Background: Role of human mitochondrial MTF mutations causing Leigh syndrome is unknown.
Results: Mutation of certain conserved residues in MTF affects enzyme activity.
Conclusion: Strategic positioning of small aliphatic amino acids is required for normal MTF function.
Significance: This work presents the first characterization of human MTF mutants leading to poor formylation of mitochondrial methionyl-tRNA and thereby reduced mitochondrial translation efficiency, causing Leigh syndrome.
Keywords: Alternative Splicing, Enzyme Kinetics, Mitochondrial Disease, Recombinant Protein Expression, Transfer RNA (tRNA), Translation Initiation, Leigh Syndrome, Methionyl-tRNA Formyltransferase, Mitochondrial Protein Synthesis, MTFMT
Abstract
N-Formylation of initiator methionyl-tRNA (Met-tRNAMet) by methionyl-tRNA formyltransferase (MTF) is important for translation initiation in bacteria, mitochondria, and chloroplasts. Unlike all other translation systems, the metazoan mitochondrial system is unique in using a single methionine tRNA (tRNAMet) for both initiation and elongation. A portion of Met-tRNAMet is formylated for initiation, whereas the remainder is used for elongation. Recently, we showed that compound heterozygous mutations within the nuclear gene encoding human mitochondrial MTF (mt-MTF) significantly reduced mitochondrial translation efficiency, leading to combined oxidative phosphorylation deficiency and Leigh syndrome in two unrelated patients. Patient P1 has a stop codon mutation in one of the MTF genes and an S209L mutation in the other MTF gene. P2 has a S125L mutation in one of the MTF genes and the same S209L mutation as P1 in the other MTF gene. Here, we have investigated the effect of mutations at Ser-125 and Ser-209 on activities of human mt-MTF and of the corresponding mutations, Ala-89 or Ala-172, respectively, on activities of Escherichia coli MTF. The S125L mutant has 653-fold lower activity, whereas the S209L mutant has 36-fold lower activity. Thus, both patients depend upon residual activity of the S209L mutant to support low levels of mitochondrial protein synthesis. We discuss the implications of these and other results for whether the effect of the S209L mutation on mitochondrial translational efficiency is due to reduced activity of the mutant mt-MTF and/or reduced levels of the mutant mt-MTF.
Introduction
One of the major energy-generating pathways in eukaryotic organisms involves the mitochondrion, where oxidation of NADH and FADH2 is coupled to phosphorylation of ADP to synthesize ATP. Mitochondria have their own protein-synthesizing system, which is different from the one in the cytoplasm (1–3). Most mitochondrial DNAs, including mammalian mitochondrial DNAs, code for a total of 13 proteins, which are subunits of the membrane complexes I and III–V involved in oxidative phosphorylation (4–6), and code for rRNAs as well as tRNAs required for mitochondrial protein synthesis. All other proteins present in mitochondria, including the other subunits of the membrane complexes involved in oxidative phosphorylation, are encoded by nuclear DNA, synthesized in the cytoplasm, and imported into mitochondria. Mutations leading to defects of either the components of the mitochondrial protein-synthesizing system or of oxidative phosphorylation complexes can give rise to multiple oxidative phosphorylation deficiencies (7). These conditions are manifested in the form of a wide variety of human diseases associated with bioenergetic defects of mitochondria and generally affect the tissues that are mostly dependent on oxidative phosphorylation.
Protein synthesis is initiated universally with the amino acid methionine or its derivative formylmethionine (1, 8–10). Of the two classes of methionine tRNA (tRNAMet)3 present in all kingdoms, the initiator tRNAMet is used exclusively for the initiation of protein synthesis, whereas the elongator tRNAMet is used for insertion of methionine internally. Bacteria and eukaryotic organelles, such as mitochondria and chloroplasts, initiate protein synthesis with formylmethionine, whereas the cytoplasmic protein-synthesizing systems of eukaryotes and archaea initiate protein synthesis with methionine. Following aminoacylation of the initiator tRNAMet with methionine in bacteria and eukaryotic organelles, the methionyl-tRNAMet (Met-tRNAMet) formed is formylated to formylmethionyl-tRNAMet (fMet-tRNAMet) by the enzyme methionyl-tRNA formyltransferase (MTF). In Escherichia coli and in many other organisms, the formyl group attached to methionine in the initiator tRNA plays an important role in initiation of protein synthesis by acting as a positive determinant for the initiation factor IF2 (11, 12) and as the second of two negative determinants for the elongation factor EF-Tu (13–15).
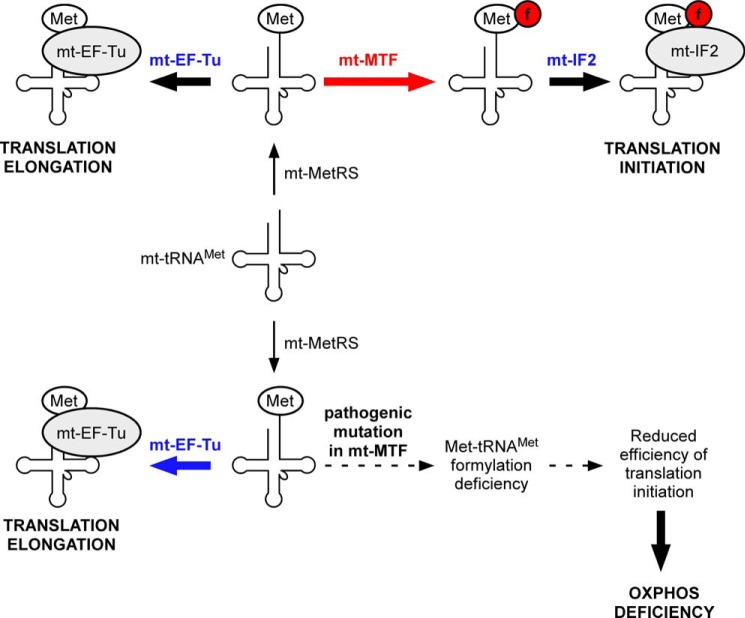
In contrast to the presence of two different methionine tRNA species in all organisms, metazoan mitochondria, including human mitochondria (1, 6), contain only a single species of methionine tRNA (mt-tRNAMet). This striking exception, combined with the lack of evidence of import of any methionine tRNA from the cytoplasm into mitochondria, led to the hypothesis that the single species of mt-tRNAMet acts both as an elongator in the form of Met-tRNAMet and an initiator in the form of fMet-tRNAMet (Fig. 1), with the Met-tRNAMet binding to the mitochondrial elongation factor mt-EF-Tu and the fMet-tRNAMet binding to the mitochondrial initiation factor mt-IF2. Recent work in vitro and in vivo has provided support for this hypothesis (16, 17). The dual role of the same tRNA in initiation and in elongation (Fig. 1, top), however, requires that the mitochondrial Met-tRNAMet be a substrate for two competing proteins, mt-MTF and mt-EF-Tu (18). How mitochondria regulate the levels of Met-tRNAMet and fMet-tRNAMet necessary for their different roles in protein synthesis is unknown.
FIGURE 1.
Top, schematic representation depicting the use of a single mt-tRNAMet for both translation initiation and elongation. The mt-tRNAMet is aminoacylated by mt-MetRS to form Met-tRNAMet. A fraction of Met-tRNAMet is formylated by mt-MTF to generate fMet-tRNAMet, which interacts with mt-IF2 to participate in translation initiation. The remaining fraction of Met-tRNAMet binds to mt-EF-Tu, which transports it to the mt-ribosome for use in translation elongation. Bottom, mutations in mt-MTF causing reduction in its activity can have a deleterious effect on translation initiation because mt-MTF and mt-EF-Tu compete for the same mitochondrial Met-tRNAMet. Because of this competition, even small changes in the activity or levels of mt-MTF could potentially cause a significant reduction in mitochondrial translation initiation, leading to different forms of oxidative phosphorylation deficiency diseases.
The importance of formylation of the mitochondrial Met-tRNAMet for its activity in the initiation of protein synthesis in human mitochondria was demonstrated recently by the finding that mutations in the nuclear gene for mt-MTF led to a defect in mitochondrial translation and to pathogenic effects resulting in Leigh syndrome (Fig. 1, bottom) (17). Compound heterozygous mutations in the nuclear gene for human mt-MTF were identified as the causative agent in two unrelated patients (referred to as P1 and P2). These mutations resulted in significant impairment of mitochondrial protein synthesis in both patients and in dramatic decrease in levels of mitochondrial fMet-tRNAMet. One of the mutant alleles in P1 replaces Ser-209 with leucine. This single mutation at the DNA level of C626 to T (c.626C→T) within the sequence GTTGTCAAG (mutated codon underlined) in exon 4 of the human MTF gene is also predicted to lead to alternative splicing of the pre-mRNA with skipping of exon 4 (19, 20), frameshifting of the reading frame, and a premature UGA termination codon leading to a protein truncated at amino acid 185 (p.R181sfs5) (17). The second allele of mt-MTF in P1 harbored a nonsense mutation c.382C→T at codon 128 leading to a protein truncated at amino acid Arg-128.
One of the mutant alleles in P2 had the same c.626C→T mutation at the DNA level found in P1. The second mutant allele had a c.374C→T mutation, which resulted in the replacement of a highly conserved serine to leucine (S125L) in the catalytic core of full-length mt-MTF. Thus, the only full-length mt-MTF protein present in P1 harbored an S209L mutation, whereas P2 could potentially have two different full-length mutant mt-MTFs containing the S209L and the S125L mutations, respectively.
Quantitative RT-PCR and sequencing of the mt-MTF mRNAs showed that fibroblasts from patient P1 had only 9% of full-length mRNA coding for the S209L mt-MTF variant and the R128X truncated variant, whereas patient P2 had 56% of full-length mRNA coding mostly for the S125L variant (17). The reduced levels of mt-MTF mRNAs in the patients is probably due to the surveillance of mRNAs by the nonsense-mediated decay pathway operating in eukaryotes (21, 22). Despite the reduced levels of mRNAs coding for the mutant proteins, fibroblasts of both patients had residual mt-MTF enzyme activity, because mass spectrometric analysis of peptides from the mitochondrially synthesized cytochrome oxidase subunit I showed that the protein contained predominantly fMet at the N terminus (17).
The finding above that both patients had significantly reduced levels of mRNA for mutant forms of mt-MTF raised the question of whether the reduction in mitochondrial translation efficiency in patients is due to reduced amount of full-length mt-MTF transcripts, reduced activity of mutant enzymes, or both. There is the possibility that the mutations have only a marginal effect on mt-MTF activity as such and that the reductions in mitochondrial translational efficiency seen with the MTF mutants are more due to reductions in mt-MTF mRNA levels and/or the unusual situation of competition between mt-MTF and mt-EF-Tu for the same substrate. Analysis of the catalytic activities of the mt-MTF mutants compared with that of the wild-type MTF is, therefore, an important first step for a better understanding of the basis for reduced mitochondrial translational efficiency and the origin of residual mt-MTF activity in the patients and development of Leigh syndrome. Although recent papers have identified 12 new patients with pathological mutations in mt-MTF (23–25), including 10 more patients with the same S209L mutation found in patients P1 and P2, there have been no biochemical analyses of the effect of any of the mutations on the function of mt-MTF.
This paper describes two sets of experiments designed to study the effects of the S125L and S209L mutations in human mt-MTF on their activities. First, we transplanted these mutations into E. coli MTF to produce the corresponding E. coli MTF mutants (A89L and A172L, respectively) and analyzed their activities in vitro and in vivo using a genetic system that we had developed previously (26, 27). Second, we expressed the wild-type and mutant human mt-MTF proteins in E. coli, purified them, and measured their activities in vitro using E. coli initiator tRNA (tRNA2fMet) and a transcript of the human mt-tRNAMet as substrates. Our results show that the S125L mutant mt-MTF (A89L in E. coli MTF), the predominant mt-MTF in fibroblasts of patient P2, has greatly reduced activity, with Vmax/Km lowered by factors of 107–653-fold for the human enzyme and 144-fold for the E. coli enzyme. The S209L mutation (A172L in E. coli) identified in patients P1 and P2 has a more moderate effect, lowering Vmax/Km by factors of 10–36 for the human enzyme and 4 for the E. coli enzyme. Thus, both patients P1 and P2 depend on activity of the S209L mutant mt-MTF to sustain a low level of translation in mitochondria.
EXPERIMENTAL PROCEDURES
Strains, Growth Conditions, and DNA Manipulations
E. coli DH5α (28) was used as host for cloning, and DH10B (Invitrogen) was used for in vivo assays. E. coli JM109 (29) and BL21 (DE3) (30) served as the host strains for overexpression of the recombinant forms of E. coli MTF and human mt-MTF variants, respectively. E. coli B105 (31) was used for overexpression and purification of E. coli initiator tRNA (tRNA2fMet). Plasmids used in this study are listed in Table 1. E. coli strains were routinely grown at 37 °C in 2× YT medium or on 2× YT agar. The following antibiotics were added to the media where appropriate: ampicillin (100 μg/ml), tetracycline (12.5 μg/ml), and chloramphenicol (50 μg/ml). Transformation of competent E. coli cells and routine DNA manipulations were performed using standard procedures (32). Site-specific mutagenesis was done by QuikChange mutagenesis using high fidelity Pfu Turbo polymerase (Stratagene). All constructs were verified by DNA sequencing.
TABLE 1.
Plasmids used in this study
| Plasmids | Description | Reference/Source |
|---|---|---|
| pRSVCATam1.2.5 trnfM U35A36/G72G73/QRS | pRSVCATam1.2.5 trnfM U35A36/G72G73 with glutaminyl-tRNA synthetase (QRS) gene; ColE1 origin; ampr | Ref. 55 |
| pACD | E. coli vector containing p15A origin of replication, tetr | Ref. 55 |
| pACD MTFs | pACD containing gene for E. coli MTF suppressor (MTFs) under lac promoter | Ref. 27 |
| pACD MTFs A89S | pACD MTFs with A89S mutation | This study |
| pACD MTFs A89L | pACD MTFs with A89L mutation | This study |
| pACD MTFs A172S | pACD MTFs with A172S mutation | This study |
| pACD MTFs A172L | pACD MTFs with A172L mutation | This study |
| pQE16 MTF | pQE16 expression vector (Qiagen) containing E. coli wild-type MTF gene; ampr | Ref. 27 |
| pQE16 MTF A89L | pQE16 MTF with A89L mutation | This study |
| pQE16 MTF A172L | pQE16 MTF with A172L mutation | This study |
| pET mt-MTF | pET19-based expression vector (Novagen) containing wild-type human mt-MTF; ampr | This study |
| pET mt-MTF S125L | pET mt-MTF with S125L mutation | This study |
| pET mt-MTF S209L | pET mt-MTF with S209L mutation | This study |
Preparation of Cell Extracts
A 5-ml culture of E. coli DH10B in 2× YT medium containing the appropriate antibiotics was inoculated with a fresh overnight culture of E. coli transformants and grown until mid-log phase at 37 °C. A cell pellet from 1.2 ml of this culture was resuspended in 120 μl of TME (25 mm Tris-HCl, pH 8.0, 2 mm β-mercaptoethanol, and 1 mm Na-EDTA) containing 0.1 mg/ml lysozyme, and the mixture was left at room temperature for 10 min. DNase I from New England Biolabs (2 units) and 10 mm MgCl2 were added, and the mixture was incubated for another 5 min to digest chromosomal DNA. Cellular debris was removed by centrifugation at 4 °C for 10 min, and the supernatant was collected. Protein concentration was estimated using Bradford reagent and BSA as the standard (33).
Assays for Chloramphenicol Acetyltransferase (CAT) and β-Lactamase Activity
The assays were done as described by Varshney and RajBhandary (26). For the CAT assay, the incubation mixture (100 μl) contained 470 mm Tris-HCl, pH 8.0, 0.8 mm acetyl CoA, 160 μm 14C-chloramphenicol (specific activity 12.5 μCi/mol; PerkinElmer Life Sciences), and varying amounts of the cell extract. Incubation was at 37 °C for 15 min, after which the reaction was stopped by adding ethyl acetate. The ethyl acetate layer was evaporated to dryness and dissolved in a small volume of ethyl acetate, and an aliquot was spotted on a silica gel thin layer chromatography plate (J. T. Baker). CAT activity was normalized with β-lactamase activity to adjust for any fluctuations in plasmid copy number. The incubation mixture for assay of β-lactamase in cell extracts contained 1 ml of a 50 μg/ml solution of Nitrocefin (Calbiochem; in 100 mm sodium phosphate buffer pH 7.0, 1 mm Na-EDTA) and 1 μg of protein. Incubation was for 10 min at room temperature. The reaction was stopped by the addition of 110 μl of 10% SDS, and the absorbance was measured at 486 nm.
Immunoblotting
Total protein (2 μg) present in crude extracts was fractionated on 15% SDS-polyacrylamide gels, electroblotted onto polyvinylidene difluoride membrane (Millipore), and probed with specific antibodies. The antibodies used in this study include rabbit anti-CAT, rabbit anti-β-lactamase (both from 5 Prime 3 Prime, Inc.), mouse anti-tetra-His (Qiagen), and a rabbit polyclonal antibody raised specifically against purified recombinant E. coli MTF (Thermo Scientific). Specific bands were visualized using either HRP-conjugated goat anti-rabbit or anti-mouse IgG (Amersham Biosciences), and antibody-coupled horseradish peroxidase activity was detected with the ECL oxidase/luminol reagents (PerkinElmer Life Sciences).
Purification of Recombinant MTF Proteins
Recombinant E. coli MTF proteins were purified as described previously (27). Recombinant human mt-MTF proteins were purified from E. coli BL21 (DE3) transformed with pET19b (Novagen)-derived plasmids of mt-MTF. A 250-ml culture was grown at 37 °C in the presence of ampicillin until the A600 reached 0.4–0.5. The transcription of the mt-MTF gene was then induced by the addition of 0.1 mm isopropyl β-d-thiogalactoside, and incubation was continued at 18 °C for 6 h. Following centrifugation, cells were resuspended in 20 ml of buffer 1 (50 mm Tris-HCl, pH 8.0, 500 mm KCl, 10 mm MgCl2, 10 mm imidazole, and 20 mm β-mercaptoethanol). The cell suspension was passed twice through a French press cell (12,000 p.s.i.), and 0.5 ml of 100 mm phenylmethylsulfonyl fluoride was added to the lysate. The lysate was clarified by centrifugation (11,000 rpm for 40 min at 4 °C) and brought to room temperature, and 0.8 ml of freshly prepared 0.25 m ATP (pH 7.5 adjusted with NaOH) was added to a final concentration of 10 mm ATP. The lysate was then incubated for 40 min at room temperature with intermittent gentle mixing and added to 1 ml of slurry of Ni2+-NTA affinity resin (Qiagen) pre-equilibrated at room temperature with buffer 1, and the mixture was further incubated for 30 min. The slurry was then poured into a column and washed with 15 ml of buffer 1. The column was then incubated with 10 ml of ATP wash buffer (20 mm Tris-HCl, pH 8.0, 1.5 m KCl, 10 mm MgCl2, 20 mm β-mercaptoethanol supplemented with 10 mm ATP) on a nutator at room temperature, and the flow-through was collected. Washing of the column with 10 mm ATP was repeated once more, followed by two washings with NaCl wash buffer (20 mm Tris-HCl, pH 8.0, 1 m NaCl). The column was then transferred to a cold room, and all subsequent steps were carried out at 4 °C. The column was washed with 15 ml of buffer 1 and then with 8 ml of 20 mm imidazole and 50 ml (for wild-type) or 15 ml (for the mutants) of 50 mm imidazole at a flow rate of 0.5 ml/min. MTF was then eluted with 1.5 ml of buffer 1 containing 100, 150, 200, and 300 mm imidazole at the same flow rate. The purity of MTF proteins was monitored in each fraction of the eluate by SDS-PAGE. Fractions containing MTF were subsequently dialyzed against storage buffer (50 mm Tris-HCl, pH 8.0, 100 mm KCl, 10 mm MgCl2, 20 mm β-mercaptoethanol, and 50% glycerol), and the protein concentration was estimated by a Bradford assay with BSA as the standard. In general, 100–200 μg of recombinant purified mt-MTF proteins were recovered from a 250-ml culture.
tRNA and Aminoacylation Reactions
E. coli tRNA2fMet was purified as described previously (34). T7 transcript of human mt-tRNAMet and purified recombinant mitochondrial methionyl-tRNA synthetase (mt-MetRS) were isolated as described previously (35, 36). Methionine acceptor activity of purified E. coli tRNA was determined by incubating the tRNA in a 30-μl reaction mixture containing 20 mm imidazole buffer, pH 7.6, 150 mm NH4Cl, 10 mm MgCl2, 10 μg/ml BSA, 0.1 mm Na-EDTA, 2 mm ATP, 0.5% (w/v) CHAPS, 0.5 μm [35S]methionine (specific activity, 1175 Ci/mmol; PerkinElmer Life Sciences), 100 μm unlabeled l-methionine, and an excess of purified E. coli MetRS for quantitative aminoacylation. Incubation was at 37 °C, and aliquots (5 μl) taken at various time points were spotted on 3MM Whatman filter paper, which had been presoaked in 2% casamino acids and 10% trichloroacetic acid (TCA) and then dried. The filters were washed once for 15 min with 2% casamino acids and 10% TCA, twice with 5% TCA, and finally with ethanol. Acid-precipitable counts were determined by scintillation counting. Aminoacylation of the E. coli tRNA2fMet was found to be essentially quantitative.
For the human mt-tRNAMet, the tRNA transcript was first denatured by incubating it at 80–85 °C for 3 min. The tRNA transcript was then added to the same reaction mixture as described above and refolded by incubation at 37 °C for 15 min prior to the addition of an excess of mt-MetRS for aminoacylation. The extent of aminoacylation of the tRNA transcript (23–26%) was used to calculate the concentration of the tRNA substrate in formylation.
Measurement of Kinetic Parameters in Formylation of tRNA
The assay for formylation used a two-step reaction. tRNA was first aminoacylated with [35S]methionine using an excess of MetRS. Subsequently, it was formylated using N10-formyl tetrahydrofolate (FTHF) and purified MTF. FTHF was prepared from folinic acid (Sigma) according to the protocol described by Kahn et al. (37). Aminoacylation was allowed to proceed for 30 min at 37 °C in a total reaction volume of 10 μl under the same experimental conditions as described above to ensure maximal aminoacylation of native E. coli initiator tRNA2fMet or the mt-tRNAMet transcript. Subsequently, 2 μl of 0.3 mm FTHF and 3 μl of an appropriately diluted amount of MTF were added to initiate the formylation reaction. Control experiments showed that the aminoacyl-ester linkage in the aminoacyl-tRNA was completely stable during the course of the formylation reaction. The reaction was terminated at different time points by mixing aliquots of the reaction mix with an equal volume of 0.36 m CuSO4 in 1.1 m Tris-HCl, pH 7.8, and further incubation for 10 min before spotting the mixture onto 3MM Whatman filter papers, presoaked in 2% casamino acids and 10% TCA. For calculation of initial velocity, acid-precipitable radioactivity was measured as described above.
Kinetic parameters (Km and Vmax) for the E. coli MTF were determined using Lineweaver-Burk plots. Because the activity of human mt-MTF was much lower than that of E. coli MTF, it was not possible to measure Vmax and Km individually. Instead, Vmax/Km of the wild-type and mutant mt-MTFs was calculated from the initial velocity of formylation using E. coli tRNA2fMet or mt-tRNAMet transcript concentrations in which the initial rate of formylation of Met-tRNAMet is proportional to the tRNA or transcript concentration. At low tRNA substrate concentrations, when [S] ≪ Km, the Michaelis-Menten equation, v = (Vmax[S])/(Km + [S]), approximates to v = (Vmax[S])/Km, where v is initial velocity and [S] is substrate concentration. Under these conditions, v ∝ [S] and v/[S] = Vmax/Km.
RESULTS
Sequence Conservation between E. coli MTF and Human mt-MTF
The crystal structure of E. coli MTF showed that it contains two domains connected by a linker region (38). The structure of the N-terminal domain is highly homologous to that of glycinamide ribonucleotide formyltransferase, which, like MTF, uses FTHF as a formyl group donor in formylation reactions. Similar to glycinamide ribonucleotide formyltransferase and all other MTFs known to date, the N-terminal domain of E. coli MTF contains all of the amino acid residues needed to bind FTHF and for catalysis of formylation, such as Asn-108, His-110, Ser/Thr-137, and Asp-146 (Fig. 7D). The C-terminal domain of E. coli MTF is structurally homologous to some other tRNA-binding proteins and can bind tRNA on its own, although in a nonspecific manner (38).
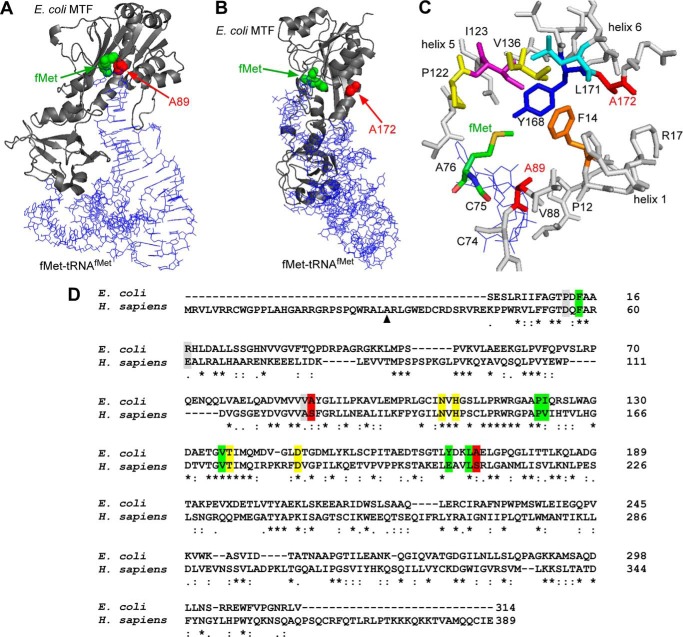
FIGURE 7.
Structural representation of Ala-89 and Ala-172 in the context of the E. coli MTF-fMet-tRNAfMet complex. Structures were drawn using PyMOL (Schrödinger, LLC, New York) and Protein Data Bank entry 2FMT. A, a backbone trace of the complex is depicted, where E. coli MTF is shown in a ribbon representation (gray) and the tRNA molecule is shown as lines (blue). The positions of the fMet moiety and Ala-89 in the methionine binding pocket are highlighted with green and red spheres, respectively. B, the same complex is shown from a different angle to highlight the positions of fMet and Ala-172, as indicated by green and red spheres, respectively. The Ala-172 residue is distal to the methionine binding pocket and belongs to helix 6. C, close-up view of amino acids (represented as sticks) lining the methionine binding pocket of E. coli MTF. The CCA end (Cys-74, Cys-75, and Cys-76) of the initiator tRNA is shown as blue lines. D, pairwise alignment of E. coli MTF and mt-MTF using ClustalW2. Ala-89 and Ala-172 (Ser-125 and Ser-209 in mt-MTF) are highlighted in red. Green, amino acids lining the methionine binding pocket (Phe-14, Ile-123, Val-136, Leu-171, Pro-122, and Tyr-168). Note that Ala-89 is part of the methionine binding pocket (39). Yellow, amino acids involved in FTHF binding and catalysis (Asn-108, His-110, Thr-137, and Asp-146) (38). Gray, additional amino acids of interest mentioned in this work (Pro-12, Val-88, and Arg-17). The numbering of residues in E. coli MTF starts with the serine residue at the N terminus of the mature protein. The numbering for human mt-MTF starts with the first amino acid methionine as encoded by the mt-MTF gene. ▴, start site of mt-MTF after removal of the mitochondrial targeting site.
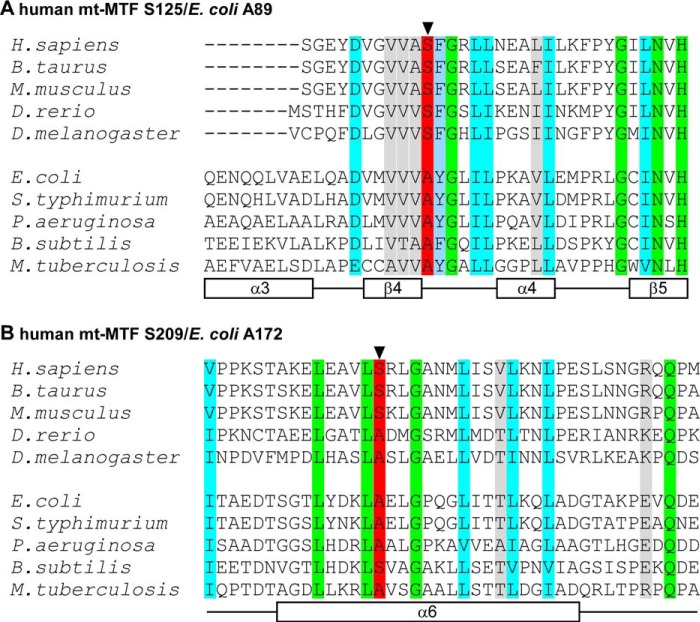
Comparison of MTF sequences of a wide variety of organisms shows a significant level of sequence homology (e.g. ∼30% sequence identity between E. coli MTF and human mt-MTF), including sequences around Ser-125 and Ser-209 mutated in the human mt-MTF of patients P1 and/or P2 (Fig. 2, A and B). Ala-89 of E. coli MTF (highlighted in red in Fig. 2A), corresponding to Ser-125 of human mt-MTF, is always conserved as alanine in bacteria and serine in eukaryotes and is part of the methionine binding pocket. Ala-172 of E. coli MTF (highlighted in red in Fig. 2B) is conserved as alanine or serine in most bacteria and eukaryotes and is part of a long α-helix (helix 6) in the N-terminal domain of MTF. Thus, the sites of the pathogenic substitution mutations in human mt-MTF being studied here consist of small amino acids. However, although E. coli MTF has been used extensively for studies of structure-function relationship and specificity in RNA-protein interaction, no mutants of Ala-89 or Ala-172 have been previously identified in genetic analyses or generated by site-specific mutagenesis (27, 39–41). Therefore, as a first step to study the effect of mutations in human mt-MTF and because of the feasibility of doing genetic and biochemical analyses of the mutants using E. coli, we transplanted the Ser-125 and Ser-209 human mt-MTF mutations into E. coli MTF and analyzed the properties of the mutant enzymes in vivo in E. coli and in vitro.
FIGURE 2.
Multiple sequence alignment of different eukaryotic and bacterial homologs of human mt-MTF highlighting the region harboring the recently identified mutations that form the genetic basis of oxidative phosphorylation disease and Leigh syndrome in humans (17). The secondary structure elements for α-helices (designated as α) and β-sheets (designated as β) are indicated by empty rectangles below the aligned sequences and numbered based on the E. coli MTF crystal structure (38). Identical residues are highlighted in green, whereas similar residues are shown in cyan. The conserved residues Ser-125 (A) and Ser-209 (B), which are sites of mutations in patients with Leigh syndrome, are indicated in red and by arrowheads. The corresponding residues in E. coli MTF are Ala-89 and Ala-172, respectively. The numbering of residues in E. coli MTF starts with the serine residue at the N terminus of the mature protein. The numbering for human mt-MTF starts with the first amino acid methionine as encoded by the mt-MTF gene.
Genetic System for Analysis of MTF Activity in E. coli
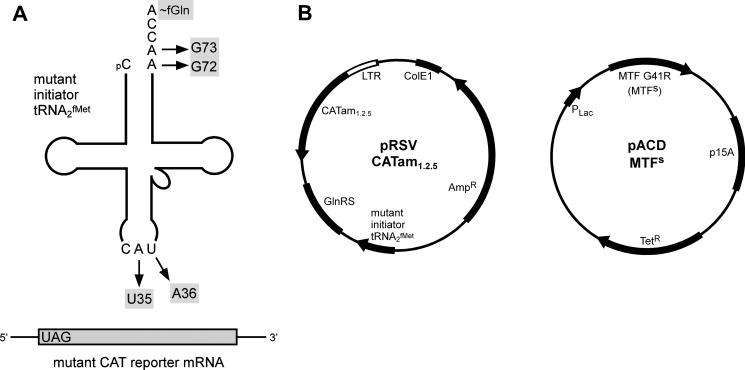
We previously described a genetic system for analyzing the activities of mutants of E. coli MTF and E. coli initiator tRNA (tRNA2fMet) without interfering with activities of endogenous wild-type MTF and wild-type tRNA2fMet (10). The system uses as reporter a mutant CAT gene, whose initiation codon AUG has been changed to UAG, a termination codon (Fig. 3B). Synthesis of CAT from this reporter gene (CATam1.2.5) depends upon the presence of a mutant tRNA2fMet, whose anticodon sequence has been changed from CAU to CUA (the U35A36 mutant) and which can read the UAG initiation codon in the mutant CAT mRNA (26). Cells carrying the mutant CAT reporter gene and the U35A36 mutant initiator tRNA are therefore chloramphenicol-resistant. Introduction of further mutations in the acceptor stem of the U35A36 mutant tRNA2fMet (e.g. G72G73) (Fig. 3A), makes the tRNA an extremely poor substrate for MTF and abolishes its activity in initiation (42). Consequently, E. coli cells carrying the U35A36/G72G73 mutant tRNA2fMet (Fig. 3A) and the CATam1.2.5 reporter gene cannot synthesize CAT and are sensitive to chloramphenicol. Using this system, we isolated previously a suppressor mutation in E. coli MTF (MTFs) carrying a G41R change (27), which allowed CAT synthesis and conferred a chloramphenicol resistance phenotype to E. coli carrying the CATam1.2.5 gene and the U35A36/G72G73 mutant tRNA2fMet gene (Fig. 3B). Here, we have used the two-plasmid system shown in Fig. 3B to study the effect of introducing further mutations at Ala-89 or Ala-172 of the E. coli MTFs on suppressor activity of MTFs in E. coli.
FIGURE 3.
A, schematic representation of the genetic system used for functional analysis of MTFs mutants in E. coli. The strategy for in vivo analysis of MTFs activity relies on a mutant CAT reporter gene (CATam1.2.5) coding for an mRNA transcript having UAG as the initiation codon. Translation of the mutant CAT mRNA requires the simultaneous presence of a mutant initiator tRNA with corresponding anticodon (U35A36) mutations. The inclusion of acceptor stem mutations (G72G73) makes the mutant initiator tRNA defective in formylation and, therefore, inactive in initiation. A suppressor mutation in MTF (MTFs) was previously shown to rescue the defect (27) and to allow the synthesis of CAT in vivo. The sites of mutations in the initiator tRNA are indicated by arrows. B, plasmids used for analysis of MTFs activity in vivo. pRSVCATam1.2.5 (left) contains the genes for the mutant CAT reporter, the mutant initiator tRNA gene, and the E. coli glutaminyl-tRNA synthetase (GlnRS) gene. The CATam1.2.5 gene has an amber mutation at codon 1 and additional mutations at codons 2 and 5. The U35A36 anticodon sequence change in the mutant initiator tRNA makes it a substrate for E. coli glutaminyl-tRNA synthetase (54). To ensure quantitative aminoacylation of the mutant tRNA in vivo, E. coli GlnRS was overproduced by cloning the glutaminyl-tRNA synthetase gene on the same plasmid. pACD MTFs (right) contains the gene for E. coli MTFs under the control of the Plac promoter. CAT activities in extracts of cells expressing the Ala-89 or Ala-172 MTFs mutants were compared to evaluate the effect of these mutations on MTF activity in vivo. LTR, long terminal repeat; AmpR, ampicillin resistance; TetR, tetracycline resistance.
Effect of Ala-89 and Ala-172 Mutations on Activity of E. coli MTFs in Vivo
Ala-89 and Ala-172 were changed to serine with a small amino acid side chain or leucine with a large amino acid side chain, respectively. The effect of mutations on CAT synthesis in vivo was studied using three different assays: (i) growth on chloramphenicol-containing plates; (ii) measurement of CAT activity in cell extracts; and (iii) immunoblot analysis for CAT protein in cell extracts using a specific antibody.
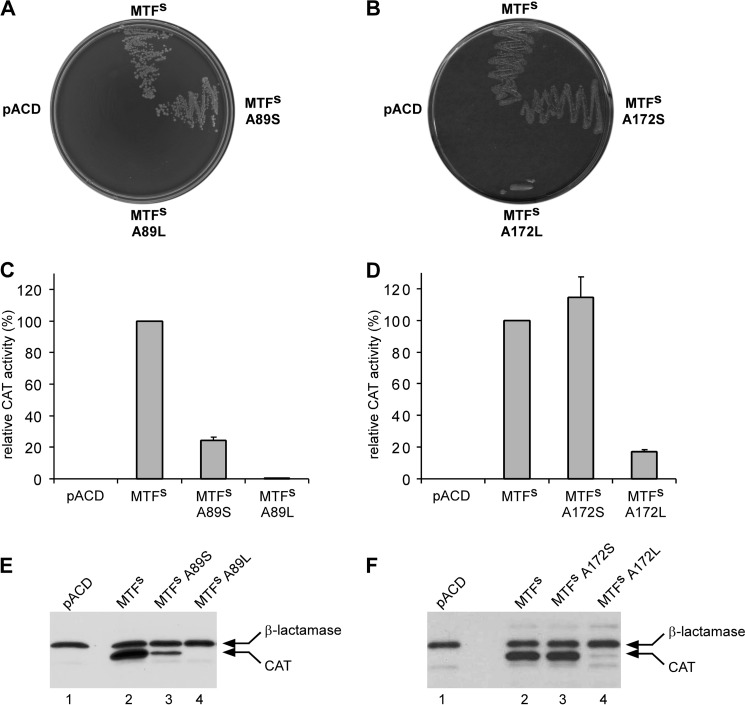
E. coli DH10B carrying the pRSVCATam1.2.5 plasmid was transformed with pACD vector containing either MTFs or MTFs mutants in which Ala-89 or Ala-172 had been changed to serine or to leucine, and the co-transformants were monitored for growth on plates containing chloramphenicol. Fig. 4, A and B, shows that co-transformants harboring MTFs grew normally upon streaking, whereas the one harboring the pACD vector control did not. This confirms earlier findings showing that formylation of the U35A36/G72G73 mutant tRNA2fMet is carried out by MTFs and not by the endogenous wild-type MTF (27, 42). The growth of cells carrying the MTFs A89S mutant on chloramphenicol plates was comparable with that of MTFs, suggesting that the ability of the mutant enzyme to formylate the U35A36/G72G73 mutant initiator tRNA2fMet is not significantly affected by the A89S mutation. In contrast, transformants harboring the MTFs A89L mutants did not grow in the presence of chloramphenicol (Fig. 4A). Analysis of the effect of the mutation at Ala-172 also showed that the growth of cells carrying the MTFs with the A172S mutation remains unaffected, whereas transformants harboring the MTFs A172L mutant showed poor growth in the presence of chloramphenicol, indicating that the mutation might have led to a reduction of enzyme activity (Fig. 4B).
FIGURE 4.
Analysis of the effect of mutations in MTFsin vivo. A and B, growth of E. coli DH10B co-transformants, containing pRSVCATam1.2.5 and one of the pACD MTFs constructs, on 2× YT agar plates containing 50 μg/ml chloramphenicol. The growth phenotypes of cells carrying different mutations at position Ala-89 (A) or Ala-172 (B) are compared with those of cells carrying native MTFs. pACD represents the empty vector control. C and D, relative CAT activities in cell extracts of different co-transformants are shown. CAT activities in cells expressing either Ala-89 (C) or Ala-172 (D) MTFs mutant proteins were compared with those expressing MTFs (set to 100%). CAT activities are normalized to β-lactamase activity in the same extracts to compensate for any fluctuations in plasmid copy number. Experiments were done in duplicate using extracts from two independent sets of transformations. Error bars, S.D. E and F, immunoblot analysis for CAT and β-lactamase in crude extracts (2 μg) prepared from co-transformants expressing either Ala-89 (E) or Ala-172 (F) MTFs mutant proteins using anti-CAT and anti-β-lactamase antibodies. Arrows, positions of bands corresponding to CAT and β-lactamase proteins in different cell extracts.
The activity of MTFs and its mutants in translation initiation was analyzed more quantitatively by measuring CAT activity in cell extracts of different co-transformants. To correct for possible variations in copy number of the pRSVCATam1.2.5 plasmid, CAT activity in each extract was normalized to β-lactamase activity. Fig. 4, C and D, compares the relative CAT activities in various extracts, setting the activity measured in cell extracts carrying MTFs as 100%. The data clearly show that the A89S mutation in MTFs resulted in a small decrease of about 4-fold in CAT activity, whereas the MTFs A89L mutation showed ∼200-fold reduction in CAT activity. On the other hand, the MTFs A172S mutant showed no discernible effect on the CAT activity compared with MTFs, whereas the A172L mutation resulted in a reduction in CAT activity of about 6-fold.
Immunoblot analysis of total cell extracts with anti-CAT antibodies was also used to estimate the cellular levels of CAT in the various co-transformants. All in all, the results agree well with those based on the CAT assays. CAT expression is high in cells carrying MTFs (Fig. 4, E and F; compare lanes 1 and 2). The MTFs A89S mutant showed a moderate decrease in CAT expression level, whereas the A89L mutant failed to show any expression of the reporter CAT enzyme within the detection limits (Fig. 4E). Similarly, CAT protein levels were unchanged in cells carrying the MTFs A172S mutant compared with MTFs. However, replacement of Ala-172 with leucine showed a marked reduction in the expression of the CAT protein consistent with measured CAT activities (Fig. 4F). Taken together, these observations corroborate the effects of these mutations on growth in the presence of chloramphenicol.
Measurement of Steady State Kinetic Parameters for E. coli MTF and Its Mutants
The genetic system for analysis of in vivo activity of E. coli MTF required the introduction of corresponding mutations in MTFs, which used a mutant initiator tRNA as substrate. To investigate more directly the effect of the A89L or A172L mutation on the activity of E. coli MTF, we also introduced these mutations into wild-type E. coli MTF and measured the kinetic parameters of wild-type or the mutant E. coli MTF proteins using native E. coli tRNA2fMet as a substrate in steady state enzyme kinetic assays. The recombinant E. coli MTF and wild-type initiator tRNA were purified to homogeneity as described under “Experimental Procedures.” The assay for MTF activity takes advantage of selective protection of fMet-tRNAMet from CuSO4-mediated cleavage of the ester bond linking methionine to tRNA in Met-tRNAMet (43, 44). The initial velocity was determined by measuring TCA-precipitable [35S]methionine counts at various tRNA substrate concentrations ranging from 0.3 to 2 μm for wild-type or A172L mutant MTF and from 0.5 to 4.5 μm for the MTF A89L mutant. Kinetic parameters were determined using Lineweaver-Burk plots. Mean and S.D. values of different kinetic parameters were deduced from multiple replicates of steady state enzyme kinetic assays.
Table 2 compares the kinetic parameters in formylation of the wild-type initiator tRNA2fMet by the purified wild-type and the mutant MTF enzymes. The Vmax/Km of MTF containing the A89L mutation was about 144-fold lower than that of the wild-type enzyme. The effect is due to both a decrease in Vmax and an increase in Km for the MTF A89L mutant compared with the wild-type MTF. With the A172L mutant MTF, there was only a 4-fold reduction in Vmax/Km with respect to the wild-type enzyme. In this case, the mutation has relatively minor effects on Vmax and Km, which together lead to moderate attenuation of enzyme activity. Overall, the effect of these mutations on wild-type E. coli MTF is similar to that of the corresponding mutations in MTFs in vivo.
TABLE 2.
Steady state kinetic parameters of formylation of E. coli initiator tRNA2fMet using wild-type and mutant E. coli MTF enzymes
Kinetic parameters were measured using Lineweaver-Burk plots. The kinetic parameters listed are the average and S.D. of three independent measurements.
| E. coli MTF | Km | Vmax | Vmax/Km | Approximate -fold decrease in Vmax/Kma |
|---|---|---|---|---|
| μm | pmol·min−1·μg−1 | |||
| Wild type | 0.51 ± 0.08 | 4080.18 ± 635.91 | 8000.35 | 1 |
| A89L | 5.87 ± 2.06 | 326.78 ± 60.51 | 55.67 | 144 |
| A172L | 1.07 ± 0.39 | 1941.09 ± 180.17 | 1814.10 | 4 |
a Approximate -fold decrease in Vmax/Km is the ratio of Vmax/Km of wild-type MTF to that of the mutant MTF, setting wild-type MTF to 1.
Purification of Recombinant Wild-type and Mutant Human mt-MTF Proteins
Analysis of the properties of the S125L and S209L human mt-MTF requires the isolation of wild-type and mutant proteins in homogenous form and, in particular, free of any MTF from E. coli. For this purpose, the genes for wild-type and mutant human mt-MTF were cloned in the plasmid vector pET19b as His-tagged recombinant proteins. The clones contain the open reading frame starting with the residue homologous to the mature form of bovine mt-MTF and lacking the first 29 residues that comprise the mitochondrial targeting sequence (18). The wild-type or mutant mt-MTF proteins expressed in E. coli were purified by chromatography on Ni2+-NTA columns as described under “Experimental Procedures.” Expression and purification of human proteins in E. coli are often hampered by problems due to insolubility, misfolding, or aggregation. Because of this, conditions for expression of recombinant human mt-MTF proteins in E. coli and their purification needed optimization. Initial attempts to purify recombinant human mt-MTF were complicated by co-purification of the chaperone GroEL (45) and poor yield of the recombinant proteins (data not shown). These problems were overcome using a combination of strategies, including (i) use of an N-terminal affinity tag with a longer stretch of histidine residues (His10 instead of His6 tag); (ii) protein expression at low levels by inducing the culture with 0.1 mm isopropyl 1-thio-β-d-galactopyranoside, followed by growth at 18 °C for 6 h (instead of 12–16 h); (iii) incubation of cleared cell lysates with ATP at room temperature to facilitate dissociation of GroEL bound to recombinant protein (46); and (iv) multiple washings of the column material containing bound recombinant human mt-MTF with buffer supplemented with ATP (see “Experimental Procedures”).
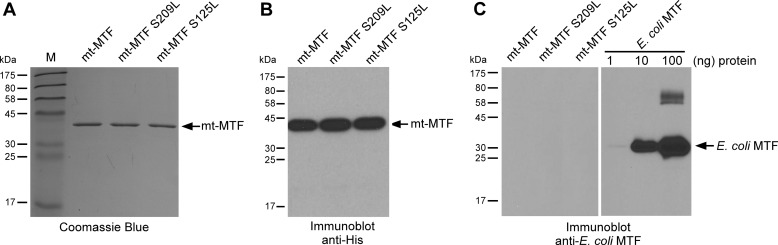
Although the yield was somewhat lower, the purity of mt-MTF proteins increased significantly under these conditions. SDS-PAGE analysis of the dialyzed fraction of each of these proteins showed that the human mt-MTF proteins were at least ∼90% pure (Fig. 5A). Also, immunoblot analysis of these protein preparations with anti-His antibody detected a single band corresponding to the expected molecular mass (∼43.2 kDa), further verifying the identity of these proteins as recombinant human mt-MTFs (Fig. 5B). To rule out the possibility of any contamination by endogenous E. coli MTF, mt-MTF protein preparations were also probed with a polyclonal antibody raised against recombinant E. coli MTF. The results showed the complete absence of detectable levels of E. coli MTF in recombinant mt-MTF preparations (Fig. 5C).
FIGURE 5.
A, a 12% SDS-polyacrylamide gel representing the purified fractions of His10-tagged recombinant human mt-MTF and mutants, as indicated. The recombinant proteins were expressed in E. coli and purified using Ni2+-NTA immobilized metal affinity chromatography (see “Experimental Procedures”). The gel was stained with Coomassie Blue R-250. The first lane represents protein molecular mass markers, and numbers at the left indicate their molecular mass in kDa. B, immunoblot analysis of purified fractions of recombinant human mt-MTF or its mutants using an anti-His monoclonal antibody. The position and molecular mass of individual marker proteins (in kDa) are indicated at the left. C, immunoblot analysis of purified human mt-MTF wild-type and its mutants (2 μg) and E. coli MTF (1, 10, and 100 ng) using an anti-E. coli MTF polyclonal antibody.
Vmax/Km of Wild-type and Mutant Human mt-MTF
Earlier studies on bovine mt-MTF, a closely related homolog of the human enzyme, showed that bovine mt-MTF formylates E. coli initiator Met-tRNAfMet with almost equal efficiency as the cognate mitochondrial Met-tRNAMet (18). We investigated the effect of S125L and S209L mutations on Vmax/Km of human mt-MTF using E. coli tRNA2fMet or a T7 transcript of human mt-tRNAMet as substrate. Similar to bovine mt-MTF (47), the activity of human mt-MTF was substantially lower than that of E. coli MTF. Therefore, the effect of mutations on human mt-MTF activity was analyzed by comparing the Vmax/Km of the wild-type and mutant mt-MTFs at low tRNA substrate concentration (2 μm) in the presence of 0.5% (w/v) CHAPS (18) instead of measuring Vmax and Km individually. The tRNA concentration used is such that it falls within the range in which the initial rate of Met-tRNAMet formylation is proportional to tRNA concentration. The data suggested that with the E. coli tRNA2fMet as substrate, Vmax/Km for the S125L mutant was greatly reduced, by about 107-fold relative to wild-type mt-MTF (Table 3); for the S209L mutant, it was only about 10-fold lower than the wild-type mt-MTF. Thus, mutations at these positions affect the activity of human mt-MTF with E. coli tRNA2fMet as substrate. As in the case of E. coli MTF, the S125L mutation had a substantially greater effect on Vmax/Km than did the S209L mutation.
TABLE 3.
Kinetic parameters of formylation of E. coli Met-tRNA2fMet or a transcript of human mitochondrial Met-RNAMet using human wild-type and mutant mt-MTF enzymes
Vmax/Km listed is the average and S.D. of three independent measurements.
| tRNA | mt-MTF | Vmax/Km | Approximate -fold decrease in Vmax/Kma |
|---|---|---|---|
| pmol·min−1·μg−1·μm−1 | |||
| E. coli tRNA2fMet | Wild type | 1.98 ± 0.181 | 1 |
| S125L | 0.0185 ± 0.002 | 107 | |
| S209L | 0.201 ± 0.029 | 10 | |
| Human mt-RNAMet | Wild type | 3.46 ± 0.389 | 1 |
| S125L | 0.0053 ± 0.0005 | 653 | |
| S209L | 0.096 ± 0.014 | 36 |
a Approximate -fold decrease in Vmax/Km is the ratio of Vmax/Km of wild-type MTF to that of the mutant MTF, setting wild-type MTF to 1.
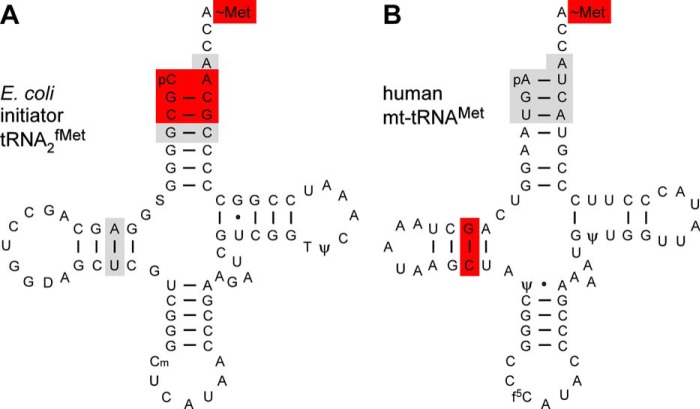
The physiological substrate for human mt-MTF is the native mt-tRNAMet, which is difficult to purify in significant amounts necessary for enzyme kinetic analyses. However, the ready availability, correct folding, and retention of function make the T7 transcript of mt-tRNAMet a good alternative substrate (35, 36). The mt-tRNAMet transcript lacks the three modified nucleotides found in the native mt-tRNAMet, two pseudouridines and a 5-formyl cytidine (f5C) found in the first position of the anticodon (Fig. 6) (48). Despite the absence of post-transcriptional modifications, the T7 transcript of human mt-tRNAMet is functionally active in aminoacylation, formylation, and binding to mt-IF2 (16). Also, a lack of these three modifications is not likely to affect our studies in any significant manner, because the mt-MTF is not expected to interact with regions of the tRNA that contain these modifications.
FIGURE 6.
Cloverleaf structures of E. coli initiator tRNA2fMet (A) and human mt-tRNAMet (B) highlighting the known identity determinants for the corresponding MTF proteins. Red, major determinants; gray, minor determinants. The assessment of major and minor identity determinants was as described earlier (10, 47). f5C, 5-formyl cytidine.
The mt-tRNAMet transcript was aminoacylated in vitro with [35S]methionine using purified mt-MetRS (data not shown). The initial velocity of formylation for the wild-type and mutant mt-MTF enzymes was determined in the presence of low Met-tRNAMet transcript concentration (2 μm). Comparison of Vmax/Km showed that, with the mt-tRNAMet transcript as the substrate, S125L mutant mt-MTF showed a strong reduction (653-fold) in enzyme activity, whereas the S209L mutant was 36-fold less active compared with the wild-type mt-MTF (Table 3).
DISCUSSION
The work described here represents the first biochemical characterization of pathogenic mutations in human mt-MTF that lead to combined oxidative phosphorylation deficiency and Leigh syndrome. Of the two patients with compound heterozygous mutations in mt-MTF (17), P1 has a nonsense (stop codon) mutation at amino acid 128 in one of the MTF genes and an S209L mutation in the other MTF gene. P2 has an S125L mutation in one of the MTF genes and the same S209L mutation as P1 in the other MTF gene. Assuming that the stop codon mutation results in a complete loss of full-length mt-MTF, mitochondrial protein synthesis in P1 depends solely upon the function of the S209L mutant mt-MTF. Our result showing that the S125L mutant mt-MTF is 653-fold less active than wild-type mt-MTF suggests that mitochondrial protein synthesis in fibroblasts of P2 also depends essentially on the activity of the S209L mutant mt-MTF.
The S209L mutation also reduces mt-MTF activity, although the effect is more moderate, down by a factor of 36. This result would explain residual mitochondrial protein synthesis, initiated predominantly with formylmethionine in fibroblasts of P1 and P2 (17). It could also explain why the c.626C→T mutation in the MTF gene, leading to the S209L change in MTF, is the most prevalent one among patients identified so far (25). Subsequent to the report of the first mutations in mt-MTF in patients P1 and P2 in 2011 (17), of the 12 new patients born to non-consanguineous parents identified with mutations in the mt-MTF gene, 10 have the same c.626C→T mutation, with one of the 10 patients also being homozygous for the c.626C→T mutation. The patient homozygous for the c.626C→T mutation must clearly depend upon the function of the S209L mutant mt-MTF for mitochondrial protein synthesis, and it is likely that many of the other patients also do (24, 25).
It was shown previously that fibroblasts from wild-type cell lines contained uncharged mt-tRNAMet and mt-fMet-tRNAMet but no mt-Met-tRNAMet, suggesting that mt-MTF is normally not limiting in mitochondria (17). It is interesting, therefore, that despite the relatively moderate effect of the S209L mutation on MTF activity, mitochondrial protein synthesis in fibroblasts of patients P1 and P2 is severely impaired (17). Also, steady state levels of fMet-tRNAMet are undetectable in mitochondria from patients P1 and P2, suggesting that fMet-tRNAMet levels are limiting and that any fMet-tRNAMet that is synthesized by the mutant MTF is used immediately for translation initiation. As pointed out above, because of the unique situation of the same tRNAMet being used for both initiation and for elongation in vertebrate mitochondria, mt-MTF and mt-EF-Tu compete for the same mt-Met-tRNAMet. Thus, it is possible that the strong effect of the S209L mutation on mitochondrial translation is not only due to the reduced activity of the mutant MTF (36-fold lower) but also due to reduced amounts of mutant MTF synthesized and competition with mt-EF-Tu for the same Met-tRNAMet substrate in mammalian mitochondria.
In trying to assess the effect of the c.626C→T (p.S209L) mutation on the severity of mitochondrial dysfunction in patients, it is important to remember that the c.626C→T mutation has multiple effects at various levels. Besides the potential for producing the full-length S209L mutant MTF, the mutant pre-mRNA can also undergo alternative splicing (19, 20), involving skipping of exon 4, which leads to shifting of the mRNA reading frame and a premature stop codon (17). Use of this stop codon in turn results in degradation of much of the alternatively spliced mRNA by nonsense-mediated decay (21, 22). It is quite likely, however, that there are tissue-specific differences in the extent of alternative splicing (49), and because of this, some tissues in patients could have different levels of full-length S209L mutant MTF compared with other tissues, such as fibroblasts, that were analyzed in previous work with patients P1 and P2 (17). Our finding that the activity of the S209L mutant protein is not as severely impaired as that of the S125L mutant could be important in limiting the severity of mitochondrial dysfunction in patients carrying the S209L mutation only to certain tissues and/or to certain individuals (50).
Amino acid sequences in and around Ser-125 and Ser-209 of human mt-MTF, the sites of pathogenic mutations in P1 and P2, are highly conserved both in eukaryotic and in bacterial MTFs (Fig. 2, A and B). The amino acids corresponding to Ser-125 and Ser-209 in human mt-MTF are Ala-89 and Ala-172 in E. coli. Remarkably, Ser-125 of human mt-MTF is invariably serine in eukaryotes, and the corresponding position is invariably alanine in bacterial MTF. Analysis of A89S and A89L mutants of E. coli MTFs in vivo showed that mutation of Ala-89 to serine had a minor effect, whereas mutation to leucine had a major effect. In contrast, mutation of Ala-172 to serine or to leucine had only a small effect (Fig. 4). Direct analysis in vitro of the effect of A89L and A172L mutations on E. coli MTF activity showed that whereas the A89L mutation reduced Vmax/Km by 144-fold, A172L mutation reduced it by only 4-fold (Table 2). These results parallel those obtained with the corresponding human mt-MTFs (Table 3) and show that mutations of alanine to leucine at Ala-89 (corresponding to Ser-125 of mt-MTF) are more deleterious than at Ala-172 (corresponding to Ser-209). Combined with the fact that the human mt-MTF has ∼30% sequence identity with the E. coli enzyme and, like all other MTFs known to date, has the highly conserved sequences possibly involved in FTHF binding and catalysis (Fig. 7D), these findings suggest that E. coli and human mt-MTF use similar mechanisms for substrate binding and catalysis (38, 39).
In trying to understand the molecular mechanism of the effect of the S125L and S209L mutations on the activity of human mt-MTF, we have superimposed these mutations on the known structure of E. coli MTF complexed to fMet-tRNAfMet (39). In E. coli MTF, the side chain of Ala-89 (corresponding to Ser-125) forms part of a hydrophobic pocket consisting of amino acids Phe-14, Ile-123, Val-136, Leu-171, Pro-122, and Tyr-168, which bind to methionine of the fMet-tRNAfMet (Fig. 7C) (39). Of the seven amino acids lining the methionine binding pocket of E. coli MTF, the human mt-MTF has four amino acids that are identical (phenylalanine, proline, valine, and leucine) and two that are similar (serine instead of alanine and isoleucine instead of valine) (Fig. 7D). Therefore, the human mt-MTF probably has a binding pocket for methionine similar to that of E. coli MTF. The strong negative effect of mutation of Ala-89 to leucine on activity of E. coli MTF most likely occurs because replacement of this absolutely conserved alanine with leucine creates unfavorable interactions with Pro-14, Pro-12, Val-88, or the methyl group of methionine (Fig. 7). Phe-14 is in the methionine binding pocket, and Pro-12 and Val-88 are nearby, and a clash of the leucine side chain with these residues and methionine would significantly perturb the methionine binding pocket of MTF.
Compared with the strong effect of the A89L mutation on E. coli MTF activity, the effect of the A172L mutation is relatively mild (4-fold). In contrast to Ala-89, which is present in the methionine binding pocket of E. coli MTF, Ala-172 is distal to the active site and is located in a long α helix (helix 6) closer to the C-terminal region of MTF, although Tyr-168 and Leu-171 in helix 6 are also part of the methionine binding pocket (Fig. 7, B and C). The small effect of the A172L mutation on E. coli MTF activity is presumably indirect. In the crystal structure of E. coli MTF, helix 6 is adjacent to helix 1, which contains Phe-14 that is close to the active site. It is possible that mutation of Ala-172 to leucine in E. coli MTF perturbs interactions between helix 1 and helix 6, leading to changes in the active site (51, 52).
Human mt-MTF has ∼30% sequence identity and long stretches of sequence homology with the E. coli MTF in the regions surrounding Ala-89 and Ala-172 (Fig. 2, A and B). Therefore, conclusions reached above with the E. coli enzyme on the molecular basis of the effect of Ala-89 to leucine and Ala-172 to leucine mutations are likely to also hold for the corresponding Ser-125 to leucine and Ser-209 to leucine mutations in the human enzyme. The effect of the S209L mutation on the activity of the human enzyme (36-fold decrease) is, however, more deleterious than with the E. coli enzyme (4-fold decrease). This could be due to the fact that in addition to methionine attached to the tRNA (Fig. 6), E. coli MTF uses many sequence elements in the acceptor stem of the initiator tRNA as critical determinants for formylation (10), whereas the human mitochondrial enzyme uses the methionine attached to the tRNA and the purine 11:pyrimidine 24 base pair in the D stem as the two most critical determinants for formylation of Met-tRNAMet (47). Therefore, the human mt-MTF could be more susceptible to relatively small changes in the methionine binding pocket and the tRNA D stem-binding region (39) of the mutant enzymes compared with the E. coli enzyme.
Finally, following the use of exome sequencing (53) for identification of pathogenic mutations in mt-MTF in patients P1 and P2 (17), the use of a similar approach has led to the identification of mutations in mt-MTF in 13 more patients, including several new mutations (23–25). It is hoped that the biochemical characterization of mutant mt-MTFs as described here will prove useful in studying the effects of newly identified mutations on activities of the mutant mt-MTFs.
Acknowledgments
We thank Annmarie McInnis for help with preparing the manuscript and Drs. Emmanuelle Schmitt and Yves Mechulam for suggestions on the possible effects of the mutations in MTF on its structure.
This work was supported, in whole or in part, by National Institutes of Health Grants GM17151 (to U. L. R.), 5RNIAG042169 (to Y. M. H.), and GM097136 (to V. K. M.).
- tRNAMet
- methionine tRNA
- Met-tRNAMet
- methionyl-tRNA
- fMet-tRNAMet
- formylmethionyl-tRNAMet
- tRNA2fMet
- E. coli initiator tRNA species 2
- MTF
- methionyl-tRNA formyltransferase
- mt-MTF
- mitochondrial MTF
- IF2
- initiation factor 2
- mt-IF2
- mitochondrial IF2
- mt-EF-Tu
- mitochondrial elongation factor Tu
- mt-tRNAMet
- human mitochondrial methionine tRNA
- CAT
- chloramphenicol acetyltransferase
- FTHF
- N10-formyl tetrahydrofolate
- MetRS
- methionyl-tRNA synthetase
- mt-MetRS
- mitochondrial MetRS
- MTFs
- suppressor mutant of E. coli methionyl-tRNA formyltransferase carrying a G41R mutation
- NTA
- nitrilotriacetic acid.
REFERENCES
- 1. Kozak M. (1983) Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol. Rev. 47, 1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Christian B. E., Spremulli L. L. (2012) Mechanism of protein biosynthesis in mammalian mitochondria. Biochim. Biophys. Acta 1819, 1035–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lightowlers R. N., Rozanska A., Chrzanowska-Lightowlers Z. M. (2014) Mitochondrial protein synthesis: figuring the fundamentals, complexities and complications, of mammalian mitochondrial translation. FEBS Lett. 588, 2496–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F., Schreier P. H., Smith A. J., Staden R., Young I. G. (1981) Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 [DOI] [PubMed] [Google Scholar]
- 5. Attardi G. (1985) Animal mitochondrial DNA: an extreme example of genetic economy. Int. Rev. Cytol. 93, 93–145 [DOI] [PubMed] [Google Scholar]
- 6. Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. (1982) Complete sequence of bovine mitochondrial DNA: conserved features of the mammalian mitochondrial genome. J. Mol. Biol. 156, 683–717 [DOI] [PubMed] [Google Scholar]
- 7. Jacobs H. T., Turnbull D. M. (2005) Nuclear genes and mitochondrial translation: a new class of genetic disease. Trends Genet. 21, 312–314 [DOI] [PubMed] [Google Scholar]
- 8. Gold L. (1988) Posttranscriptional regulatory mechanisms in Escherichia coli. Annu. Rev. Biochem. 57, 199–233 [DOI] [PubMed] [Google Scholar]
- 9. Gualerzi C. O., Pon C. L. (1990) Initiation of mRNA translation in prokaryotes. Biochemistry 29, 5881–5889 [DOI] [PubMed] [Google Scholar]
- 10. RajBhandary U. L. (1994) Initiator transfer RNAs. J. Bacteriol. 176, 547–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Canonaco M. A., Calogero R. A., Gualerzi C. O. (1986) Mechanism of translational initiation in prokaryotes. Evidence for a direct effect of IF2 on the activity of the 30 S ribosomal subunit. FEBS Lett. 207, 198–204 [DOI] [PubMed] [Google Scholar]
- 12. Sundari R. M., Stringer E. A., Schulman L. H., Maitra U. (1976) Interaction of bacterial initiation factor 2 with initiator tRNA. J. Biol. Chem. 251, 3338–3345 [PubMed] [Google Scholar]
- 13. Nissen P., Kjeldgaard M., Thirup S., Polekhina G., Reshetnikova L., Clark B. F., Nyborg J. (1995) Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science 270, 1464–1472 [DOI] [PubMed] [Google Scholar]
- 14. Woriax V. L., Spremulli G. H., Spremulli L. L. (1996) Nucleotide and aminoacyl-tRNA specificity of the mammalian mitochondrial elongation factor EF-Tu·Ts complex. Biochim. Biophys. Acta 1307, 66–72 [DOI] [PubMed] [Google Scholar]
- 15. Seong B. L., RajBhandary U. L. (1987) Mutants of Escherichia coli formylmethionine tRNA: a single base change enables initiator tRNA to act as an elongator in vitro. Proc. Natl. Acad. Sci. U.S.A. 84, 8859–8863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spencer A. C., Spremulli L. L. (2004) Interaction of mitochondrial initiation factor 2 with mitochondrial fMet-tRNA. Nucleic Acids Res. 32, 5464–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tucker E. J., Hershman S. G., Köhrer C., Belcher-Timme C. A., Patel J., Goldberger O. A., Christodoulou J., Silberstein J. M., McKenzie M., Ryan M. T., Compton A. G., Jaffe J. D., Carr S. A., Calvo S. E., RajBhandary U. L., Thorburn D. R., Mootha V. K. (2011) Mutations in MTFMT underlie a human disorder of formylation causing impaired mitochondrial translation. Cell Metab. 14, 428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takeuchi N., Kawakami M., Omori A., Ueda T., Spremulli L. L., Watanabe K. (1998) Mammalian mitochondrial methionyl-tRNA transformylase from bovine liver. Purification, characterization, and gene structure. J. Biol. Chem. 273, 15085–15090 [DOI] [PubMed] [Google Scholar]
- 19. Fairbrother W. G., Yeh R. F., Sharp P. A., Burge C. B. (2002) Predictive identification of exonic splicing enhancers in human genes. Science 297, 1007–1013 [DOI] [PubMed] [Google Scholar]
- 20. Wang Z., Rolish M. E., Yeo G., Tung V., Mawson M., Burge C. B. (2004) Systematic identification and analysis of exonic splicing silencers. Cell 119, 831–845 [DOI] [PubMed] [Google Scholar]
- 21. Kervestin S., Jacobson A. (2012) NMD: a multifaceted response to premature translational termination. Nat. Rev. Mol. Cell Biol. 13, 700–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Isken O., Maquat L. E. (2007) Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 21, 1833–1856 [DOI] [PubMed] [Google Scholar]
- 23. Neeve V. C., Pyle A., Boczonadi V., Gomez-Duran A., Griffin H., Santibanez-Koref M., Gaiser U., Bauer P., Tzschach A., Chinnery P. F., Horvath R. (2013) Clinical and functional characterisation of the combined respiratory chain defect in two sisters due to autosomal recessive mutations in MTFMT. Mitochondrion 13, 743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Haack T. B., Haberberger B., Frisch E. M., Wieland T., Iuso A., Gorza M., Strecker V., Graf E., Mayr J. A., Herberg U., Hennermann J. B., Klopstock T., Kuhn K. A., Ahting U., Sperl W., Wilichowski E., Hoffmann G. F., Tesarova M., Hansikova H., Zeman J., Plecko B., Zeviani M., Wittig I., Strom T. M., Schuelke M., Freisinger P., Meitinger T., Prokisch H. (2012) Molecular diagnosis in mitochondrial complex I deficiency using exome sequencing. J. Med. Genet. 49, 277–283 [DOI] [PubMed] [Google Scholar]
- 25. Haack T. B., Gorza M., Danhauser K., Mayr J. A., Haberberger B., Wieland T., Kremer L., Strecker V., Graf E., Memari Y., Ahting U., Kopajtich R., Wortmann S. B., Rodenburg R. J., Kotzaeridou U., Hoffmann G. F., Sperl W., Wittig I., Wilichowski E., Schottmann G., Schuelke M., Plecko B., Stephani U., Strom T. M., Meitinger T., Prokisch H., Freisinger P. (2014) Phenotypic spectrum of eleven patients and five novel MTFMT mutations identified by exome sequencing and candidate gene screening. Mol. Genet. Metab. 111, 342–352 [DOI] [PubMed] [Google Scholar]
- 26. Varshney U., RajBhandary U. L. (1990) Initiation of protein synthesis from a termination codon. Proc. Natl. Acad. Sci. U.S.A. 87, 1586–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramesh V., Gite S., Li Y., RajBhandary U. L. (1997) Suppressor mutations in Escherichia coli methionyl-tRNA formyltransferase: role of a 16-amino acid insertion module in initiator tRNA recognition. Proc. Natl. Acad. Sci. U.S.A. 94, 13524–13529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanahan D. (1985) in DNA Cloning: A Practical Approach (Glover D. M., ed.) pp. 109–135, IRL Press, Oxford [Google Scholar]
- 29. Yanisch-Perron C., Vieira J., Messing J. (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33, 103–119 [DOI] [PubMed] [Google Scholar]
- 30. Studier F. W., Moffatt B. A. (1986) Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189, 113–130 [DOI] [PubMed] [Google Scholar]
- 31. Mandal N., RajBhandary U. L. (1992) Escherichia coli B lacks one of the two initiator tRNA species present in E. coli K-12. J. Bacteriol. 174, 7827–7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 33. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 34. Seong B. L., RajBhandary U. L. (1987) Escherichia coli formylmethionine tRNA: mutations in GGG:CCC sequence conserved in anticodon stem of initiator tRNAs affect initiation of protein synthesis and conformation of anticodon loop. Proc. Natl. Acad. Sci. U.S.A. 84, 334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Spencer A. C., Heck A., Takeuchi N., Watanabe K., Spremulli L. L. (2004) Characterization of the human mitochondrial methionyl-tRNA synthetase. Biochemistry 43, 9743–9754 [DOI] [PubMed] [Google Scholar]
- 36. Hou Y. M. (2012) High-purity enzymatic synthesis of site-specifically modified tRNA. Methods Mol. Biol. 941, 195–212 [DOI] [PubMed] [Google Scholar]
- 37. Kahn D., Fromant M., Fayat G., Dessen P., Blanquet S. (1980) Methionyl-transfer-RNA transformylase from Escherichia coli: purification and characterisation. Eur. J. Biochem. 105, 489–497 [DOI] [PubMed] [Google Scholar]
- 38. Schmitt E., Blanquet S., Mechulam Y. (1996) Structure of crystalline Escherichia coli methionyl-tRNA(f)Met formyltransferase: comparison with glycinamide ribonucleotide formyltransferase. EMBO J. 15, 4749–4758 [PMC free article] [PubMed] [Google Scholar]
- 39. Schmitt E., Panvert M., Blanquet S., Mechulam Y. (1998) Crystal structure of methionyl-tRNAfMet transformylase complexed with the initiator formyl-methionyl-tRNAfMet. EMBO J. 17, 6819–6826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramesh V., Gite S., RajBhandary U. L. (1998) Functional interaction of an arginine conserved in the sixteen amino acid insertion module of Escherichia coli methionyl-tRNA formyltransferase with determinants for formylation in the initiator tRNA. Biochemistry 37, 15925–15932 [DOI] [PubMed] [Google Scholar]
- 41. Gite S., Li Y., Ramesh V., RajBhandary U. L. (2000) Escherichia coli methionyl-tRNA formyltransferase: role of amino acids conserved in the linker region and in the C-terminal domain on the specific recognition of the initiator tRNA. Biochemistry 39, 2218–2226 [DOI] [PubMed] [Google Scholar]
- 42. Varshney U., Lee C. P., Seong B. L., RajBhandary U. L. (1991) Mutants of initiator tRNA that function both as initiators and elongators. J. Biol. Chem. 266, 18018–18024 [PubMed] [Google Scholar]
- 43. Schofield P., Zamecnik P. C. (1968) Cupric ion catalysis in hydrolysis of aminoacyl-tRNA. Biochim. Biophys. Acta 155, 410–416 [DOI] [PubMed] [Google Scholar]
- 44. Guillon J. M., Mechulam Y., Schmitter J. M., Blanquet S., Fayat G. (1992) Disruption of the gene for Met-tRNA(fMet) formyltransferase severely impairs growth of Escherichia coli. J. Bacteriol. 174, 4294–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martin J., Mayhew M., Langer T., Hartl F. U. (1993) The reaction cycle of GroEL and GroES in chaperonin-assisted protein folding. Nature 366, 228–233 [DOI] [PubMed] [Google Scholar]
- 46. Thain A., Gaston K., Jenkins O., Clarke A. R. (1996) A method for the separation of GST fusion proteins from co-purifying GroEL. Trends Genet. 12, 209–210 [DOI] [PubMed] [Google Scholar]
- 47. Takeuchi N., Vial L., Panvert M., Schmitt E., Watanabe K., Mechulam Y., Blanquet S. (2001) Recognition of tRNAs by methionyl-tRNA transformylase from mammalian mitochondria. J. Biol. Chem. 276, 20064–20068 [DOI] [PubMed] [Google Scholar]
- 48. Moriya J., Yokogawa T., Wakita K., Ueda T., Nishikawa K., Crain P. F., Hashizume T., Pomerantz S. C., McCloskey J. A., Kawai G. (1994) A novel modified nucleoside found at the first position of the anticodon of methionine tRNA from bovine liver mitochondria. Biochemistry 33, 2234–2239 [DOI] [PubMed] [Google Scholar]
- 49. Nilsen T. W., Graveley B. R. (2010) Expansion of the eukaryotic proteome by alternative splicing. Nature 463, 457–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lalonde E., Ha K. C., Wang Z., Bemmo A., Kleinman C. L., Kwan T., Pastinen T., Majewski J. (2011) RNA sequencing reveals the role of splicing polymorphisms in regulating human gene expression. Genome Res. 21, 545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nilsson A. I., Zorzet A., Kanth A., Dahlström S., Berg O. G., Andersson D. I. (2006) Reducing the fitness cost of antibiotic resistance by amplification of initiator tRNA genes. Proc. Natl. Acad. Sci. U.S.A. 103, 6976–6981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li Y., Ramesh V., Mangroo D., Taneja C., RajBhandary U. L. (2000) Suppressor mutations in Escherichia coli methionyl-tRNA formyltransferase that compensate for the formylation defect of a mutant tRNA aminoacylated with lysine. Biochemistry 39, 8039–8046 [DOI] [PubMed] [Google Scholar]
- 53. Calvo S. E., Tucker E. J., Compton A. G., Kirby D. M., Crawford G., Burtt N. P., Rivas M., Guiducci C., Bruno D. L., Goldberger O. A., Redman M. C., Wiltshire E., Wilson C. J., Altshuler D., Gabriel S. B., Daly M. J., Thorburn D. R., Mootha V. K. (2010) High-throughput, pooled sequencing identifies mutations in NUBPL and FOXRED1 in human complex I deficiency. Nat. Genet. 42, 851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Schulman L. H., Pelka H. (1985) In vitro conversion of a methionine to a glutamine-acceptor tRNA. Biochemistry 24, 7309–7314 [DOI] [PubMed] [Google Scholar]
- 55. Mangroo D., RajBhandary U. L. (1995) Mutants of Escherichia coli initiator tRNA defective in initiation. Effects of overproduction of methionyl-tRNA transformylase and the initiation factors IF2 and IF3. J. Biol. Chem. 270, 12203–12209 [DOI] [PubMed] [Google Scholar]