medium containing 10 μm EGCG for 30 min. cAMP production was evaluated using alohaLISA. Error bars, S.D. in vitro or S.E. in vivo (n = 3 per group in vitro). *, p < 0.05; **, p < 0.01; ***, p < 0.001. n.s., not significant; Cont., control; scr, scrambled.
Cyclic adenosine-3′,5′-monophosphate (cAMP) is a second messenger produced in melanocytes with a crucial role in cell differentiation (25). Melanoma cells have elevated cAMP phosphodiesterase activity that inhibits cAMP signaling and allows hyperproliferation (26). Reactivating the cAMP pathway by promoting cAMP production or preventing phosphodiesterase activity inhibits melanoma progression (27). cAMP activity is largely dependent on the cAMP effector, protein kinase A (PKA), which activates PP2A (28). Our data showed that treatment with H-89, a specific PKA inhibitor, attenuated EGCG-elicited PP2A activation (Fig. 2G). Additionally, EGCG dose-dependently increased intracellular cAMP production, whereas a tea polyphenol epicatechin lacking anti-melanoma activity did not elevate cAMP in B16 cells (Fig. 2H). Thus, EGCG-elicited PP2A activation is mediated by the cAMP/PKA pathway. To investigate whether cAMP mediates EGCG-induced anti-melanoma activity, we examined the effect of the cell-permeable cAMP analog dibutyryl-cAMP on B16 cell proliferation. Treatment with dibutyryl-cAMP inhibited B16 cell proliferation; however, PP2A silencing abrogated the inhibitory effect of EGCG on cell growth (Fig. 2I). Adenylate cyclase (AC) is activated in response to melanocytic agonists such as melanocyte-stimulating hormone to produce cAMP (29). Therefore, EGCG-induced cAMP production may be due to AC activation. AC inhibitor SQ22536 prevented both EGCG-elicited PP2A activation and B16 cell growth inhibition (Fig. 2, J and K). Moreover, silencing of 67LR induced by doxycycline treatment blocked EGCG-elicited cAMP production (Fig. 2L). Taken together, EGCG functions as a Merlin activator by 67LR/cAMP/PKA/PP2A-mediating CPI-17 inhibition.
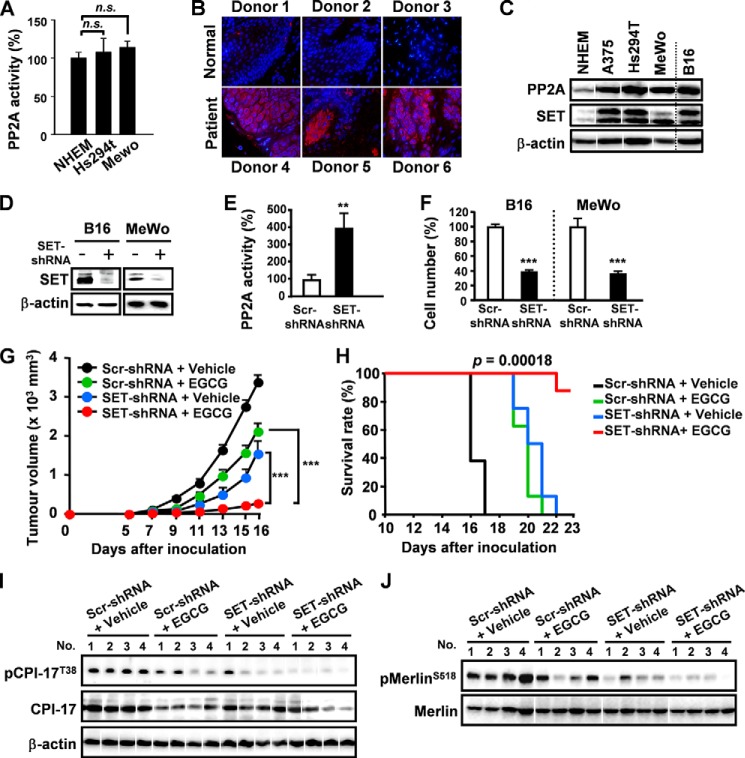
Silencing of SET Significantly Enhanced EGCG-induced Anti-melanoma Activity
SET (Suvar3–9, enhancer-of-zeste, trithorax) protein, isolated from a chromosomal rearrangement at 9q34 in a patient with acute undifferentiated leukemia (30), is a potent physiologic inhibitor of PP2A and is highly expressed in various human tumors, including chronic myeloid leukemia and head and neck squamous cell carcinoma (31–33). Nevertheless, the expression of SET in melanoma is unclear. PP2A was overexpressed in Hs294T and MeWo cell lines compared with NHEM cells, whereas the basal enzyme activities of PP2A were similar (Fig. 3A). Compared with normal skin tissue, SET was abnormally elevated in human melanoma tissues and four melanoma cell lines (A375, Hs294T, MeWo, B16) (Fig. 3, B and C). Thus, SET may attenuate PP2A activity in B16 cells. To examine the effect of overexpressed SET, B16 cells were transfected with lentivirus encoding SET shRNA (Fig. 3D). Remarkably, silencing of SET enhanced PP2A activity and significantly suppressed B16 cell proliferation (Fig. 3, E and F). Importantly, SET was highly expressed in MeWo (non-BRAF mutant melanoma, which has no target for clinical trials) cells, and silencing of SET strongly suppressed MeWo cell proliferation (Fig. 3, C and F, right). Therefore, targeting PP2A pathway might be a promising treatment strategy to melanoma progression with non-BRAF mutant melanoma. To evaluate the influence of silencing SET on EGCG-induced tumor suppression, SET-knockdown B16 cells were injected subcutaneously into C57BL/6N mice. Silencing of SET significantly potentiated EGCG-induced suppression of tumor growth and increased survival rate (Fig. 3, G and H). CPI-17 was inhibited by EGCG in SET knockdown tumor cells, resulting in potent Merlin activation (Fig. 3, I and J). Thus, SET might be a potential combinational target to enhance the effect of PP2A-dependent anti-melanoma chemotherapy.
FIGURE 3.
Silencing of SET significantly enhanced EGCG-induced anti-melanoma activity. A, PP2A activities in different melanoma cell lines. B, SET expression was evaluated by immunofluorescence staining on malignant melanoma tissues and normal skin tissues. C, PP2A and SET expression in different cell lines. D, cells transfected with lentivirus SET shRNA. E, silencing of SET potentiated PP2A activity in B16 cells. F, silencing of SET suppressed cell proliferation of B16 and MeWo (non BRAF mutant) cells. G and H, silencing of SET potentiated EGCG-induced tumor growth suppression and increased survival (n = 8). I, SET expression on EGCG-induced phosphorylation of Merlin in tumor cells. J, phosphorylation levels of CPI-17 were evaluated. Error bars, S.D. (n = 3 per group in vitro). n.s., not significant; Scr, scrambled. ***, p < 0.001.
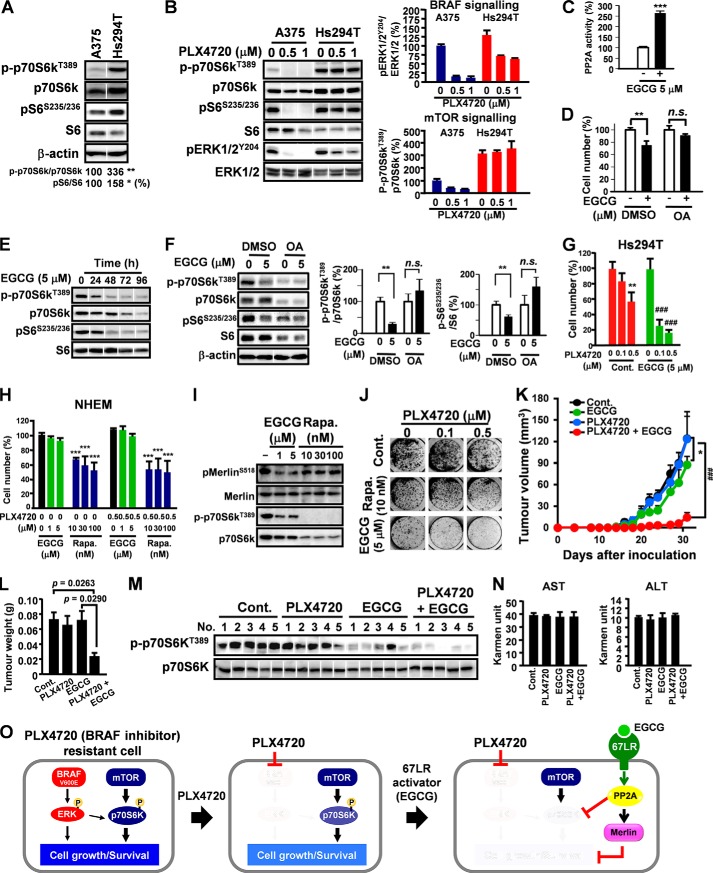
EGCG-induced PP2A Activation Regained the Sensitivity of BRAF Inhibitor PLX4720
Recent studies suggested sustained activation of S6 and p70S6k caused drug resistance to BRAF inhibitor PLX4720 (4). We observed that p70S6k/S6 signaling activity was abnormally increased in Hs294T cells, which exhibited PLX4720 resistance, compared with A375 (PLX4720 sensitive) cells (Fig. 4A). Although PLX4720 increased dephosphorylation of ERK1/2 in A375 and Hs294T cells, phospho-p70S6k was not influenced by PLX4720 in Hs294T cells (Fig. 4B). This suggests that PLX4720 can inhibit BRAF signaling but has no effect on mTOR signaling in Hs294T cells, which can then contribute to PLX4720 resistance. Rapamycin is a potent mTOR inhibitor, nevertheless, clinical trials reported ∼30–50% of patients discontinued it during follow-up due to adverse effects (8). Thus, effective strategies for inhibiting mTOR signaling without increasing toxicity in cancer treatment are urgently required. PP2A is a negative regulator of mTOR signaling by inhibiting p70S6k (13). Therefore, EGCG may suppress p70S6k by activating PP2A. EGCG activated PP2A in Hs294T cells (Fig. 4C). Treatment with OA abolished EGCG-elicited cell growth inhibition in Hs294T (Fig. 4D). Moreover, EGCG promoted dephosphorylation of P70S6k and S6 in a time-dependent manner (Fig. 4E), whereas treatment with OA abolished EGCG-elicited dephosphorylation of P70S6k and S6 (Fig. 4F). Interestingly, EGCG increased PLX4720 sensitivity in Hs294T cells (Fig. 4G). Importantly, combined EGCG and PLX4720 had no adverse effects on NHEM cells, whereas rapamycin was toxic to the normal cells (Fig. 4H). In Hs294T cells, EGCG inhibited p70S6k and activated Merlin, whereas rapamycin only inhibited p70S6k (Fig. 4I). Moreover, combination EGCG and PLX4720 more efficiently prevented colony formation than rapamycin in Hs294T cells (Fig. 4J). To evaluate the in vivo activity of combined EGCG/PLX4720, Hs294T cells were injected subcutaneously into female nude mice. EGCG/PLX4720 treatment significantly suppressed tumor growth in mice compared with mice treated with EGCG or PLX4720 alone (Fig. 4, K and L). EGCG/PLX4720 combination treatment elicited potent inhibition of p70S6k in tumor cells (Fig. 4 m) with no adverse effects on serum AST/ALT activity (Fig. 4N).
FIGURE 4.
EGCG-induced PP2A activation regained the sensitivity of BRAF inhibitor (PLX4720). A, phosphorylation level and expression of P70S6k and S6 were analyzed by Western blot analysis. B, melanoma cells were treated with PLX4720 of for 48 h, and the phosphorylation level of P70S6k, S6 and ERK1/2 were analyzed by Western blot analysis. C, Hs294T cells were treated with EGCG for 24 h, and the activity of PP2A was measured. D, Hs294T cells were treated with EGCG for 96 h in the presence or absence of OA. E, Hs294T cells were treated with EGCG (5 μm) of and the phosphorylation levels of P70S6k and S6 were analyzed by Western blot analysis. F, Hs294T cells were treated with EGCG for 48 h in the presence or absence of OA, and the phosphorylation levels of P70S6k and S6 were measured. G, Hs294T cells were treated with EGCG, PLX4720, or EGCG/PLX4720 in combination for 96 h. H, NHEM were treated with EGCG or rapamycin (Rapa.) alone or combined with PLX4720 for 96h. I, Hs294T cells were treated with EGCG or rapamycin for 24 h. J, Hs294T cells were treated with EGCG, rapamycin, or PLX4720 alone, or in combination for 14 days for long term colony formation assay. K–N, effect of PLX4720 and EGCG combination on the tumor growth (K), tumor weight (L), p70S6k and ERK phosphorylation (n = 4) (M), aspartate transaminase (AST) and alanine aminotransferase (ALT) (N) of mice on day 33 after inoculation. O, signaling pathway schematic. Error bars, S.D. in vitro or S.E. in vivo (n = 3 per group in vitro or n = 7 per group in vivo). *, p < 0.05; **, p < 0.01; *** or ###, p < 0.001. DMSO, dimethyl sulfoxide; n.s., not significant.
DISCUSSION
A well balanced network of kinases and phosphatases is crucial for cells to react efficiently to their environment and for cells to divide correctly. It has been demonstrated that many oncogenes and proto-oncogenes are kinases and that inappropriately increased or decreased levels of their enzymatic activities may contribute to the process of tumorigenic transformation (14). There are many different kinases, each subject to its own specific regulation. However, only a limited number of serine/threonine (Ser/Thr) phosphatase catalytic subunits have been shown to antagonize kinases (14). The specific regulation of Ser/Thr phosphatases in cancer cells may therefore provide a new insight for cancer treatment.
PP2A is a major Ser/Thr phosphatase and has a critical role in cellular processes such as cell proliferation, signal transduction, and apoptosis. PP2A is considered to be a tumor suppressor and is thought to be functionally inactivated in cancer (14, 15). It negatively regulates many proliferative signaling pathways associated with cancer progression by dephosphorylating crucial proteins and decreasing oncogene expression (28). However, no ideal agent regulating PP2A in a cancer-specific manner has yet been identified. Although interactions between EGCG and PP2A have been reported previously (34–36), the concentrations of EGCG used were much higher than the physiologic concentration of EGCG in human plasma (∼1 μm). In contrast, we demonstrated that tumor-overexpressed 67LR mediated the PP2A-activating pathway in melanoma cells and that EGCG activated 67LR/PP2A signaling at physiological concentrations as low as 1 μm.
Melanoma is a very difficult disease to treat, and long term survivors are rare (1, 2). Although BRAF inhibitors represent a major clinical agent for treating melanomas, the mTOR pathway is aberrantly activated in these tumors, contributing to chemotherapeutic resistance (5). PP2A activity is decreased in tumor cells, including melanomas (37). Additionally, PP2A directly interacts with p70S6k and negatively regulates mTOR signaling (13). PP2A may therefore be an ideal target for overcoming unresponsiveness to BRAF inhibition in drug-resistant melanoma. Our data showed that the PP2A activator EGCG down-regulated mTOR signaling and had strong synergy with the investigational BRAF inhibitor PLX4720 in BRAF-resistant melanoma cells. Long term treatment (>72 h) with EGCG decreased expression of both phospho- and total p70S6k and S6. The effects on total p70S6k and S6 might have been caused by instability induced by dephosphorylation. However, although the precise mechanisms whereby total p70S6k and S6 were decreased are unknown, the reduced expression levels of both phospho and total p70S6k and S6 resulted in down-regulation of mTOR signaling by EGCG and renewed sensitivity to the BRAF inhibitor PLX4720.
There are numerous benefits associated with the use of mTOR inhibitors. However, these agents are also associated with a number of potential adverse effects that can reduce patient quality of life. Thus, ∼30–50% of patients receiving mTOR inhibitor therapy discontinue it during follow-up (8). Interestingly, the combination of EGCG and PLX4720 had no adverse effects on NHEM cells, whereas rapamycin, a potent mTOR inhibitor, was toxic to normal cells. 67LR is a non-integrin cell-surface receptor for laminin and functions as a receptor for viruses. Its role as a laminin receptor makes it an important molecule for cell adhesion to the basement membrane and for the metastasis of tumor cells. Several clinical studies have shown that overexpression of 67LR correlates with tumor progression (38–40), and recent findings also indicated that 67LR acts as a tumor-specific death receptor mediating the EGCG signaling pathway (11, 18–20). It has therefore been suggested that 67LR plays a significant role in tumor progression, and studies aimed at clarifying the 67LR signaling pathway could help in the development of new approaches to tumor-selective anticancer therapy. In this study, we showed that 67LR was overexpressed in malignant melanoma and that EGCG activated PP2A through this cell surface receptor, suggesting that targeting 67LR/PP2A signaling may represent a safe targeting strategy in melanoma. cAMP is a second messenger synthesized by adenylyl cyclase, a membrane-associated enzyme regulated by G proteins. We showed that 67LR activated PP2A through the AC/cAMP/PKA pathway in melanomas. However, although 67LR is a cell surface receptor, it is not a G-protein-coupled receptor, though it may in turn regulate G-protein-coupled receptors. Further studies are thus needed to clarify the mechanisms whereby 67LR activates AC.
Merlin isoform I is a tumor suppressor protein encoded by the NF2 gene, which shares significant sequence similarities with the ezrin, radixin, moesin family of cytoskeletal linker proteins (21). Defects caused by mutations of the NF2 gene give rise to NF2 disease and numerous non-NF2-associated tumors including melanoma. Dephosphorylation of Merlin at Ser-518 activates its growth inhibitory activity as a tumor suppressor. Increasing evidence indicates that Merlin regulates the functions and activities of cell surface receptor tyrosine kinases and adhesion/extracellular matrix receptors and serves as a key regulator of several important signaling pathways that regulate cell motility, proliferation, and survival (21). Although the activity of Merlin can be modulated through PKA, p21-activated kinase 1 and 2 (PAK1/2) or MYPT1, there are no currently available agents that activate Merlin in a cancer-selective manner (21). However, the results of this study demonstrate that EGCG activates Merlin through 67LR/PP2A signaling in melanoma cells, suggesting that EGCG may be an ideal natural activator of Merlin and that this 67LR agonist may be a promising agent for cancer-selective Merlin-targeted therapy. Additionally, Merlin negatively regulates CD44 function and contributes to cancer stem cell behavior (21). This may explain why the combination of EGCG and PLX4720 therapy was more effective than rapamycin in preventing colony formation in Hs294T cells, namely, because EGCG inhibited p70S6k and activated Merlin, whereas rapamycin only inhibited p70S6k.
SET is an oncogene that directly binds and inhibits PP2A. It is highly expressed in various human tumors, including chronic myeloid leukemia and head and neck squamous cell carcinoma (31–33). We found that SET was overexpressed in malignant melanoma compared with normal skin, and it dramatically suppressed the 67LR/PP2A-mediated melanoma-specific mTOR inhibition pathway. Importantly, silencing of SET strongly suppressed cell proliferation of non-BRAF mutant melanoma, suggesting that SET inhibitors might be promising agents for treating melanomas with non-BRAF mutations.
In conclusion, our findings demonstrate that targeting 67LR/PP2A signaling might offer a promising approach to the melanoma-specific mTOR inhibition pathway, which can overcome unresponsiveness to BRAF inhibition. Crucially, our results predict that 67LR/PP2A-targeted therapy might be effective even in melanomas with non-BRAF mutations, for which no specific inhibitors currently exist. Potent activators of 67LR might thus represent ideal tumor-specific agents for melanoma treatment.
Acknowledgments
We appreciate the technical assistance from The Research Support Center, Research Center for Human Disease Modeling, Kyushu University Graduate School of Medical Sciences.
This work was supported in part by Grants-in-aid for Scientific Research (S) Grant 22228002 (to H. T.) and Research Fellowship for Young Scientists from the Japan Society for the Promotion of Science DC2 (to S. T.) and DC1 (to Y. H.).
- EGCG
- (−)-epigallocatechin-3-O-gallate
- 67LR
- 67-kDa laminin receptor
- PP2A
- protein phosphatase 2A
- GSE
- genetic suppressor element
- p70S6k
- p70S6 kinase
- NHEM
- normal human epidermal melanocyte
- OA
- okadaic acid
- MYPT
- myosin phosphatase
- PKA
- protein kinase A
- AC
- adenylate cyclase.
REFERENCES
- 1. Chin L., Garraway L. A., Fisher D. E. (2006) Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 20, 2149–2182 [DOI] [PubMed] [Google Scholar]
- 2. Salama A. K., Flaherty K. T. (2013) BRAF in Melanoma: Current strategies and future directions. Clin. Cancer Res. 19, 4326–4334 [DOI] [PubMed] [Google Scholar]
- 3. Davies H., Bignell G. R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M. J., Bottomley W., Davis N., Dicks E., Ewing R., Floyd Y., Gray K., Hall S., Hawes R., Hughes J., Kosmidou V., Menzies A., Mould C., Parker A., Stevens C., Watt S., Hooper S., Wilson R., Jayatilake H., Gusterson B. A., Cooper C., Shipley J., Hargrave D., Pritchard-Jones K., Maitland N., Chenevix-Trench G., Riggins G. J., Bigner D. D., Palmieri G., Cossu A., Flanagan A., Nicholson A., Ho J. W., Leung S. Y., Yuen S. T., Weber B. L., Seigler H. F., Darrow T. L., Paterson H., Marais R., Marshall C. J., Wooster R., Stratton M. R., Futreal P. A. (2002) Mutations of the BRAF gene in human cancer. Nature 417, 949–954 [DOI] [PubMed] [Google Scholar]
- 4. Chapman P. B., Hauschild A., Robert C., Haanen J. B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., Hogg D., Lorigan P., Lebbe C., Jouary T., Schadendorf D., Ribas A., O'Day S. J., Sosman J. A., Kirkwood J. M., Eggermont A. M., Dreno B., Nolop K., Li J., Nelson B., Hou J., Lee R. J., Flaherty K. T., McArthur G. A., and BRIM-3 Study Group (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nazarian R., Shi H., Wang Q., Kong X., Koya R. C., Lee H., Chen Z., Lee M. K., Attar N., Sazegar H., Chodon T., Nelson S. F., McArthur G., Sosman J. A., Ribas A., Lo R. S. (2010) Melanomas acquire resistance to B-RAF (V600E) inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng W., Gopal Y. N., Scott A., Chen G., Woodman S. E., Davies M. A. (2012) Role and therapeutic potential of PI3K-mTOR signaling in de novo resistance to BRAF inhibition. Pigment Cell Melanoma Res. 25, 248–258 [DOI] [PubMed] [Google Scholar]
- 7. Meier F., Schittek B., Busch S., Garbe C., Smalley K., Satyamoorthy K., Li G., Herlyn M. (2005) The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front Biosci. 10, 2986–3001 [DOI] [PubMed] [Google Scholar]
- 8. Merkel S., Mogilevskaja N., Mengel M., Haller H., Schwarz A. (2006) Side effects of sirolimus. Transplant Proc. 38, 714–715 [DOI] [PubMed] [Google Scholar]
- 9. Nihal M., Ahmad N., Mukhtar H., Wood G. S. (2005) Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int. J. Cancer 114, 513–521 [DOI] [PubMed] [Google Scholar]
- 10. Singh T., Katiyar S. K. (2011) Green tea catechins reduce invasive potential of human melanoma cells by targeting COX-2, PGE2 receptors and epithelial-to-mesenchymal transition. PLoS One 6, e25224. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11. Umeda D., Yano S., Yamada K., Tachibana H. (2008) Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J. Biol. Chem. 283, 3050–3058 [DOI] [PubMed] [Google Scholar]
- 12. Holzmayer T. A., Pestov D. G., Roninson I. B. (1992) Isolation of dominant negative mutants and inhibitory antisense RNA sequences by expression selection of random DNA fragments. Nucleic Acids Res. 20, 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janssens V., Goris J., Van Hoof C. (2005) PP2A: the expected tumor suppressor. Curr. Opin. Genet. Dev. 15, 34–41 [DOI] [PubMed] [Google Scholar]
- 14. Peterson R. T., Desai B. N., Hardwick J. S., Schreiber S. L. (1999) Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc. Natl. Acad. Sci. U.S.A. 96, 4438–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Q., Claret F. X. (2012) Phosphatases: the new brakes for cancer development? Enzyme Res. 2012, 659649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nelson J., McFerran N. V., Pivato G., Chambers E., Doherty C., Steele D., Timson D. J. (2008) The 67 kDa laminin receptor: structure, function and role in disease. Biosci. Rep. 28, 33–48 [DOI] [PubMed] [Google Scholar]
- 17. Scheiman J., Tseng J. C., Zheng Y., Meruelo D. (2010) Multiple functions of the 37/67-kd laminin receptor make it a suitable target for novel cancer gene therapy. Mol. Ther. 18, 63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tsukamoto S., Hirotsu K., Kumazoe M., Goto Y., Sugihara K., Suda T., Tsurudome Y., Suzuki T., Yamashita S., Kim Y., Huang Y., Yamada K., Tachibana H. (2012) Green tea polyphenol EGCG induces lipid-raft clustering and apoptotic cell death by activating protein kinase Cδ and acid sphingomyelinase through a 67 kDa laminin receptor in multiple myeloma cells. Biochem. J. 443, 525–534 [DOI] [PubMed] [Google Scholar]
- 19. Kumazoe M, Sugihara K, Tsukamoto S, Huang Y, Tsurudome Y, Suzuki T, Suemasu Y, Ueda N, Yamashita S, Kim Y, Yamada K, Tachibana H. (2013) 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J. Clin. Invest. 123, 787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tachibana H., Koga K., Fujimura Y., Yamada K. (2004) A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 11, 380–381 [DOI] [PubMed] [Google Scholar]
- 21. Stamenkovic I., Yu Q. (2010) Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr. Protein Pept. Sci. 11, 471–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin H., Sperka T., Herrlich P., Morrison H. (2006) Tumorigenic transformation by CPI-17 through inhibition of a Merlin phosphatase. Nature 442, 576–579 [DOI] [PubMed] [Google Scholar]
- 23. Takizawa N., Niiro N., Ikebe M. (2002) Dephosphorylation of the two regulatory components of myosin phosphatase, MBS and CPI17. FEBS Lett. 515, 127–132 [DOI] [PubMed] [Google Scholar]
- 24. Murray L. B., Lau Y. K., Yu Q. (2012) Merlin is a negative regulator of human melanoma growth. PLoS One 7, e43295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cho-Chung Y. S. (1990) Role of cyclic AMP receptor proteins in growth, differentiation, and suppression of malignancy: new approaches to therapy. Cancer Res. 50, 7093–7100 [PubMed] [Google Scholar]
- 26. Watanabe Y., Murata T., Shimizu K., Morita H., Inui M., Tagawa T. (2012) Phosphodiesterase 4 regulates the migration of B16-F10 melanoma cells. Exp. Ther. Med. 4, 205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marquette A., André J., Bagot M., Bensussan A., Dumaz N. (2011) ERK and PDE4 cooperate to induce RAF isoform switching in melanoma. Nat. Struct. Mol. Biol. 18, 584–591 [DOI] [PubMed] [Google Scholar]
- 28. Eichhorn P. J., Creyghton M. P., Bernards R. (2009) Protein phosphatase 2A regulatory subunits and cancer. Biochim. Biophys. Acta 1795, 1–15 [DOI] [PubMed] [Google Scholar]
- 29. Bennett D. C., Cooper P. J., Hart I. R. (1987) A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int. J. Cancer. 39, 414–418 [DOI] [PubMed] [Google Scholar]
- 30. Li M., Makkinje A., Damuni Z. (1996) The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J. Biol. Chem. 271, 11059–11062 [DOI] [PubMed] [Google Scholar]
- 31. Neviani P., Santhanam R., Trotta R., Notari M., Blaser B. W., Liu S., Mao H., Chang J. S., Galietta A., Uttam A., Roy D. C., Valtieri M., Bruner-Klisovic R., Caligiuri M. A., Bloomfield C. D., Marcucci G., Perrotti D. (2005) The tumor suppressor PP2A is functionally inactivated in blast crisis CML through the inhibitory activity of the BCR/ABL-regulated SET protein. Cancer Cell 8, 355–368 [DOI] [PubMed] [Google Scholar]
- 32. ten Klooster J. P., Leeuwen I. v., Scheres N., Anthony E. C., Hordijk P. L. (2007) Rac1-induced cell migration requires membrane recruitment of the nuclear oncogene SET. EMBO J. 26, 336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel V., Hood B. L., Molinolo A. A., Lee N. H., Conrads T. P., Braisted J. C., Krizman D. B., Veenstra T. D., Gutkind J. S. (2008) Proteomic analysis of laser-captured paraffin-embedded tissues: a molecular portrait of head and neck cancer progression. Clin. Cancer Res. 14, 1002–1014 [DOI] [PubMed] [Google Scholar]
- 34. Kiss A., Bécsi B., Kolozsvári B., Komáromi I., Kövér K. E., Erdődi F. (2013) Epigallocatechin-3-gallate and penta-O-galloyl-β-d-glucose inhibit protein phosphatase-1. FEBS J. 280, 612–626 [DOI] [PubMed] [Google Scholar]
- 35. Kitano K., Nam K. Y., Kimura S., Fujiki H., Imanishi Y. (1997) Sealing effects of (-)-epigallocatechin gallate on protein kinase C and protein phosphatase 2A. Biophys. Chem. 65, 157–164 [DOI] [PubMed] [Google Scholar]
- 36. Qin J., Chen H. G., Yan Q., Deng M., Liu J., Doerge S., Ma W., Dong Z., Li D. W. (2008) Protein phosphatase-2A is a target of epigallocatechin-3-gallate and modulates p53-Bak apoptotic pathway. Cancer Res. 68, 4150–4162 [DOI] [PubMed] [Google Scholar]
- 37. Tay K. H., Jin L., Tseng H. Y., Jiang C. C., Ye Y., Thorne R. F., Liu T., Guo S. T., Verrills N. M., Hersey P., Zhang X. D. (2012) Suppression of PP2A is critical for protection of melanoma cells upon endoplasmic reticulum stress. Cell Death Dis. 3, e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanjuán X., Fernández P. L., Miquel R., Muñoz J., Castronovo V., Ménard S., Palacín A., Cardesa A., Campo E. (1996) Overexpression of the 67-kD laminin receptor correlates with tumor progression in human colorectal carcinoma. J. Pathol. 179, 376–380 [DOI] [PubMed] [Google Scholar]
- 39. al-Saleh W., Delvenne P., van den Brule F. A., Menard S., Boniver J., Castronovo V. (1997) Expression of the 67 KD laminin receptor in human cervical preneoplastic and neoplastic squamous epithelial lesions: an immunohistochemical study. J. Pathol. 181, 287–293 [DOI] [PubMed] [Google Scholar]
- 40. Viacava P., Naccarato A. G., Collecchi P., Ménard S., Castronovo V., Bevilacqua G. (1997) The spectrum of 67-kD laminin receptor expression in breast carcinoma progression. J. Pathol. 182, 36–44 [DOI] [PubMed] [Google Scholar]