Abstract
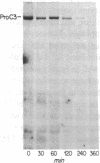
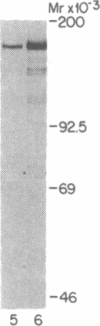
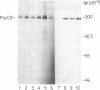
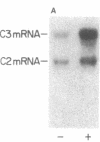
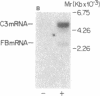
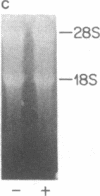
The third component of complement (C3) is a plasma glycoprotein with a variety of biologic functions in the initiation and maintenance of host response to infectious agents. While the hepatocyte is the primary source of plasma C3, mononuclear phagocytes contribute to the regulation of tissue availability of C3. Lipopolysaccharide (LPS), a constituent of cell walls of gram-negative bacteria, consists of a polysaccharide moiety (core polysaccharide and O antigen) covalently linked to a lipid portion (lipid A). Using metabolic labeling with [35S]methionine, immunoprecipitation, and SDS-polyacrylamide gel electrophoresis, we examined the effects of LPS on synthesis of C3 by human mononuclear phagocytes as well as synthesis of the second component of complement (C2), factor B, lysozyme, and total protein. LPS increased C3 synthesis 5-30-fold without affecting the kinetics of secretion of C3 or the synthesis of C2, lysozyme, or total protein. Factor B synthesis was consistently increased by LPS. Experiments with lipid A-inactivated LPS (alkaline treated), LPS from a polysaccharide mutant strain, and lipid X (a lipid A precursor) indicated that the lipid A portion is the structural element required for this effect. Northern blot analysis demonstrated at least a fivefold increase in C3 mRNA in LPS-treated monolayers, which suggests that the regulation of the increase in C3 synthesis is pretranslational. C2 mRNA and factor B mRNA were increased approximately twofold. The availability of specific gene products in human mononuclear phagocytes that respond to LPS should permit understanding of the molecular regulation of more complex functions of these cells elicited by LPS in which multiple gene products are coordinately expressed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Evans R. Endotoxin and double stranded RNA render macrophages cytotoxic. Nat New Biol. 1971 Jul 21;232(29):76–78. doi: 10.1038/newbio232076a0. [DOI] [PubMed] [Google Scholar]
- Brade V., Hall R. E., Colten H. R. Biosynthesis of pro-C3, a precursor of the third component of complement. J Exp Med. 1977 Sep 1;146(3):759–765. doi: 10.1084/jem.146.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole F. S., Auerbach H. S., Goldberger G., Colten H. R. Tissue-specific pretranslational regulation of complement production in human mononuclear phagocytes. J Immunol. 1985 Apr;134(4):2610–2616. [PubMed] [Google Scholar]
- Cooper P. H., Mayer P., Baggiolini M. Stimulation of phagocytosis in bone marrow-derived mouse macrophages by bacterial lipopolysaccharide: correlation with biochemical and functional parameters. J Immunol. 1984 Aug;133(2):913–922. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Hoffman J. S., Quaife C. J., Benditt E. P., Chen H. Y., Brinster R. L., Palmiter R. D. Induction of mouse metallothionein-I mRNA by bacterial endotoxin is independent of metals and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1053–1056. doi: 10.1073/pnas.81.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein L. P., Hansen P. J., Ballow M., Davis A. E., 3rd, Davis J. S., 4th, Alper C. A., Rosen F. S., Colten H. R. Biosynthesis of the third component of complement (C3) in vitro by monocytes from both normal and homozygous C3-deficient humans. J Clin Invest. 1977 Nov;60(5):963–969. doi: 10.1172/JCI108876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein L. P., Schneeberger E. E., Colten H. R. Synthesis of the second component of complement by long-term primary cultures of human monocytes. J Exp Med. 1976 Jan 1;143(1):114–126. doi: 10.1084/jem.143.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Sim R. B., Hill M., Gordon S. Local opsonization by secreted macrophage complement components. Role of receptors for complement in uptake of zymosan. J Exp Med. 1984 Jan 1;159(1):244–260. doi: 10.1084/jem.159.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger G., Thomas M. L., Tack B. F., Williams J., Colten H. R., Abraham G. N. NH2-terminal structure and cleavage of guinea pig pro-C3, the precursor of the third complement component. J Biol Chem. 1981 Dec 25;256(24):12617–12619. [PubMed] [Google Scholar]
- Gordon S., Unkeless J. C., Cohn Z. A. Induction of macrophage plasminogen activator by endotoxin stimulation and phagocytosis: evidence for a two-stage process. J Exp Med. 1974 Oct 1;140(4):995–1010. doi: 10.1084/jem.140.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie C. C., Musson R. A., Henson P. M. Production of growth factor activity for fibroblasts by human monocyte-derived macrophages. J Leukoc Biol. 1984 Aug;36(2):143–159. doi: 10.1002/jlb.36.2.143. [DOI] [PubMed] [Google Scholar]
- Miyama A., Kawamoto Y., Ichikawa H., Okamoto K., Hara S., Inoue T. Complement proteins and macrophages. II. The secretion of factor B by lipopolysaccharide-stimulated macrophages. Microbiol Immunol. 1980;24(12):1223–1232. doi: 10.1111/j.1348-0421.1980.tb02926.x. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Musson R. A., Shafran H., Henson P. M. Intracellular levels and stimulated release of lysosomal enzymes from human peripheral blood monocytes and monocyte-derived macrophages. J Reticuloendothel Soc. 1980 Sep;28(3):249–264. [PubMed] [Google Scholar]
- Nelson K. J., Mather E. L., Perry R. P. Lipopolysaccharide-induced transcription of the kappa immunoglobulin locus occurs on both alleles and is independent of methylation status. Nucleic Acids Res. 1984 Feb 24;12(4):1911–1923. doi: 10.1093/nar/12.4.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima M., Amano F., Akamatsu Y., Akagawa K., Tokunaga T., Raetz C. R. Macrophage activation by monosaccharide precursors of Escherichia coli lipid A. Proc Natl Acad Sci U S A. 1985 Jan;82(2):282–286. doi: 10.1073/pnas.82.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Passwell J. H., Colten H. R., Schneeberger E. L., Marom Z., Merler E. Modulation of human monocyte functions by Fc fragments of IgG: a comparison to other monocyte 'activators'. Immunology. 1980 Sep;41(1):217–225. [PMC free article] [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. The biochemistry of complement. Nature. 1978 Oct 26;275(5682):699–704. doi: 10.1038/275699a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Colten H. R. Rheumatoid arthritis. Biosynthesis of complement proteins by synovial tissues. N Engl J Med. 1974 Jun 6;290(23):1284–1288. doi: 10.1056/NEJM197406062902304. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Strunk R. C., Kunke K. S., Giclas P. C. Human peripheral blood monocyte-derived macrophages produce haemolytically active C3 in vitro. Immunology. 1983 May;49(1):169–174. [PMC free article] [PubMed] [Google Scholar]
- Strunk R. C., Kunke K. S., Musson R. A. Lack of requirement for spreading for macrophages to synthesize complement. J Reticuloendothel Soc. 1980 Nov;28(5):483–493. [PubMed] [Google Scholar]
- Strunk R. C., Tashjian A. H., Jr, Colten H. R. Complement biosynthesis in vitro by rat hepatoma cell strains. J Immunol. 1975 Jan;114(1 Pt 2):331–335. [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Sundsmo J. S., Chin J. R., Papin R. A., Fair D. S., Werb Z. Factor B, the complement alternative pathway serine proteinase, is a major constitutive protein synthesized and secreted by resident and elicited mouse macrophages. J Exp Med. 1985 Feb 1;161(2):306–322. doi: 10.1084/jem.161.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P., Nashed M. A., Anderson L., Raetz C. R. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A. Complete structure of A diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J Biol Chem. 1983 Jun 25;258(12):7379–7385. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Madonna G. S., Wahl L. M., Rick P. D. In vitro stimulation of C3H/HeJ spleen cells and macrophages by a lipid A precursor molecule derived from Salmonella typhimurium. J Immunol. 1984 Jan;132(1):347–353. [PubMed] [Google Scholar]
- Vogel S. N., Weedon L. L., Moore R. N., Rosenstreich D. L. Correction of defective macrophage differentiation in C3H/HeJ mice by an interferon-like molecule. J Immunol. 1982 Jan;128(1):380–387. [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by endotoxin-activated macrophages. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3598–3601. doi: 10.1073/pnas.71.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetsel R. A., Lundwall A., Davidson F., Gibson T., Tack B. F., Fey G. H. Structure of murine complement component C3. II. Nucleotide sequence of cloned complementary DNA coding for the alpha chain. J Biol Chem. 1984 Nov 25;259(22):13857–13862. [PubMed] [Google Scholar]
- Wightman P. D., Raetz C. R. The activation of protein kinase C by biologically active lipid moieties of lipopolysaccharide. J Biol Chem. 1984 Aug 25;259(16):10048–10052. [PubMed] [Google Scholar]
- Woods D. E., Edge M. D., Colten H. R. Isolation of a complementary DNA clone for the human complement protein C2 and its use in the identification of a restriction fragment length polymorphism. J Clin Invest. 1984 Aug;74(2):634–638. doi: 10.1172/JCI111461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Markham A. F., Ricker A. T., Goldberger G., Colten H. R. Isolation of cDNA clones for the human complement protein factor B, a class III major histocompatibility complex gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5661–5665. doi: 10.1073/pnas.79.18.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Tucker P. W. Effect of lipopolysaccharide stimulation on the transcription and translation of messenger RNA for cell surface immunoglobulin M. J Exp Med. 1982 Oct 1;156(4):962–974. doi: 10.1084/jem.156.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer B., Hartung H. P., Scharfenberger G., Bitter-Suermann D., Hadding U. Quantitative studies of the secretion of complement component C3 by resident, elicited and activated macrophages. Comparison with C2, C4 and lysosomal enzyme release. Eur J Immunol. 1982 May;12(5):426–430. doi: 10.1002/eji.1830120513. [DOI] [PubMed] [Google Scholar]